Plant Protein versus Dairy Proteins: A pH-Dependency Investigation on Their Structure and Functional Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physicochemical Properties
2.2.1. Chemical Composition
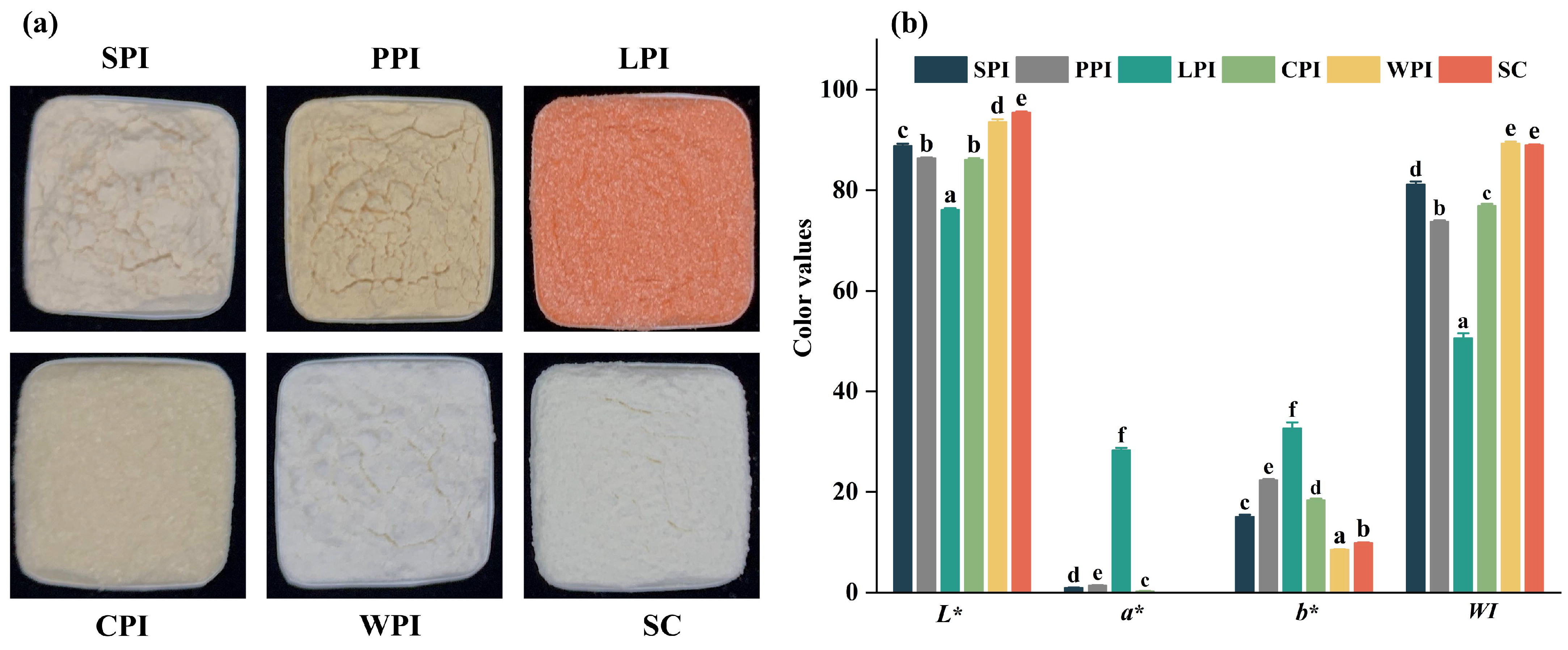
2.2.2. Color Properties
2.3. Structure Characteristics
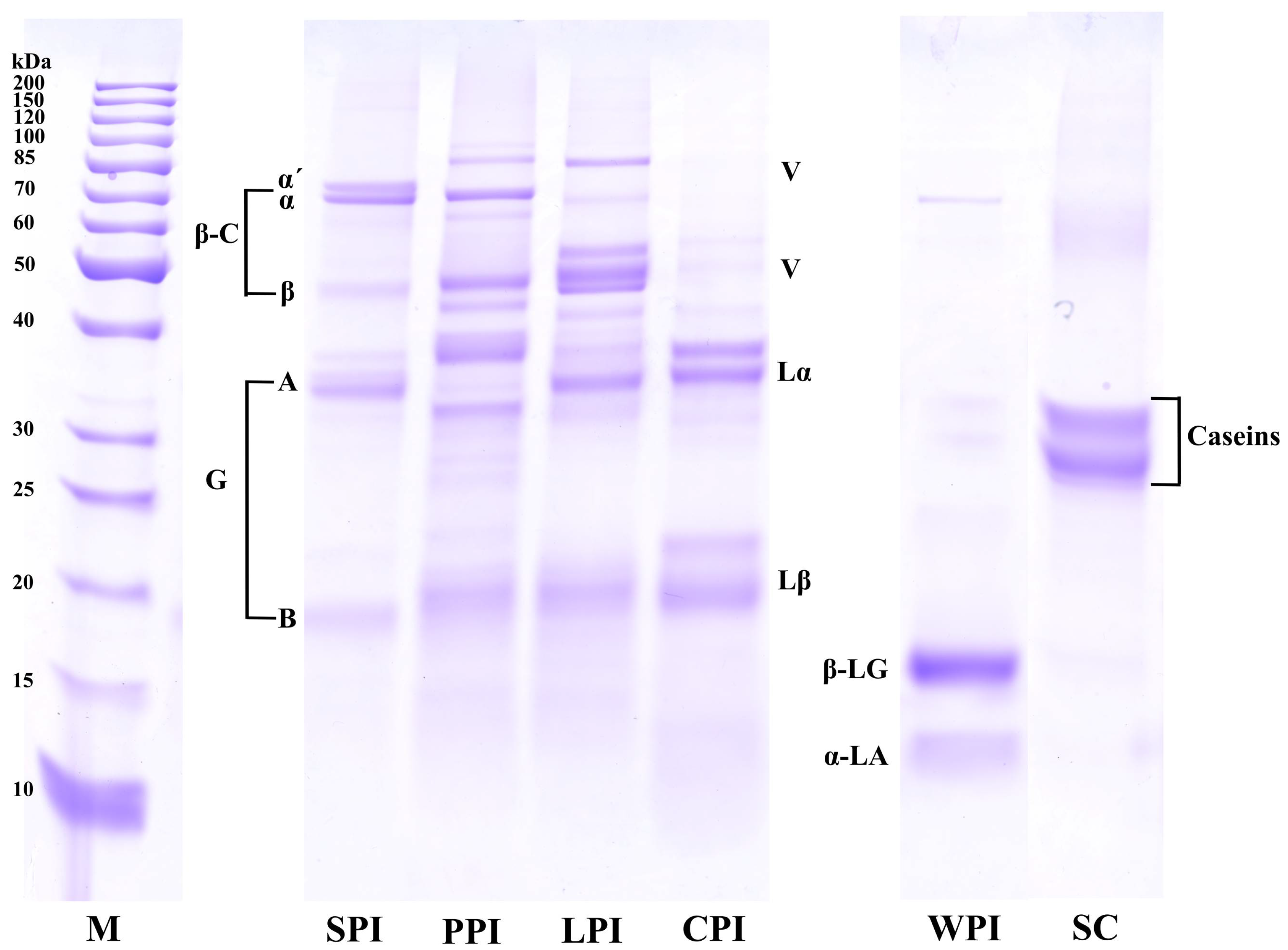
2.3.1. Sodium Dodecyl Sulfide-Polyacrylamide Gel Electrophoresis (SDS–PAGE)
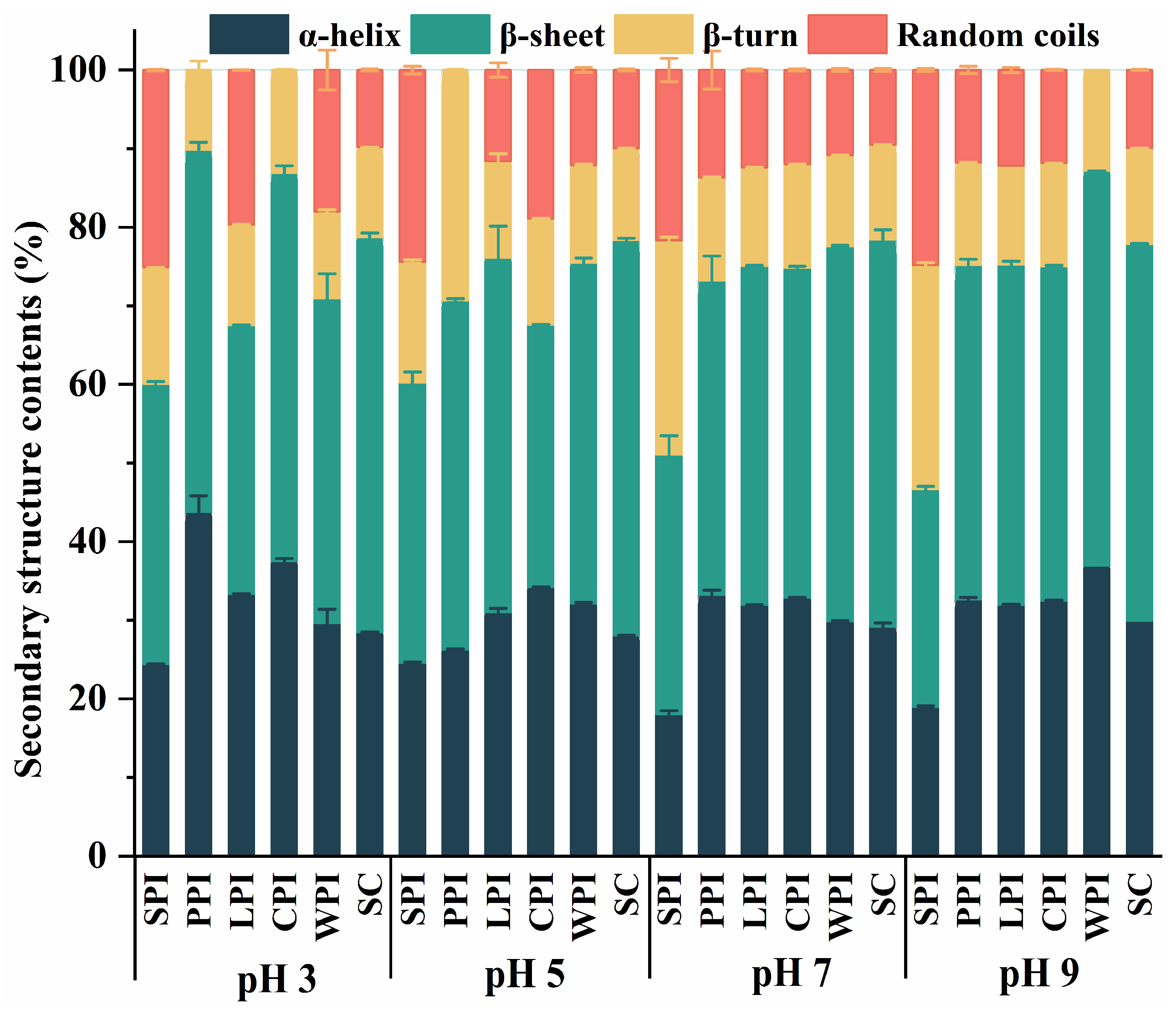
2.3.2. Fourier Transform Infrared Spectroscopy Analysis (FT-IR)
2.4. Surface Characteristics
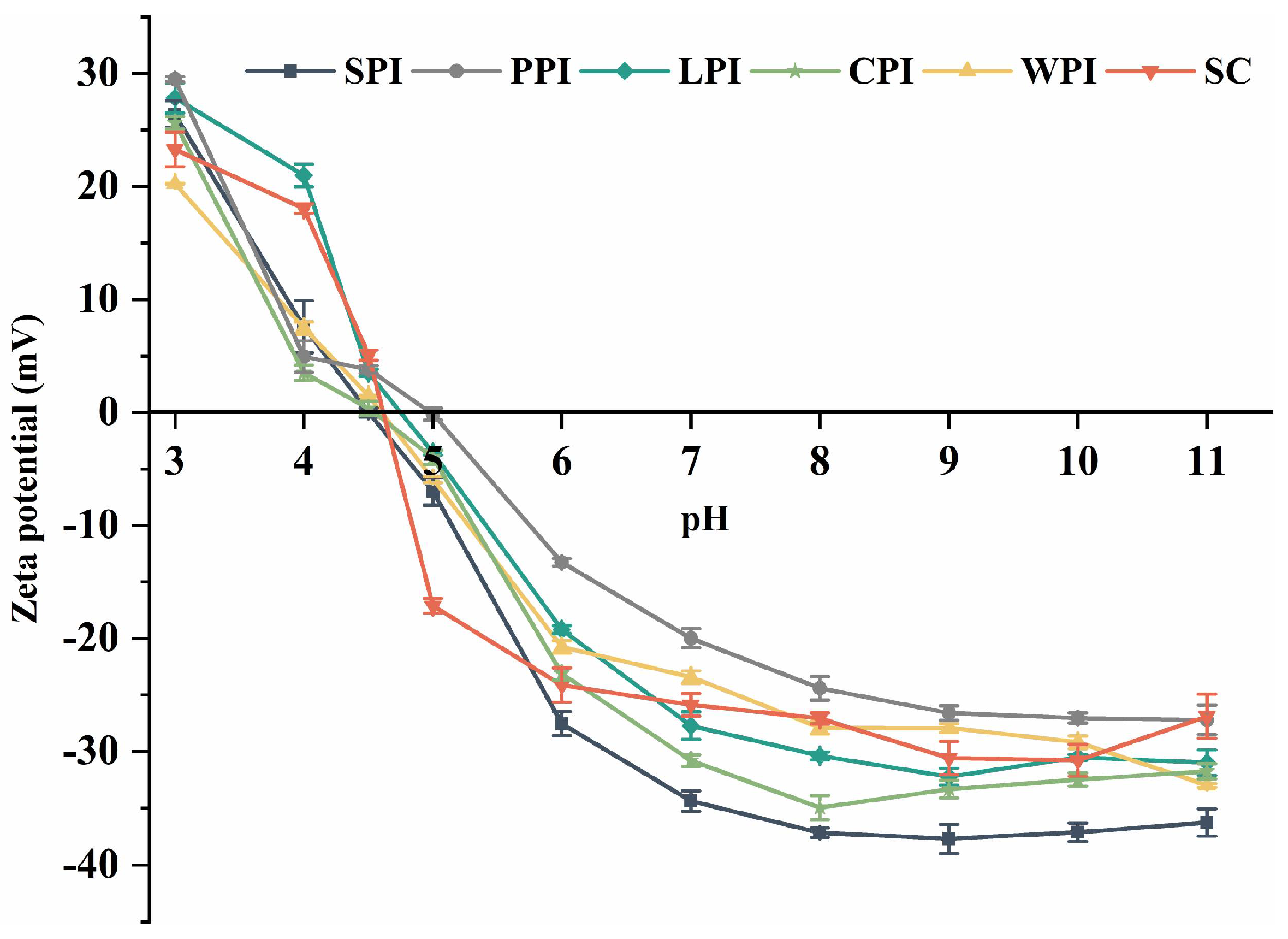
2.4.1. Surface Charge
2.4.2. Surface Hydrophobicity and Intrinsic Fluorescence
2.5. Techno-Functional Properties
2.5.1. Protein Solubility
2.5.2. Emulsifying Properties
2.5.3. Foaming Properties
2.5.4. Water-Holding Capacity (WHC) and Oil-Holding Capacity (OHC)
2.6. Hierarchical Clustering Analysis (HCA)
2.7. Statistical Analysis
3. Results and Discussions
3.1. Physicochemical Characteristics
3.2. Structural Analysis
3.2.1. Protein Profile Analysis (SDS-PAGE)
3.2.2. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
3.3. Surface Characteristics
3.3.1. Surface Charge
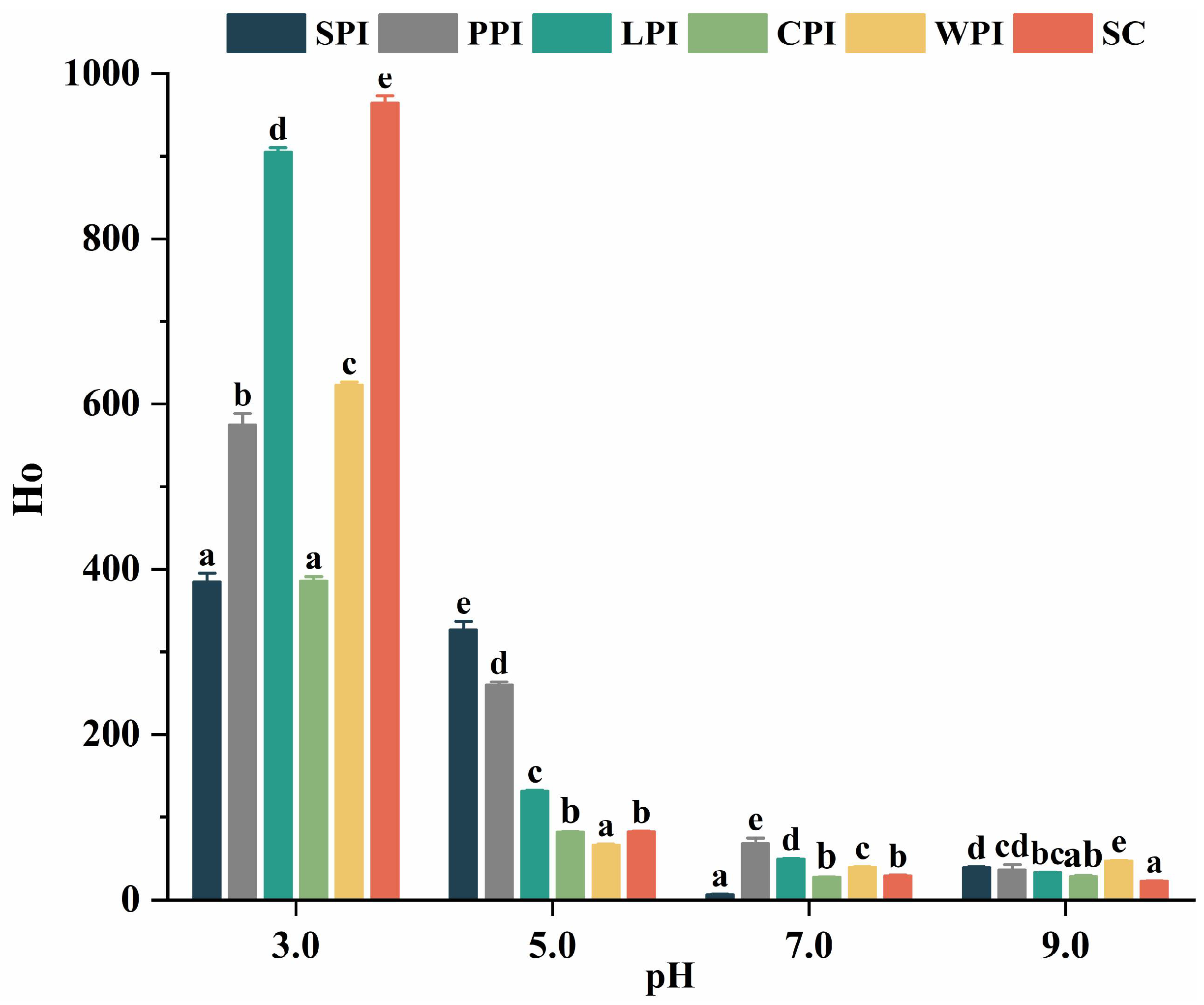
3.3.2. Effect of pH on Surface Hydrophobicity (Ho)
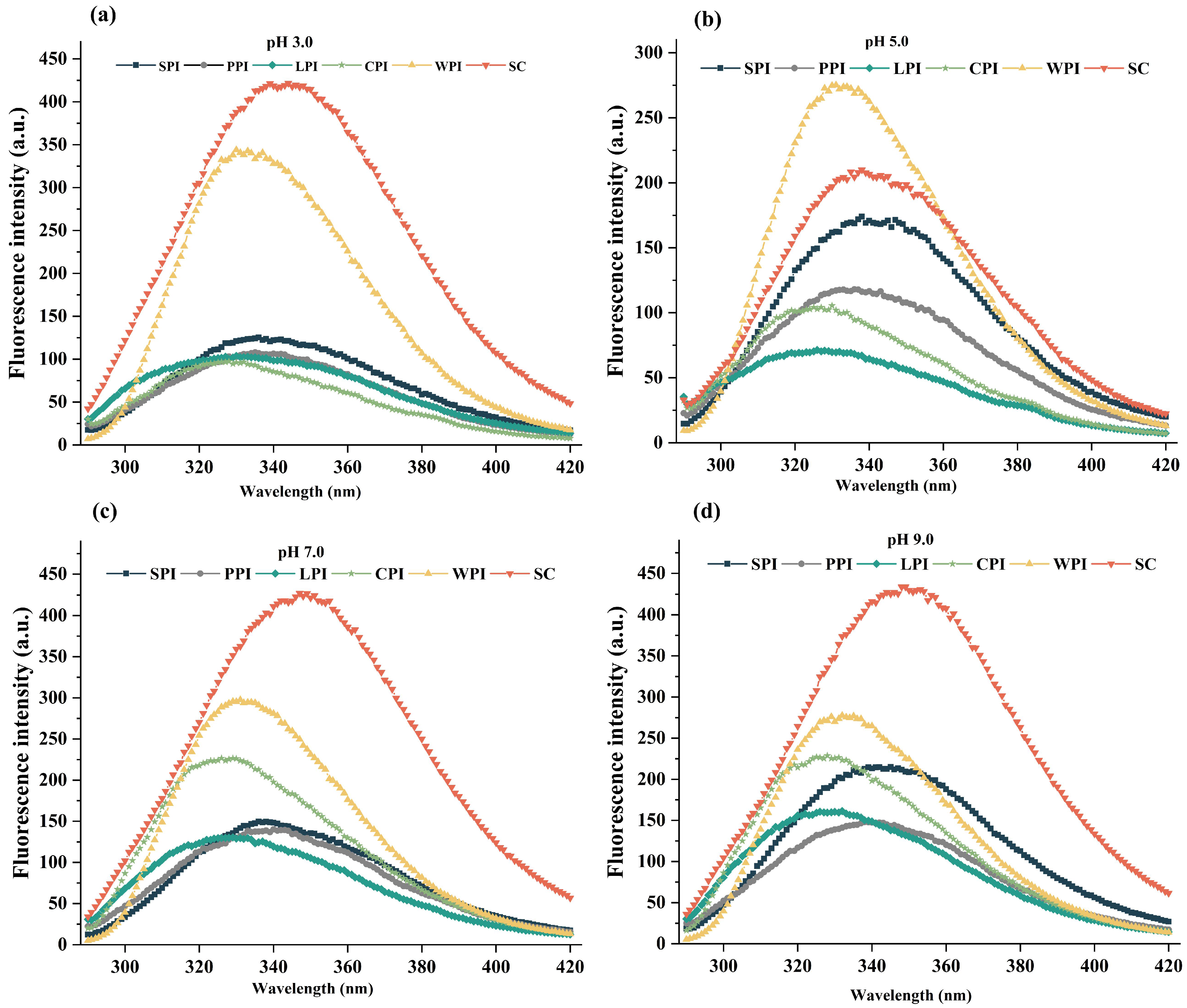
3.3.3. Intrinsic Fluorescence Analysis
3.4. Techno-Functional Properties
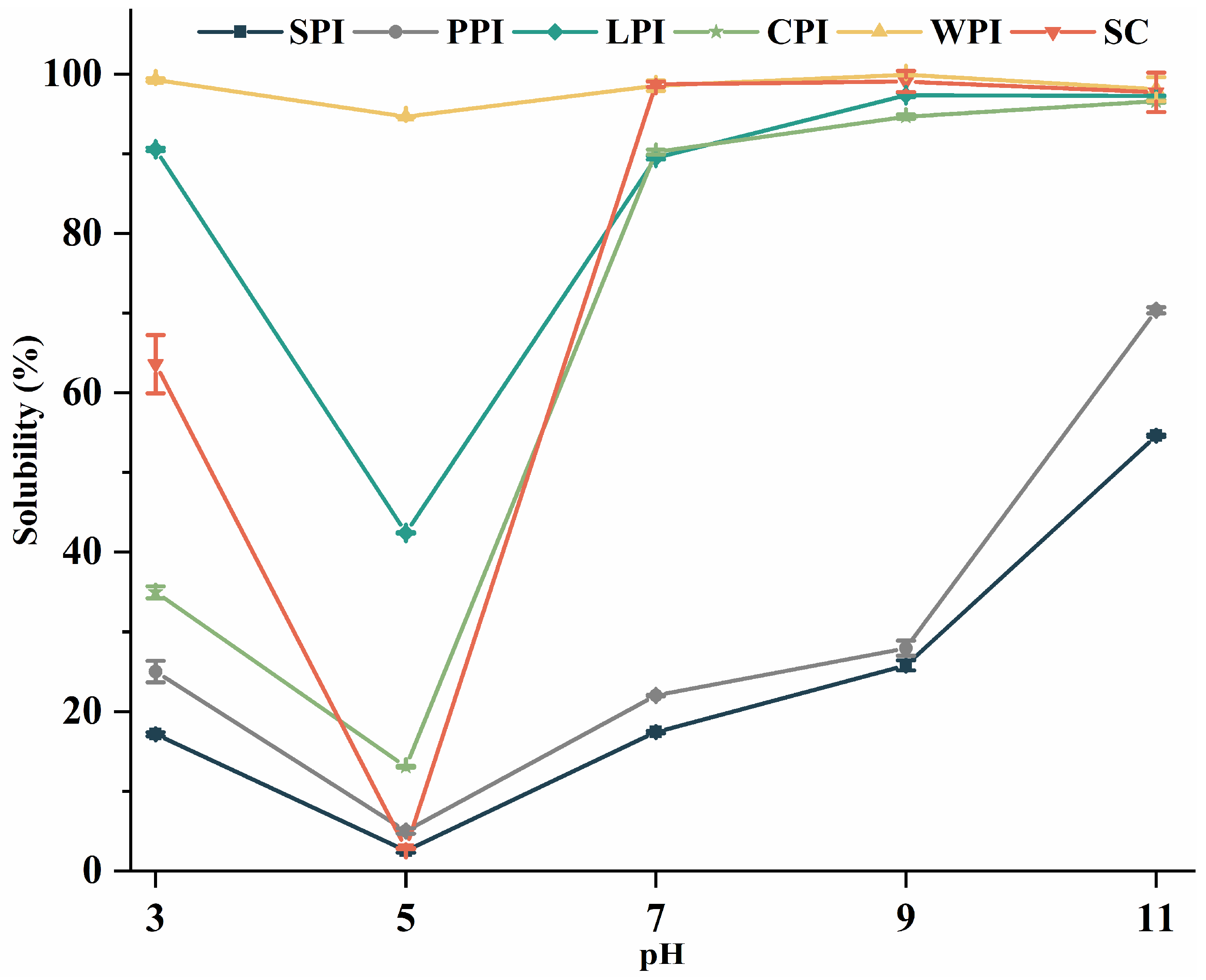
3.4.1. Effect of pH on Protein Solubility
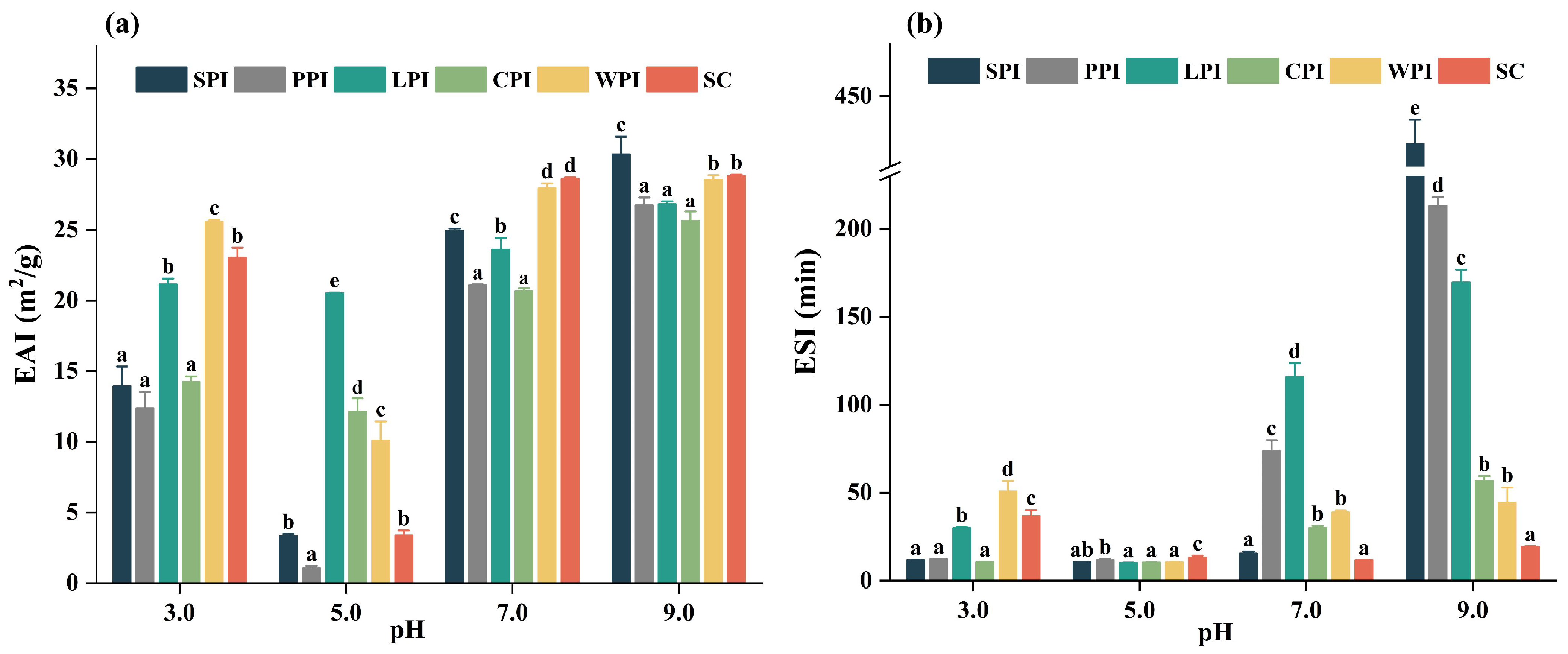
3.4.2. Effect of pH on Emulsifying Properties
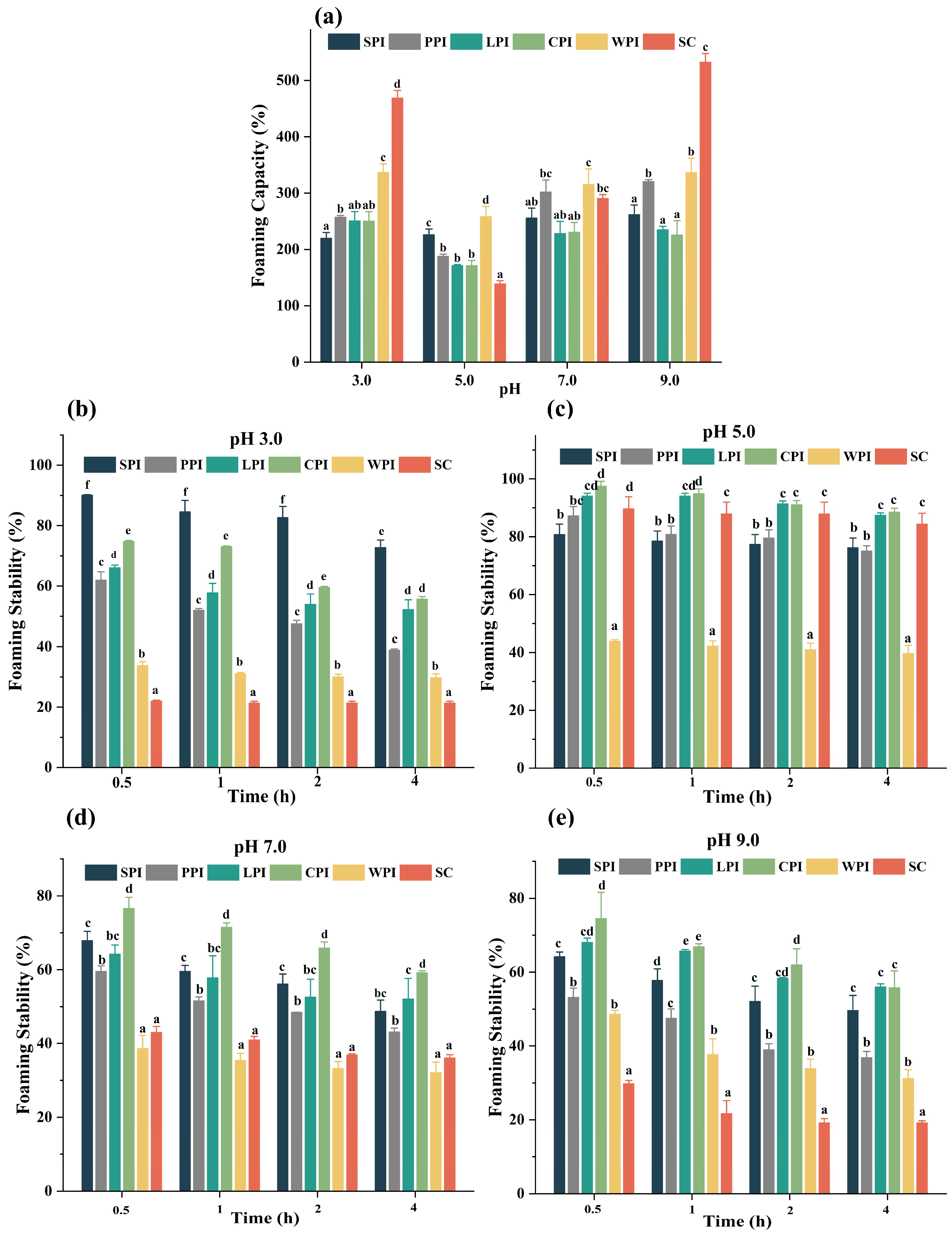
3.4.3. Effect of pH Foaming Properties
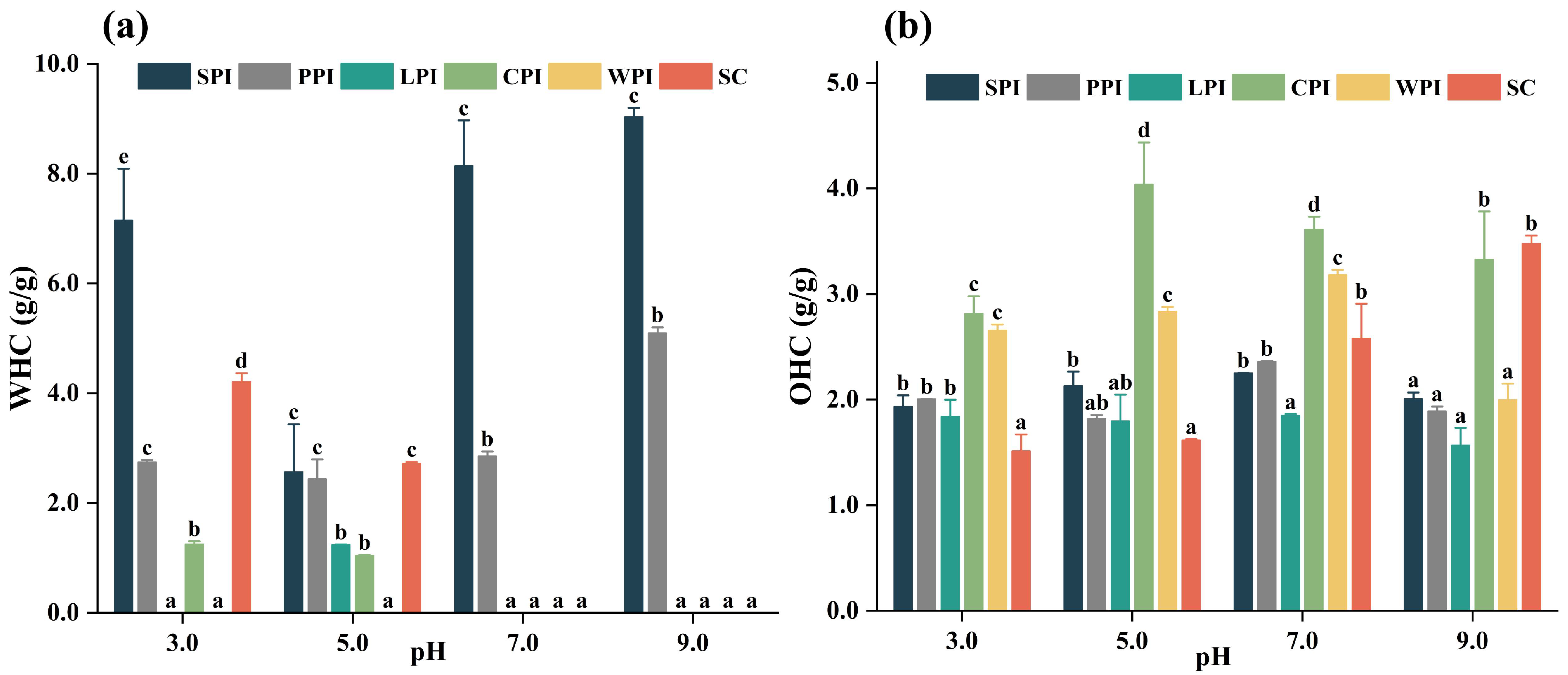
3.4.4. Effect of pH on Water-Holding Capacity (WHC) and Oil-Holding Capacity (OHC)
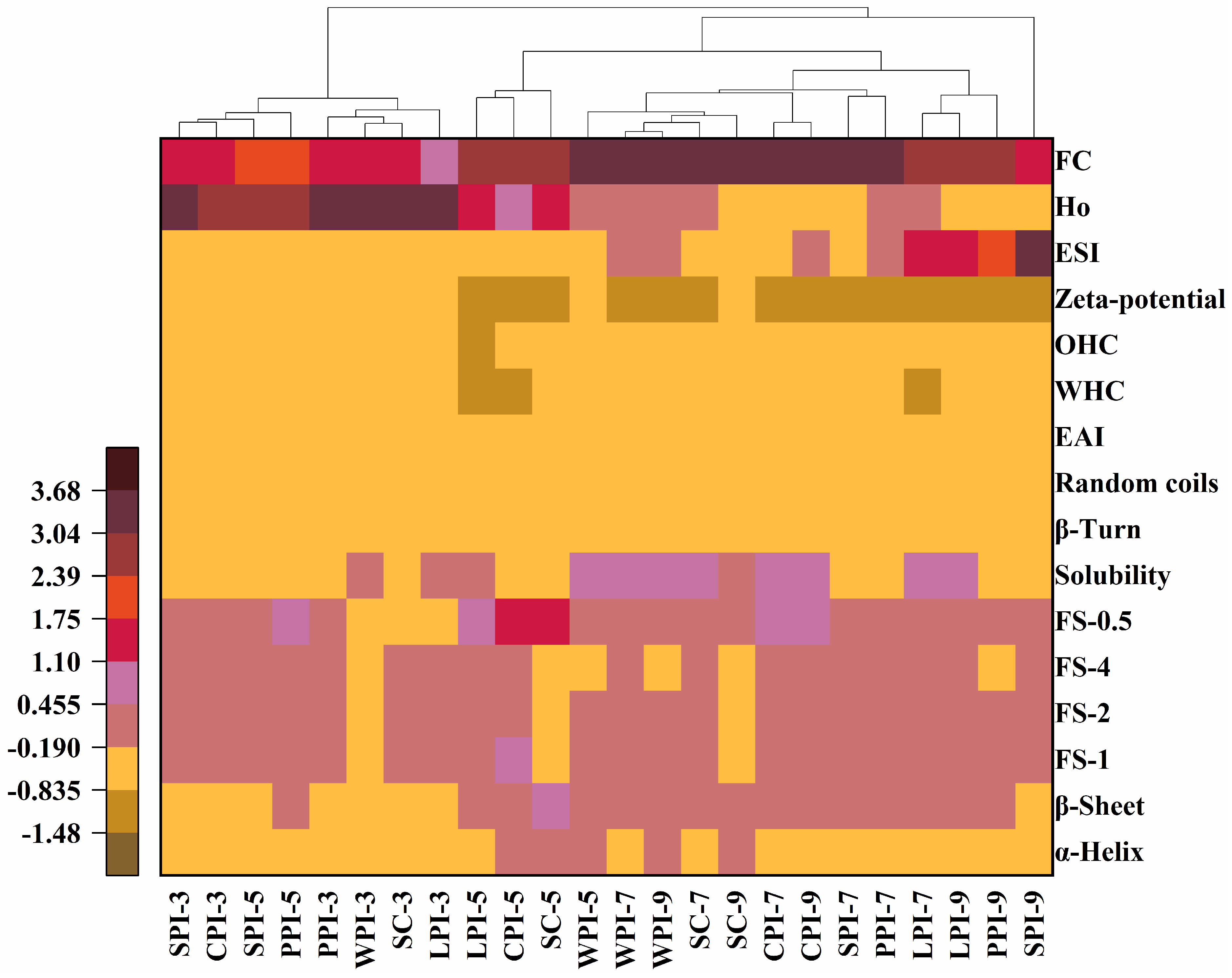
3.5. Hierarchical Cluster Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kanakis, C.; Hasni, I.; Bourassa, P.; Tarantilis, P.; Polissiou, M.; Tajmir-Riahi, H.-A. Milk β-lactoglobulin complexes with tea polyphenols. Food Chem. 2011, 127, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Lo, B.; Gorczyca, E.; Kasapis, S.; Zisu, B. Effect of low-frequency ultrasound on the particle size, solubility and surface charge of reconstituted sodium caseinate. Ultrason. Sonochem. 2019, 58, 104525. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.; Menossi, M.; Cunha, R. The effect of the glucono-δ-lactone/caseinate ratio on sodium caseinate gelation. Int. Dairy J. 2006, 16, 389–398. [Google Scholar] [CrossRef]
- Nicolai, T.; Chassenieux, C. Heat-induced gelation of casein micelles. Food Hydrocoll. 2021, 118, 106755. [Google Scholar] [CrossRef]
- Hoy, A.Q. Agricultural advances draw opposition that blunts innovation. Science 2018, 360, 1413–1414. [Google Scholar] [CrossRef]
- Stehfest, E.; Bouwman, L.; van Vuuren, D.P.; den Elzen, M.G.J.; Eickhout, B.; Kabat, P. Climate benefits of changing diet. Clim. Chang. 2009, 95, 83–102. [Google Scholar] [CrossRef]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.; Castel, V.; Rosales, M.; Rosales, M.; de Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; Food & Agriculture Organization: Rome, Italy, 2006. [Google Scholar]
- Aiking, H. Future protein supply. Trends Food Sci. Technol. 2011, 22, 112–120. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Structural and functional characterization of kidney bean and field pea protein isolates: A comparative study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Yuan, D.-B.; Min, W.; Yang, X.-Q.; Tang, C.-H.; Huang, K.-L.; Guo, J.; Wang, J.-M.; Wu, N.-N.; Zheng, H.-G.; Qi, J.-R. An improved isolation method of soy β-conglycinin subunits and their characterization. J. Am. Oil Chem. Soc. 2010, 87, 997–1004. [Google Scholar] [CrossRef]
- González-Pérez, S.; Vereijken, J.M.; Merck, K.B.; van Koningsveld, G.A.; Gruppen, H.; Voragen, A.G. Conformational states of sunflower (Helianthus annuus) helianthinin: Effect of heat and pH. J. Agric. Food Chem. 2004, 52, 6770–6778. [Google Scholar] [CrossRef]
- Mckenzie, H.A.; Sawyer, W.H. Effect of pH on β-lactoglobulins. Nature 1967, 214, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Sittikijyothin, W.; Sampaio, P.; Gonçalves, M.P. Heat-induced gelation of β-lactoglobulin at varying pH: Effect of tara gum on the rheological and structural properties of the gels. Food Hydrocoll. 2007, 21, 1046–1055. [Google Scholar] [CrossRef]
- Lakemond, C.M.; de Jongh, H.H.; Hessing, M.; Gruppen, H.; Voragen, A.G. Soy glycinin: Influence of pH and ionic strength on solubility and molecular structure at ambient temperatures. J. Agric. Food Chem. 2000, 48, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Kazmierski, M.; Corredig, M. Characterization of soluble aggregates from whey protein isolate. Food Hydrocoll. 2003, 17, 685–692. [Google Scholar] [CrossRef]
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S.H. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- Lam, R.S.H.; Nickerson, M.T. The effect of pH and temperature pre-treatments on the physicochemical and emulsifying properties of whey protein isolate. LWT-Food Sci. Technol. 2015, 60, 427–434. [Google Scholar] [CrossRef]
- Kim, J.H.J.; Varankovich, N.V.; Nickerson, M.T. The effect of pH on the gelling behaviour of canola and soy protein isolates. Food Res. Int. 2016, 81, 31–38. [Google Scholar] [CrossRef]
- Gao, H.; Ma, L.; Li, T.; Sun, D.; Hou, J.; Li, A.; Jiang, Z. Impact of ultrasonic power on the structure and emulsifying properties of whey protein isolate under various pH conditions. Process Biochem. 2019, 81, 113–122. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.X.; Mata, A.; Corke, H.; Gan, R.Y.; Fang, Y.P. Physicochemical and pH-dependent functional properties of proteins isolated from eight traditional Chinese beans. Food Hydrocoll. 2021, 112, 106288. [Google Scholar] [CrossRef]
- Ettoumi, Y.L.; Chibane, M.; Romero, A. Emulsifying properties of legume proteins at acidic conditions: Effect of protein concentration and ionic strength. LWT-Food Sci. Technol. 2016, 66, 260–266. [Google Scholar] [CrossRef]
- Sugiarto, M.; Ye, A.; Singh, H. Characterisation of binding of iron to sodium caseinate and whey protein isolate. Food Chem. 2009, 114, 1007–1013. [Google Scholar] [CrossRef]
- Niknam, R.; Mousavi, M.; Kiani, H. Comprehensive evaluation of emulsifying and foaming properties of Gleditsia caspica seed galactomannan as a new source of hydrocolloid: Effect of extraction method. Food Hydrocoll. 2022, 131, 107758. [Google Scholar] [CrossRef]
- Naik, A.; Raghavendra, S.; Raghavarao, K. Production of coconut protein powder from coconut wet processing waste and its characterization. Appl. Biochem. Biotechnol. 2012, 167, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Tang, X.; Li, Y. Drying methods affect physicochemical and functional properties of quinoa protein isolate. Food Chem. 2021, 339, 127823. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Boeck, T.; Zannini, E.; Sahin, A.W.; Bez, J.; Arendt, E.K. Nutritional and rheological features of lentil protein isolate for yoghurt-like application. Foods 2021, 10, 1692. [Google Scholar] [CrossRef] [PubMed]
- Ingrassia, R.; Palazolo, G.G.; Wagner, J.R.; Risso, P.H. Heat treatments of defatted soy flour: Impact on protein structure, aggregation, and cold-set gelation properties. Food Struct. 2019, 22, 100130. [Google Scholar] [CrossRef]
- Guldiken, B.; Stobbs, J.; Nickerson, M. Heat induced gelation of pulse protein networks. Food Chem. 2021, 350, 129158. [Google Scholar] [CrossRef]
- Alonso-Miravalles, L.; Jeske, S.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; Wriessnegger, C.L.; O’Mahony, J.A.; Zannini, E.; Arendt, E.K. Membrane filtration and isoelectric precipitation technological approaches for the preparation of novel, functional and sustainable protein isolate from lentils. Eur. Food Res. Technol. 2019, 245, 1855–1869. [Google Scholar] [CrossRef]
- Withana-Gamage, T.S.; Wanasundara, J.P.; Pietrasik, Z.; Shand, P.J. Physicochemical, thermal and functional characterisation of protein isolates from Kabuli and Desi chickpea (Cicer arietinum L.): A comparative study with soy (Glycine max) and pea (Pisum sativum L.). J. Sci. Food Agric. 2011, 91, 1022–1031. [Google Scholar] [CrossRef]
- Ramani, A.; Kushwaha, R.; Malaviya, R.; Kumar, R.; Yadav, N. Molecular, functional and nutritional properties of chickpea (Cicer arietinum L.) protein isolates prepared by modified solubilization methods. J. Food Meas. Charact. 2021, 15, 2352–2368. [Google Scholar] [CrossRef]
- Siddique, M.A.B.; Maresca, P.; Pataro, G.; Ferrari, G. Influence of pulsed light treatment on the aggregation of whey protein isolate. Food Res. Int. 2017, 99, 419–425. [Google Scholar] [CrossRef] [PubMed]
- McGrath, B.A.; Kinsella, M.; Huppertz, T.; McSweeney, P.L.; Kelly, A.L. Proteomic characterisation of heat-induced hydrolysis of sodium caseinate. Int. Dairy J. 2016, 53, 51–59. [Google Scholar] [CrossRef]
- Sharma, N.; Sahu, J.K.; Choudhary, A.; Meenu, M.; Bansal, V. High intensity ultrasound (HIU)-induced functionalization of foxtail millet protein and its fractions. Food Hydrocoll. 2023, 134, 108083. [Google Scholar] [CrossRef]
- Dai, S.; Lian, Z.; Qi, W.; Chen, Y.; Tong, X.; Tian, T.; Lyu, B.; Wang, M.; Wang, H.; Jiang, L. Non-covalent interaction of soy protein isolate and catechin: Mechanism and effects on protein conformation. Food Chem. 2022, 384, 132507. [Google Scholar] [CrossRef]
- Li, Y.P.; Sukmanov, V.; Kang, Z.L.; Ma, H. Effect of soy protein isolate on the techno-functional properties and protein conformation of low-sodium pork meat batters treated by high pressure. J. Food Process Eng. 2019, 43, e13343. [Google Scholar] [CrossRef]
- Yang, L.; Jia, J.; Zhou, X.; Liu, M.; Zhang, Q.; Tian, L.; Tan, W.; Yang, Y.; Liu, X.; Duan, X. Phosvitin-wheat gluten complex catalyzed by transglutaminase in the presence of Na2SO3: Formation, cross-link behavior and emulsifying properties. Food Chem. 2021, 346, 128903. [Google Scholar] [CrossRef]
- Rahmati, N.F.; Koocheki, A.; Varidi, M.; Kadkhodaee, R. Introducing Speckled sugar bean (Phaseolus vulgaris) protein isolates as a new source of emulsifying agent. Food Hydrocoll. 2018, 79, 498–508. [Google Scholar] [CrossRef]
- Tang, C.-H.; Sun, X. A comparative study of physicochemical and conformational properties in three vicilins from Phaseolus legumes: Implications for the structure–function relationship. Food Hydrocoll. 2011, 25, 315–324. [Google Scholar] [CrossRef]
- Ma, X.; Chen, W.; Yan, T.; Wang, D.; Hou, F.; Miao, S.; Liu, D. Comparison of citrus pectin and apple pectin in conjugation with soy protein isolate (SPI) under controlled dry-heating conditions. Food Chem. 2020, 309, 125501. [Google Scholar] [CrossRef]
- Alizadeh-Pasdar, N.; Li-Chan, E.C. Comparison of protein surface hydrophobicity measured at various pH values using three different fluorescent probes. J. Agric. Food Chem. 2000, 48, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Kinsella, J. pH dependent emulsifying properties of b-lactoglobulin. J. Dispers. Sci. 1989, 10, 77–102. [Google Scholar] [CrossRef]
- Chang, C.; Tu, S.; Ghosh, S.; Nickerson, M. Effect of pH on the inter-relationships between the physicochemical, interfacial and emulsifying properties for pea, soy, lentil and canola protein isolates. Food Res. Int. 2015, 77, 360–367. [Google Scholar] [CrossRef]
- Penzer, G. The dependence of emission spectra on molecular conformation studied by fluorescence and proton magnetic resonance. Europ. J. Biochem. 1972, 25, 218. [Google Scholar] [CrossRef]
- Tontul, I.; Kasimoglu, Z.; Asik, S.; Atbakan, T.; Topuz, A. Functional properties of chickpea protein isolates dried by refractance window drying. Int. J. Biol. Macromol. 2018, 109, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Mune, M.A.M.; Sogi, D.S. Emulsifying and foaming properties of protein concentrates prepared from cowpea and Bambara bean using different drying methods. Int. J. Food Prop. 2016, 19, 371–384. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, Q.; Wang, Y.; Hu, J.; Xiong, H.; Zhao, Q. Effects of sequential enzymatic hydrolysis and transglutaminase crosslinking on functional, rheological, and structural properties of whey protein isolate. LWT 2022, 153, 112415. [Google Scholar] [CrossRef]
- Liu, J.; Ru, Q.; Ding, Y. Glycation a promising method for food protein modification: Physicochemical properties and structure, a review. Food Res. Int. 2012, 49, 170–183. [Google Scholar] [CrossRef]
- Chen, W.; Wang, W.; Ma, X.; Lv, R.; Balaso Watharkar, R.; Ding, T.; Ye, X.; Liu, D. Effect of pH-shifting treatment on structural and functional properties of whey protein isolate and its interaction with (-)-epigallocatechin-3-gallate. Food Chem. 2019, 274, 234–241. [Google Scholar] [CrossRef]
- Vivian, J.T.; Callis, P.R. Mechanisms of tryptophan fluorescence shifts in proteins. Biophys. J. 2001, 80, 2093–2109. [Google Scholar] [CrossRef]
- Ma, X.; Chi, C.; Pu, Y.; Miao, S.; Liu, D. Conjugation of soy protein isolate (SPI) with pectin: Effects of structural modification of the grafting polysaccharide. Food Chem. 2022, 387, 132876. [Google Scholar] [CrossRef]
- Keerati-u-rai, M.; Miriani, M.; Iametti, S.; Bonomi, F.; Corredig, M. Structural changes of soy proteins at the oil–water interface studied by fluorescence spectroscopy. Colloids Surf. B Biointerfaces 2012, 93, 41–48. [Google Scholar] [CrossRef]
- Tan, H.L.; McGrath, K.M. Na-caseinate/oil/water systems: Emulsion morphology diagrams. J. Colloid Interface Sci. 2012, 381, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X. Binding, stability, and antioxidant activity of quercetin with soy protein isolate particles. Food Chem. 2015, 188, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Ten, Z.; Wang, X.S.; Yang, X.Q. Physicochemical and functional properties of hemp (Cannabis sativa L.) protein isolate. J. Agric. Food Chem. 2006, 54, 8945–8950. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Xie, J.; Gong, B.; Xu, X.; Tang, W.; Li, X.; Li, C.; Xie, M. Extraction, physicochemical characteristics and functional properties of Mung bean protein. Food Hydrocoll. 2018, 76, 131–140. [Google Scholar] [CrossRef]
- Elsohaimy, S.A.; Refaay, T.M.; Zaytoun, M.A.M. Physicochemical and functional properties of quinoa protein isolate. Ann. Agric. Sci. 2015, 60, 297–305. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Chang, C.; Chen, J.; Cao, F.; Zhao, J.; Zheng, Y.; Zhu, J. Physicochemical and functional properties of proteins extracted from three microalgal species. Food Hydrocoll. 2019, 96, 510–517. [Google Scholar] [CrossRef]
- Onsaard, E.; Vittayanont, M.; Srigam, S.; McClements, D.J. Properties and stability of oil-in-water emulsions stabilized by coconut skim milk proteins. J. Agric. Food Chem. 2005, 53, 5747–5753. [Google Scholar] [CrossRef]
- Zayas, J.F. Solubility of proteins. In Functionality of Proteins in Food; Springer: Berlin/Heidelberg, Germany, 1997; pp. 6–75. [Google Scholar]
- Jahaniaval, F.; Kakuda, Y.; Abraham, V.; Marcone, M.F. Soluble protein fractions from pH and heat treated sodium caseinate: Physicochemical and functional properties. Food Res. Int. 2000, 33, 637–647. [Google Scholar] [CrossRef]
- Ma, X.; Chatterton, D.E. Strategies to improve the physical stability of sodium caseinate stabilized emulsions: A literature review. Food Hydrocoll. 2021, 119, 106853. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Lee, K.; Ryu, H.; Rhee, K. Protein solubility characteristics of commercial soy protein products. J. Am. Oil Chem. Soc. 2003, 80, 85–90. [Google Scholar] [CrossRef]
- Sui, X.; Sun, H.; Qi, B.; Zhang, M.; Li, Y.; Jiang, L. Functional and conformational changes to soy proteins accompanying anthocyanins: Focus on covalent and non-covalent interactions. Food Chem. 2018, 245, 871–878. [Google Scholar] [CrossRef]
- Aryee, A.N.A.; Agyei, D.; Udenigwe, C.C. Impact of processing on the chemistry and functionality of food proteins. In Proteins in Food Processing; Woodhead Publishing: Sarsaparilla, UK, 2018; pp. 27–45. [Google Scholar]
- Benelhadj, S.; Gharsallaoui, A.; Degraeve, P.; Attia, H.; Ghorbel, D. Effect of pH on the functional properties of Arthrospira (Spirulina) platensis protein isolate. Food Chem. 2016, 194, 1056–1063. [Google Scholar] [CrossRef]
- Cheung, L.; Wanasundara, J.; Nickerson, M.T. Effect of pH and NaCl on the emulsifying properties of a napin protein isolate. Food Biophys. 2015, 10, 30–38. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Morr, C.V.; Ha, E.Y. Structural and functional properties of caseinate and whey protein isolate as affected by temperature and pH. J. Food Sci. 1992, 57, 1210–1229. [Google Scholar] [CrossRef]
- Adebiyi, A.P.; Aluko, R.E. Functional properties of protein fractions obtained from commercial yellow field pea (Pisum sativum L.) seed protein isolate. Food Chem. 2011, 128, 902–908. [Google Scholar] [CrossRef]
- Hu, X.-Z.; Cheng, Y.-Q.; Fan, J.-F.; Lu, Z.-H.; Yamaki, K.; Li, L.-T. Effects of Drying Method on Physicochemical and Functional Properties of Soy Protein Isolates. J. Food Process. Preserv. 2009, 34, 520–540. [Google Scholar] [CrossRef]
- Sagis, L.M.; Yang, J. Protein-stabilized interfaces in multiphase food: Comparing structure-function relations of plant-based and animal-based proteins. Curr. Opin. Food Sci. 2022, 43, 53–60. [Google Scholar] [CrossRef]
- Sai-Ut, S.; Ketnawa, S.; Chaiwut, P.; Rawdkuen, S. Biochemical and functional properties of proteins from red kidney, navy and adzuki beans. Asian J. Food Agro-Ind. 2009, 2, 493–504. [Google Scholar]
| Samples | Composition (%) | ||||
|---|---|---|---|---|---|
| Protein | Moisture | Ash | Lipid | Carbohydrates | |
| SPI | 89.96 c ± 0.26 | 4.27 b ± 0.02 | 4.74 c ± 0.36 | 0.94 b ± 0.02 | 0.09 a ± 0.08 |
| PPI | 78.66 a ± 0.04 | 7.59 e ± 0.01 | 4.30 bc ± 0.03 | 3.64 c ± 0.09 | 5.88 d ±0.01 |
| LPI | 89.07 b ± 0.79 | 1.48 a ± 0.14 | 4.22 b ± 0.02 | 0.67 ab ± 0.03 | 4.54 c ± 0.76 |
| CPI | 87.16 c ± 0.64 | 1.42 a ± 0.02 | 4.68 c ± 0.01 | 4.61 d ± 0.42 | 2.44 b ± 0.82 |
| WPI | 86.93 b ± 0.40 | 5.37 c ± 0.05 | 2.72 a ± 0.22 | 0.36 a ± 0.07 | 4.58 c ± 0.25 |
| SC | 87.51 b ± 0.49 | 6.51 d ± 0.01 | 4.14 b ± 0.02 | 0.66 ab ± 0.04 | 1.16 a ± 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Q.; Roos, Y.H.; Miao, S. Plant Protein versus Dairy Proteins: A pH-Dependency Investigation on Their Structure and Functional Properties. Foods 2023, 12, 368. https://doi.org/10.3390/foods12020368
Tang Q, Roos YH, Miao S. Plant Protein versus Dairy Proteins: A pH-Dependency Investigation on Their Structure and Functional Properties. Foods. 2023; 12(2):368. https://doi.org/10.3390/foods12020368
Chicago/Turabian StyleTang, Qi, Yrjö H. Roos, and Song Miao. 2023. "Plant Protein versus Dairy Proteins: A pH-Dependency Investigation on Their Structure and Functional Properties" Foods 12, no. 2: 368. https://doi.org/10.3390/foods12020368
APA StyleTang, Q., Roos, Y. H., & Miao, S. (2023). Plant Protein versus Dairy Proteins: A pH-Dependency Investigation on Their Structure and Functional Properties. Foods, 12(2), 368. https://doi.org/10.3390/foods12020368







