A Highly Sensitive Method for the Detection of Hydrolyzed Gluten in Beer Samples Using LFIA
Abstract
1. Introduction
2. Materials and Methods
2.1. Reference Standards
2.2. Beer Samples
2.3. Spiked Samples
2.4. Techniques Employed
2.4.1. Development of a New Method
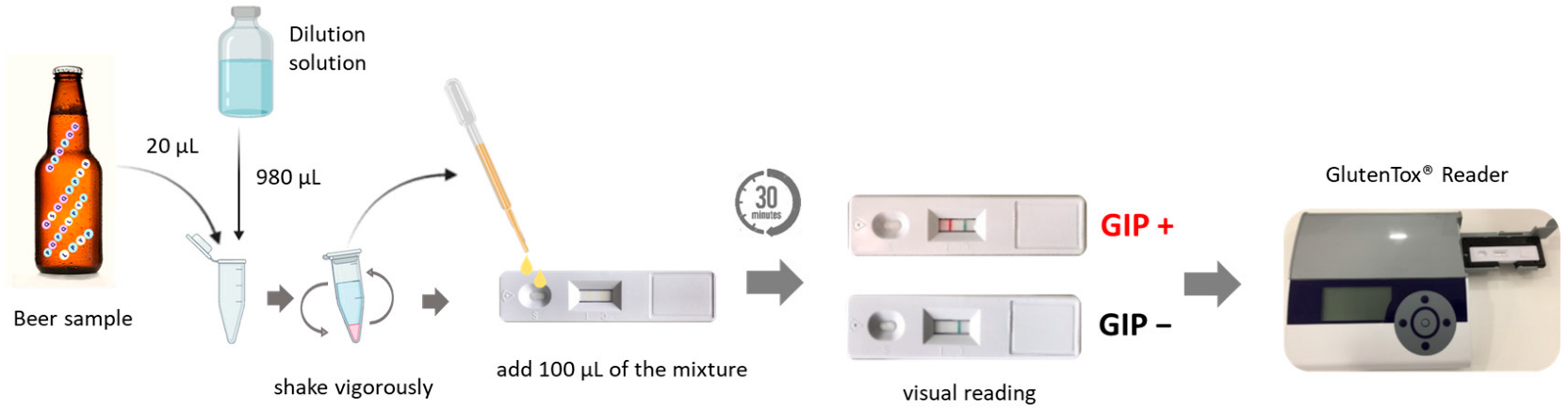
Lateral Flow Immunochromatographic Assays (LFIAs)
Analysis of Samples and Data
2.4.2. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Statistical Anlysis
3. Results and Discussion
3.1. Validation of the New Analytical Method
3.1.1. Immunochromatographic Strip Sensitivity and the LOD
3.1.2. Strip Reader Detection Capacity
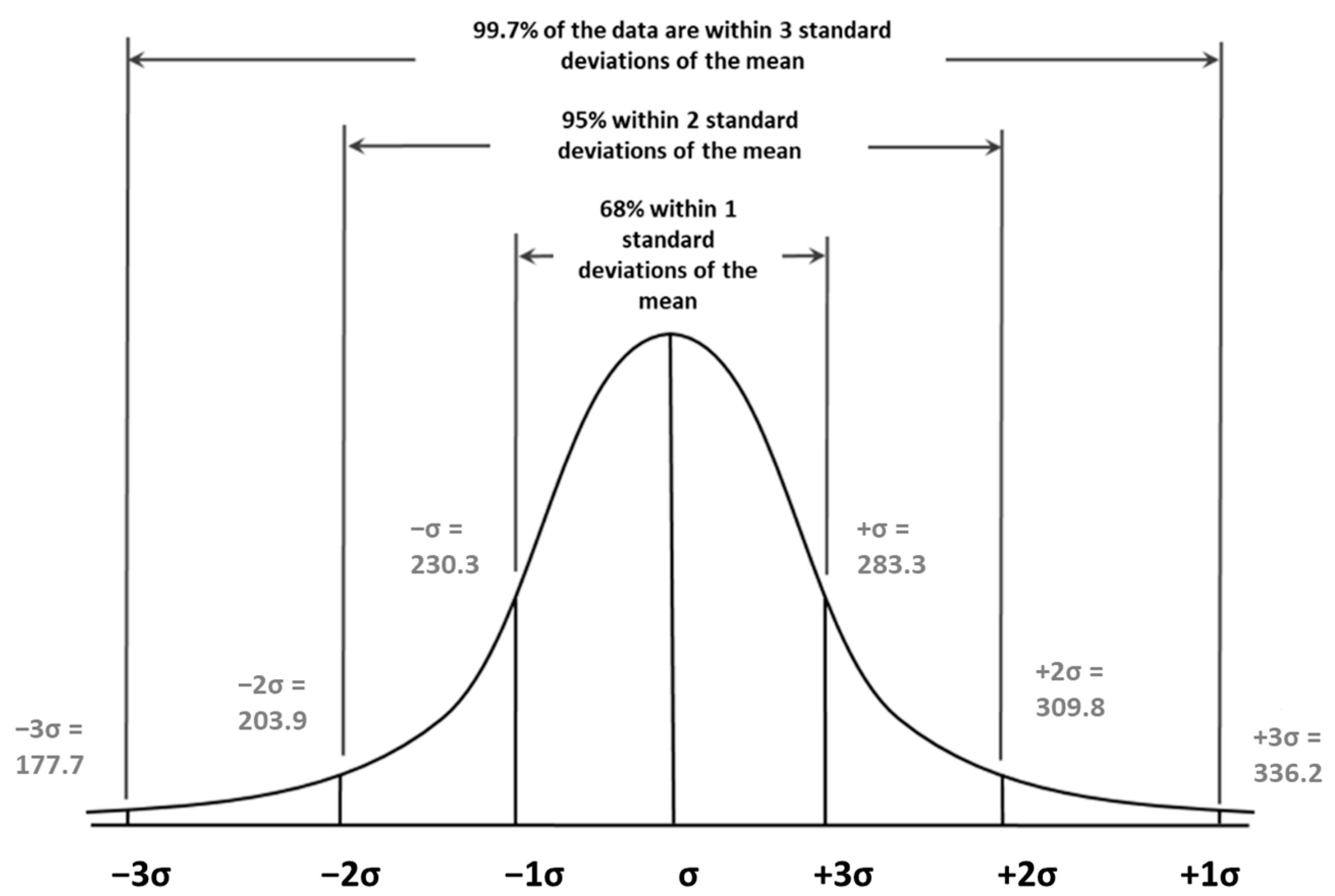
3.1.3. Accuracy and Precision
3.2. Determination of the Relative Affinity from Different Reference Standards
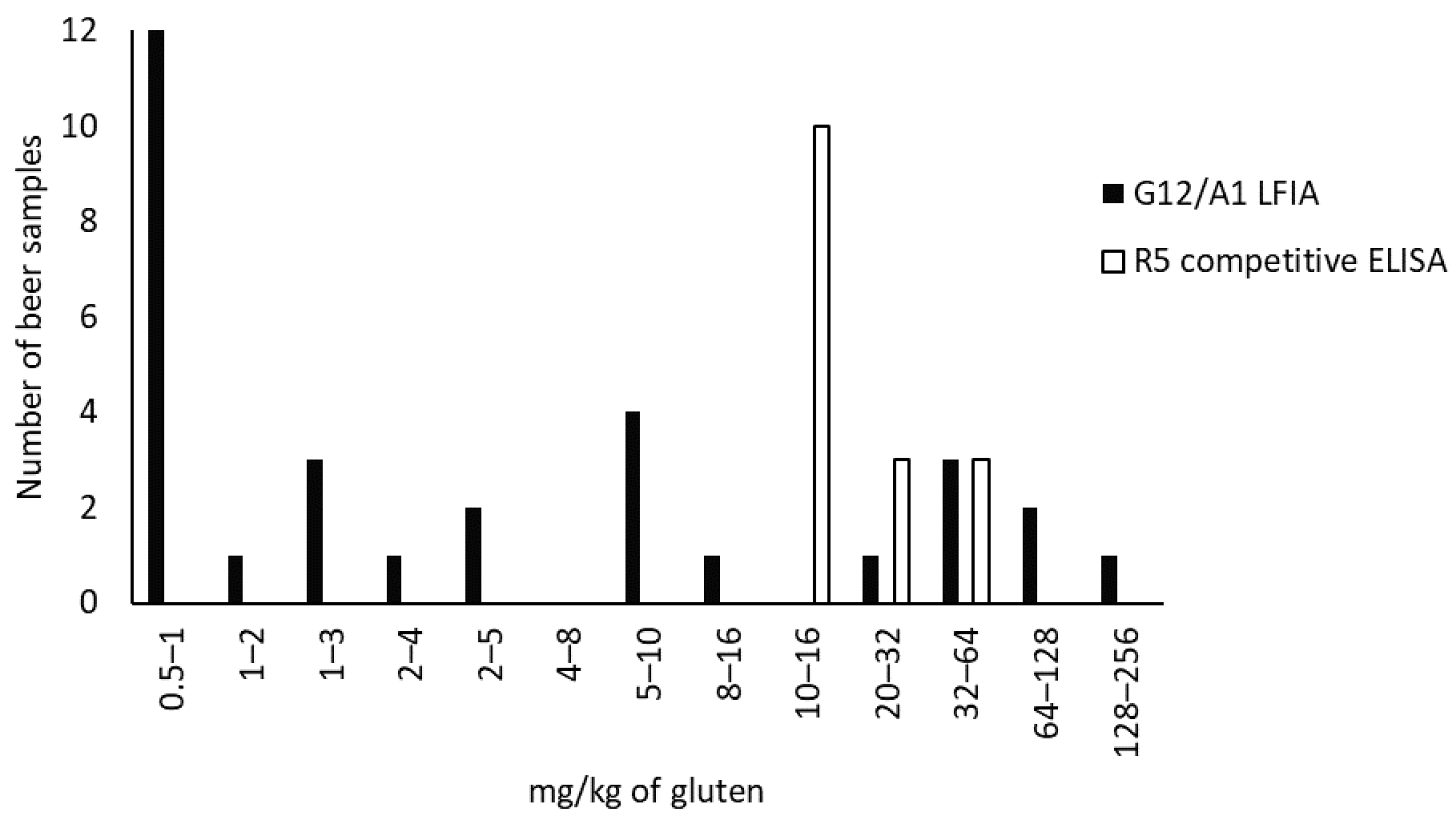
3.3. Gluten Analysis in Beer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Molina, M.D.; Giménez, M.J.; Sánchez-León, S.; Barro, F. Gluten Free Wheat: Are We There? Nutrients 2019, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Swaroop, A.; Bagchi, D. An overview of gluten-free foods and related disorders. In Nutraceutical and Functional Food Regulations in the States and around the World; Elsevier: Amsterdam, The Netherlands, 2019; pp. 75–85. [Google Scholar]
- Elli, L.; Branchi, F.; Tomba, C.; Villalta, D.; Norsa, L.; Ferretti, F.; Roncoroni, L.; Bardella, M.T. Diagnosis of gluten related disorders: Celiac disease, wheat allergy and non-celiac gluten sensitivity. World J. Gastroenterol. 2015, 21, 7110–7119. [Google Scholar] [CrossRef] [PubMed]
- Bascuñán, K.A.; Roncoroni, L.; Branchi, F.; Doneda, L.; Scricciolo, A.; Ferretti, F.; Araya, M.; Elli, L. The 5 Ws of a gluten challenge for gluten-related disorders. Nutr. Rev. 2018, 76, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Taraghikhah, N.; Ashtari, S.; Asri, N.; Shahbazkhani, B.; Al-Dulaimi, D.; Rostami-Nejad, M.; Rezaei-Tavirani, M.; Razzaghi, M.R.; Zali, M.R. An updated overview of spectrum of gluten-related disorders: Clinical and diagnostic aspects. BMC Gastroenterol. 2020, 20, 258. [Google Scholar] [CrossRef]
- Yang, D.; Gao, X. Progress of the use of alternatives to malt in the production of gluten-free beer. Crit. Rev. Food Sci. Nutr. 2020, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef]
- Murray, J.A.; Frey, M.R.; Oliva-Hemker, M. Celiac Disease. Gastroenterology 2018, 154, 2005–2008. [Google Scholar] [CrossRef]
- Lebwohl, B.; Rubio-Tapia, A. Epidemiology, presentation, and diagnosis of celiac disease. Gastroenterology 2021, 160, 63–75. [Google Scholar] [CrossRef]
- Comino, I.; Sousa, C. Advances in celiac disease and gluten-free diet. Nutrients 2022, 14, 570. [Google Scholar] [CrossRef]
- CODEX Stan 118-1979; Codex Standard for Foods for Special Dietary Use for Persons Intolerant Gluten. CODEX. Codex Alimentarius: 2008. Revised 2008. Available online: http://www.codexalimentarius.net/download/standards/29.1/cxs_118e.pdf (accessed on 7 March 2022).
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef]
- Lindfors, K.; Ciacci, C.; Kurppa, K.; Lundin, K.E.A.; Makharia, G.K.; Mearin, M.L.; Murray, J.A.; Verdu, E.F.; Kaukinen, K. Coeliac disease. Nat. Rev. Dis. Prim. 2019, 5, 3. [Google Scholar] [CrossRef]
- Wieser, H.; Segura, V.; Ruiz-Carnicer, Á.; Sousa, C.; Comino, I. Food safety and cross-contamination of gluten-free products: A narrative review. Nutrients 2021, 13, 2244. [Google Scholar] [CrossRef]
- Smeets, P.A.M.; de Graaf, C. Brain responses to anticipation and consumption of beer with and without alcohol. Chem. Senses 2019, 44, 51–60. [Google Scholar] [CrossRef]
- Donadini, G.; Bertuzzi, T.; Kordialik-Bogacka, E.; Cywińska, D.; Rossi, F.; Spigno, G.; Porretta, S. Investigating patterns of millennials’ interest in gluten-free beer in Poland: A question of beer price and alcohol content. J. Food Sci. 2020, 85, 182–191. [Google Scholar] [CrossRef]
- Cao, W.; Baumert, J.L.; Downs, M.L. Compositional and immunogenic evaluation of fractionated wheat beers using mass spectrometry. Food Chem. 2020, 333, 127379. [Google Scholar] [CrossRef]
- Taylor, S.L.; Baumert, J.L. Worldwide food allergy labeling and detection of allergens in processed foods. Chem. Immunol. Allergy 2015, 101, 227–234. [Google Scholar] [CrossRef]
- Watson, H.; Decloedt, A.; Vanderputten, D.; Van Landschoot, A. Variation in gluten protein and peptide concentrations in Belgian barley malt beers. J. Inst. Brew 2018, 124, 148–157. [Google Scholar] [CrossRef]
- Watson, H.; Vanderputten, D.; Van Landschoot, A.; Decloedt, A. Applicability of different brewhouse technologies and gluten-minimization treatments for the production of gluten-free (barley) malt beers: Pilot-to industrial-scale. J. Food Eng. 2019, 245, 33–42. [Google Scholar] [CrossRef]
- Kok, Y.J.; Ye, L.; Muller, J.; Ow, D.S.; Bi, X. Brewing with malted barley or raw barley: What makes the difference in the processes? Appl. Microbiol. Biotechnol. 2019, 103, 1059–1067. [Google Scholar] [CrossRef]
- Comino, I.; Real, A.; Moreno, M.d.L.; Montes, R.; Cebolla, A.; Sousa, C. Immunological determination of gliadin 33-mer equivalent peptides in beers as a specific and practical analytical method to assess safety for celiac patients. J. Sci. Food Agric. 2013, 93, 933–943. [Google Scholar] [CrossRef]
- Real, A.; Comino, I.; Moreno, M.d.L.; López-Casado, M.Á.; Lorite, P.; Torres, M.I.; Cebolla, Á.; Sousa, C. Identification and in vitro reactivity of celiac immunoactive peptides in an apparent gluten-free beer. PLoS ONE 2014, 9, e100917. [Google Scholar] [CrossRef] [PubMed]
- Picariello, G.; Mamone, G.; Cutignano, A.; Fontana, A.; Zurlo, L.; Addeo, F.; Ferranti, P. Proteomics, peptidomics, and immunogenic potential of wheat beer (Weissbier). J. Agric. Food Chem. 2015, 63, 3579–3586. [Google Scholar] [CrossRef]
- Spada, V.; Di Stasio, L.; Picascia, S.; Messina, B.; Gianfrani, C.; Mamone, G.; Picariello, G. Immunogenic potential of beer types brewed with hordeum and triticum spp. malt disclosed by proteomics. Front. Nutr. 2020, 7, 98. [Google Scholar] [CrossRef]
- Diaz-Amigo, C.; Popping, B. Accuracy of ELISA detection methods for gluten and reference materials: A realistic assessment. J. Agric. Food Chem. 2013, 61, 5681–5688. [Google Scholar] [CrossRef]
- Yu, J.M.; Lee, J.H.; Park, J.D.; Choi, Y.S.; Sung, J.M.; Jang, H.W. Analyzing gluten content in various food products using diferent types of elisa test kits. Foods 2021, 10, 108. [Google Scholar] [CrossRef]
- Panda, R.; Fiedler, K.L.; Cho, C.Y.; Cheng, R.; Stutts, W.L.; Jackson, L.S.; Garber, E.A. Effects of a proline endopeptidase on the detection and quantitation of gluten by antibody-based methods during the fermentation of a model sorghum beer. J. Agric. Food Chem. 2015, 63, 10525–10535. [Google Scholar] [CrossRef]
- Colgrave, M.L.; Byrne, K.; Howitt, C.A. LC-MS analysis reveals hydrolysed gluten in beers crafted to remove gluten. J. Agric. Food Chem. 2017, 65, 9715–9725. [Google Scholar] [CrossRef]
- Fernández-Gil, M.D.P.; Simon, E.; Gibert, A.; Miranda, J.; Roger-Alcoba, E.; Martínez, O.; Vilchez-Cerezo, E.; Bustamante, M.Á. Gluten assessment in beers: Comparison by different commercial elisa kits and evaluation of nir analysis as a complementary technique. Foods 2021, 10, 1170. [Google Scholar] [CrossRef]
- Cebolla, Á.; Moreno, M.L.; Coto, L.; Sousa, C. Gluten immunogenic peptides as standard for the evaluation of potential harmful prolamin content in food and human specimen. Nutrients 2018, 10, 1927. [Google Scholar] [CrossRef]
- Comino, I.; Real, A.; Vivas, S.; Síglez, M.Á.; Caminero, A.; Nistal, E.; Casqueiro, J.; Rodríguez-Herrera, A.; Cebolla, A.; Sousa, C. Monitoring of gluten-free diet compliance in celiac patients by assessment of gliadin 33-mer equivalent epitopes in feces. Am. J. Clin. Nutr. 2012, 95, 670–677. [Google Scholar] [CrossRef]
- Akeroyd, M.; van Zandycke, S.; Den Hartog, J.; Mutsaers, J.; Edens, L.; van Den Berg, M.; Christis, C. AN-PEP, proline-specific endopeptidase, degrades all known immunostimulatory gluten peptides in beer made from barley malt. J. Am. Soc. Brew Chem. 2016, 74, 91–99. [Google Scholar] [CrossRef]
- Knorr, V.; Wieser, H.; Koehler, P. Production of gluten-free beer by peptidase treatment. Eur. Food Res. Technol. 2016, 242, 1129–1140. [Google Scholar] [CrossRef]
- Di Ghionno, L.; Marconi, O.; Sileoni, V.; De Francesco, G.; Perretti, G. Brewing with prolyl endopeptidase from Aspergillus niger: The impact of enzymatic treatment on gluten levels, quality attributes and sensory profile. Int. J. Food Sci. Technol. 2017, 52, 1367–1374. [Google Scholar] [CrossRef]
- Liao, Y.S.; Kuo, J.H.; Chen, B.L.; Tsuei, H.W.; Lin, C.Y.; Lin, H.Y.; Cheng, H.F. Development and validation of the detection method for wheat and barley glutens using mass spectrometry in processed foods. Food Anal. Methods 2017, 10, 2839–2847. [Google Scholar] [CrossRef]
- Fiedler, K.L.; Panda, R.; Croley, T.R. Analysis of gluten in a wheat-gluten-incurred sorghum beer brewed in the food additives & contaminants: PART A 1161 presence of proline endopeptidase by LC/MS/MS. Anal. Chem. 2018, 90, 2111–2118. [Google Scholar] [CrossRef]
- Hnasko, R.M.; Jackson, E.S.; Lin, A.V.; Haff, R.P.; McGarvey, J.A. A rapid and sensitive lateral flow immunoassay (LFIA) for the detection of gluten in foods. Food Chem. 2021, 355, 129514. [Google Scholar] [CrossRef]
- Síglez, M.A.; Nocea, B.; Pérez, M.d.M.; García, E.M.; León, L.; Galera, C. GlutenTox® Pro test for the detection of gluten in select foods and surfaces. J. AOAC Int. 2015, 98, 1608–1627. [Google Scholar] [CrossRef]
- Gautam, P.B.; Sharma, R.; Lata, K.; Rajput, Y.S.; Mann, B. Construction of a lateral flow strip for detection of soymilk in milk. J. Food Sci. Technol. 2017, 54, 4213–4219. [Google Scholar] [CrossRef]
- Álvarez-Simón, D.; Gómez-Ollés, S. Development of a dipstick assay for soy allergens: Inexpensive detection to control allergen exposure. Methods Mol. Biol. 2020, 2019, 175–184. [Google Scholar] [CrossRef]
- Senyuva, H.Z.; Jones, I.B.; Sykes, M.; Baumgartner, S. A critical review of the specifications and performance of antibody and DNA-based methods for detection and quantification of allergens in foods. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 507–547. [Google Scholar] [CrossRef]
- Magnusson, B.; Örnemark, U. (Eds.) Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; 2014; ISBN 978-91-87461-59-0. Available online: www.eurachem.org (accessed on 10 March 2022).
- Segura, V.; Díaz, J.; Ruiz-Carnicer, Á.; Muñoz-Suano, A.; Carrillo-Carrión, C.; Sousa, C.; Cebolla, Á.; Comino, I. Rapid, effective, and versatile extraction of gluten in food with application on different immunological methods. Foods 2021, 10, 652. [Google Scholar] [CrossRef]
- Shah, V.P.; Midha, K.K.; Findlay, J.W.; Hill, H.M.; Hulse, J.D.; McGilveray, I.J.; McKay, G.; Miller, K.J.; Patnaik, R.N.; Powell, M.L.; et al. Bioanalytical method validation--a revisit with a decade of progress. Pharm. Res. 2000, 17, 1551–1557. [Google Scholar] [CrossRef]
- Lakshmi, M.R.; Angala, S.; Gopinath, C. A review on GC-MS and method development and validation. Int. J. Pharm. Qual. Assur. 2013, 4, 42–51. [Google Scholar]
- Tiwari, G.; Tiwari, R. Bioanalytical method validation: An updated review. Pharm. Methods 2010, 1, 25–38. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 5th ed.; Pearson Education Limited: London, UK, 2010; ISBN 0 131 29192 0. [Google Scholar]
- Ellison, S.L.R.; Williams, A. EURACHEM/CITAC Guide Quantifying Uncertainty in Analytical Measurement, 3rd ed.; This Guide Constitutes CITAC Guide Number 4; 2012; ISBN 978-0-948926-30-3. Available online: www.eurachem.org (accessed on 15 March 2022).
- Torgler, C.; Síglez, M.A.; Vilchez, F.; Cebolla, A.; Sousa, C. Analytical tools to detect gluten immunotoxic fractions in food based on monoclonal antibodies raised against the gliadin 33-mer peptide. In Proceedings of the 24th Meeting Working Group on Prolamin Analysis and Toxicity (PWG), Ancona, Italy, 30 September–2 October 2011; p. 47. [Google Scholar]
- Zhang, J.; Portela, S.B.; Horrell, J.B.; Leung, A.; Weitmann, D.R.; Artiuch, J.B.; Wilson, S.M.; Cipriani, M.; Slakey, L.K.; Burt, A.M.; et al. An integrated, accurate, rapid, and economical handheld consumer gluten detector. Food Chem. 2019, 275, 446–456. [Google Scholar] [CrossRef]
- Morón, B.; Bethune, M.T.; Comino, I.; Manyani, H.; Ferragud, M.; López, M.C.; Cebolla, A.; Khosla, C.; Sousa, C. Toward the assessment of food toxicity for celiac patients: Characterization of monoclonal antibodies to a main immunogenic gluten peptide. PLoS ONE 2008, 3, e2294. [Google Scholar] [CrossRef]
- Morón, B.; Cebolla, A.; Manyani, H.; Alvarez-Maqueda, M.; Megías, M.; Thomas, M.C.; López, M.C.; Sousa, C. Sensitive detection of cereal fractions that are toxic to celiac disease patients by using monoclonal antibodies to a main immunogenic wheat peptide. Am. J. Clin. Nutr. 2008, 87, 405–414. [Google Scholar] [CrossRef]
- Ehren, J.; Morón, B.; Martin, E.; Bethune, M.T.; Gray, G.M.; Khosla, C. A food-grade enzyme preparation with modest gluten detoxification properties. PLoS ONE 2009, 4, e6313. [Google Scholar] [CrossRef]
- Comino, I.; Real, A.; de Lorenzo, L.; Cornell, H.; López-Casado, M.Á.; Barro, F.; Lorite, P.; Torres, M.I.; Cebolla, A.; Sousa, C. Diversity in oat potential immunogenicity: Basis for the selection of oat varieties with no toxicity in coeliac disease. Gut 2011, 60, 915–922. [Google Scholar] [CrossRef]
- Moreno, M.L.; Muñoz-Suano, A.; López-Casado, M.Á.; Torres, M.I.; Sousa, C.; Cebolla, Á. Selective capture of most celiac immunogenic peptides from hydrolyzed gluten proteins. Food Chem. 2016, 205, 36–42. [Google Scholar] [CrossRef]
- Tanner, G.J.; Colgrave, M.L.; Blundell, M.J.; Goswami, H.P.; Howitt, C.A. Measuring hordein (gluten) in beer—A comparison of ELISA and mass spectrometry. PLoS ONE 2013, 8, e56452. [Google Scholar] [CrossRef]
- Osman, A.A.; Uhlig, H.H.; Valdes, I.; Amin, M.; Méndez, E.; Mothes, T. A monoclonal antibody that recognizes a potential coeliac-toxic repetitive pentapeptide epitope in gliadins. Eur. J. Gastroenterol. Hepatol. 2001, 13, 1189–1193. [Google Scholar] [CrossRef]
- Kanerva, P.; Sontag-Strohm, T.; Lehtonen, P. Determinación de prolaminas en cervezas por elisa y sds-page. Revista del Instituto de Elaboración de Cerveza 2005, 111, 61–64. [Google Scholar]
- Food Labeling, Gluten-Free Labeling of fermented or Hydrolyzed Foods, Food and Drug Administration. 2020. Available online: https://www.regulations.gov/document/FDA-2014-N-1021-0560 (accessed on 13 March 2022).
- Rostami, K.; Bold, J.; Parr, A.; Johnson, M.W. Gluten-Free diet indications, safety, quality, labels, and challenges. Nutrients 2017, 9, 846. [Google Scholar] [CrossRef]
- Bascuñán, K.A.; Vespa, M.C.; Araya, M. Celiac disease: Understanding the gluten-free diet. Eur. J. Nutr. 2017, 56, 449–459. [Google Scholar] [CrossRef]
- Cohen, I.S.; Day, A.S.; Shaoul, R. Gluten in celiac disease-more or less? Rambam Maimonides Med. J. 2019, 10, e0007. [Google Scholar] [CrossRef]

| Beer Samples | Spiked Sample | Peak Area |
|---|---|---|
| Beer I | 1 | 282.0 |
| 2 | 278.5 | |
| 3 | 270.2 | |
| 4 | 227.0 | |
| 5 | 274.6 | |
| Beer II | 6 | 250.6 |
| 7 | 251.7 | |
| 8 | 263.6 | |
| 9 | 273.9 | |
| 10 | 309.0 | |
| Beer III | 11 | 288.0 |
| 12 | 232.3 | |
| 13 | 279.6 | |
| 14 | 214.3 | |
| 15 | 228.1 | |
| Beer IV | 16 | 233.3 |
| 17 | 279.2 | |
| 18 | 244.8 | |
| 19 | 234.8 | |
| 20 | 220.8 |
| Day | Beer | Extraction | Analysis | Peak Area | Inside the Range | Accuracy (%) | Precision (%) |
|---|---|---|---|---|---|---|---|
| Day 1 | Beer I | Beer Ia | Beer Ia | 277.5 | yes | 88.9% | 94.5% |
| Beer Ia | 305.1 | yes | |||||
| Beer Ia | 290.4 | yes | |||||
| Beer Ib | Beer Ib | 196.9 | no | ||||
| Beer Ib | 278.1 | yes | |||||
| Beer Ib | 267.3 | yes | |||||
| Beer Ic | Beer Ic | 186.9 | no | ||||
| Beer Ic | 279.0 | yes | |||||
| Beer Ic | 217.9 | yes | |||||
| Beer II | Beer IIa | Beer IIa | 255.9 | yes | |||
| Beer IIa | 259.2 | yes | |||||
| Beer IIa | 268.9 | yes | |||||
| Beer IIb | Beer IIb | 248.2 | yes | ||||
| Beer IIb | 297.3 | yes | |||||
| Beer IIb | 265.4 | yes | |||||
| Beer IIc | Beer IIc | 235.7 | yes | ||||
| Beer IIc | 221.7 | yes | |||||
| Beer IIc | 270.2 | yes | |||||
| Day 2 | Beer I | Beer Ia | Beer Ia | 282.0 | yes | 100% | |
| Beer Ia | 278.5 | Yes | |||||
| Beer Ia | 270.2 | Yes | |||||
| Beer Ib | Beer Ib | 248.3 | Yes | ||||
| Beer Ib | 303.7 | yes | |||||
| Beer Ib | 289.5 | yes | |||||
| Beer Ic | Beer Ic | 301.9 | yes | ||||
| Beer Ic | 271.5 | yes | |||||
| Beer Ic | 226.6 | yes | |||||
| Beer II | Beer IIa | Beer IIa | 288.0 | yes | |||
| Beer IIa | 232.3 | yes | |||||
| Beer IIa | 279.6 | yes | |||||
| Beer IIb | Beer IIb | 248.2 | yes | ||||
| Beer IIb | 295.3 | yes | |||||
| Beer IIb | 268.7 | yes | |||||
| Beer IIc | Beer IIc | 208.9 | yes | ||||
| Beer IIc | 283.3 | yes | |||||
| Beer IIc | 229.4 | yes |
| Number of Beers | Positive for Gluten | % (Positive/Number of Beer) | % (Positive/Total Beer Samples) | |
|---|---|---|---|---|
| Total beer samples | 107 | |||
| Yeast style | ||||
| Lager | 49 | 10 | 20 (10/49) | 9 (10/107) |
| Ale | 46 | 16 | 35 (16/46) | 15 (16/107) |
| Unknown | 12 | 5 | 42 (5/12) | 5 (5/107) |
| Label | ||||
| Low in gluten or with traces of gluten | 12 | 4 | 33 (4/12) | 4 (4/107) |
| Gluten-free | 88 | 26 | 30 (26/88) | 24 (26/107) |
| Unknown | 7 | 1 | 14 (1/7) | 1 (1/107) |
| Ingredients | ||||
| Contains cereals with gluten | 70 | 24 | 34 (24/70) | 22 (24/107) |
| Barley | 59 | 16 | 27 (16/59) | 15 (16/107) |
| Wheat | 1 | 0 | 0 (0/1) | 0 (0/107) |
| Oats | 1 | 1 | 100 (1/1) | 1 (1/107) |
| Barley and wheat | 4 | 3 | 75 (3/4) | 3 (3/107) |
| Barley and rye | 1 | 0 | 0 (0/1) | 0 (0/107) |
| Barley and oats | 2 | 2 | 100 (2/2) | 2 (2/107) |
| Wheat, barley, and rye | 1 | 0 | 0 (0/1) | 0 (0/107) |
| Wheat, barley, and oats | 1 | 1 | 100 (1/1) | 1 (1/107) |
| Unknown | 15 | 6 | 40 (6/15) | 6 (6/107) |
| Contains cereals with gluten and gluten-free | 18 | 2 | 11.1 (2/18) | 3 (3/107) |
| Barley and maize | 7 | 1 | 14 (1/7) | 1 (1/107) |
| Barley and millet | 2 | 0 | 0 (0/2) | 0 (0/107) |
| Wheat and millet | 1 | 0 | 0 (0/1) | 0 (0/107) |
| Barley, wheat, and quinoa | 1 | 0 | 0 (0/1) | 0 (0/107) |
| Barley and rice | 3 | 0 | 0 (0/3) | 0 (0/107) |
| Wheat, barley, rye, and rice | 1 | 0 | 0 (0/1) | 0 (0/107) |
| Barley, oats, and maize | 1 | 0 | 0 (0/1) | 0 (0/107) |
| Barley, rice, and maize | 1 | 0 | 0 (0/1) | 0 (0/107) |
| Barley, rice, and quinoa | 1 | 1 | 100 (1/1) | 1 (1/107) |
| 1 | ||||
| Contains only gluten-free cereals | 5 | 2 | 40 (2/5) | 2 (2/107) |
| Maize | 2 | 0 | 0 (0/2) | 0 (0/107) |
| Millet | 2 | 1 | 50 (1/2) | 1 (1/107) |
| Buckwheat and rice | 1 | 1 | 100 (1/1) | 1 (1/107) |
| Country of origin | ||||
| Argentina | 1 | 0 | 0 (0/1) | 0 (0/107) |
| Belgium | 10 | 1 | 10 (1/10) | 1 (1/107) |
| Czech Republic | 1 | 0 | 0 (0/1) | 0 (0/107) |
| England | 1 | 0 | 0 (0/1) | 0 (0/107) |
| Finland | 15 | 8 | 53 (8/15) | 7 (8/107) |
| France | 3 | 1 | 33 (1/3) | 1 (1/107) |
| Germany | 3 | 1 | 33 (1/3) | 1 (1/107) |
| Italy | 5 | 0 | 0 (0/5) | 0 (0/107) |
| Luxembourg | 2 | 2 | 100 (2/2) | 2 (2/107) |
| Mexico | 3 | 0 | 0 (0/3) | 0 (0/107) |
| Poland | 4 | 0 | 0 (0/4) | 0 (0/107) |
| Portugal | 1 | 0 | 0 (0/1) | 0 (0/107) |
| Scotland | 2 | 1 | 50 (1/2) | 1 (1/107) |
| Spain | 43 | 16 | 37 (16/43) | 15 (16/107) |
| Switzerland | 13 | 1 | 8 (1/13) | 8 (1/107) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segura, V.; Siglez, M.Á.; Ruiz-Carnicer, Á.; Martín-Cabrejas, I.; van der Hofstadt, M.; Mellado, E.; Comino, I.; Sousa, C. A Highly Sensitive Method for the Detection of Hydrolyzed Gluten in Beer Samples Using LFIA. Foods 2023, 12, 160. https://doi.org/10.3390/foods12010160
Segura V, Siglez MÁ, Ruiz-Carnicer Á, Martín-Cabrejas I, van der Hofstadt M, Mellado E, Comino I, Sousa C. A Highly Sensitive Method for the Detection of Hydrolyzed Gluten in Beer Samples Using LFIA. Foods. 2023; 12(1):160. https://doi.org/10.3390/foods12010160
Chicago/Turabian StyleSegura, Verónica, Miguel Ángel Siglez, Ángela Ruiz-Carnicer, Izaskun Martín-Cabrejas, María van der Hofstadt, Encarnación Mellado, Isabel Comino, and Carolina Sousa. 2023. "A Highly Sensitive Method for the Detection of Hydrolyzed Gluten in Beer Samples Using LFIA" Foods 12, no. 1: 160. https://doi.org/10.3390/foods12010160
APA StyleSegura, V., Siglez, M. Á., Ruiz-Carnicer, Á., Martín-Cabrejas, I., van der Hofstadt, M., Mellado, E., Comino, I., & Sousa, C. (2023). A Highly Sensitive Method for the Detection of Hydrolyzed Gluten in Beer Samples Using LFIA. Foods, 12(1), 160. https://doi.org/10.3390/foods12010160







