Impact of Processing and Preservation Methods and Storage on Total Phenolics, Flavonoids, and Antioxidant Activities of Okra (Abelmoschus esculentus L.)
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Sample Preparation
- (1)
- Freezing (BF): A batch of 500 g underwent blanching for a duration of 5 min, followed by gradual cooling to room temperature (25 °C), kept in plastic bags, and stored in a standard home freezer at −18 °C for 3 months.
- (2)
- Freezing (FF): Another 500 g batch was stir-fried with corn oil in a frying pan over medium-high heat (170 °C) for 5 min (F), when the okra became golden brown, it was removed. The fried okra was then drained on a paper towel-lined plate to facilitate the removal of excess absorbed oil, cooled at RT, and kept frozen in plastic bags at −18 °C in a normal home freezer for 3 months.
- (3)
- Sun drying (SD): A batch of 500 g of okra was spread out in a single layer on perforated stainless-steel trays and sun dried at 25–35 °C in an area that receives direct sunlight and good airflow for 3 months. The okra was regularly turned twice a day during the sun-drying process to ensure uniform drying.
- (4)
- Convection oven drying (OD): A batch of 500 g of okra was arranged on perforated stainless-steel trays and dried through the application of hot air in a conventional oven (Gallenkamp, UK) at 70 °C for 5 days. Following the drying period, the dehydrated okra was stored in a sealed glass jar at room temperature for 3 months.
2.3. Extraction of Okra Samples
2.4. Determination of Total Phenolic Content
2.5. Determination of Total Flavonoid Content
2.6. Antioxidant Activities
2.6.1. DPPH Free Radical Scavenging Assay
2.6.2. Reducing Power Activity (%)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effects of Processing, Preservation Methods, and Storage on Total Phenolic and Flavonoid Contents of Okra
3.2. Effects of Processing, Preservation Methods, and Storage on Antioxidant Activity of Okra
3.2.1. DPPH Free Radical Scavenging Activity
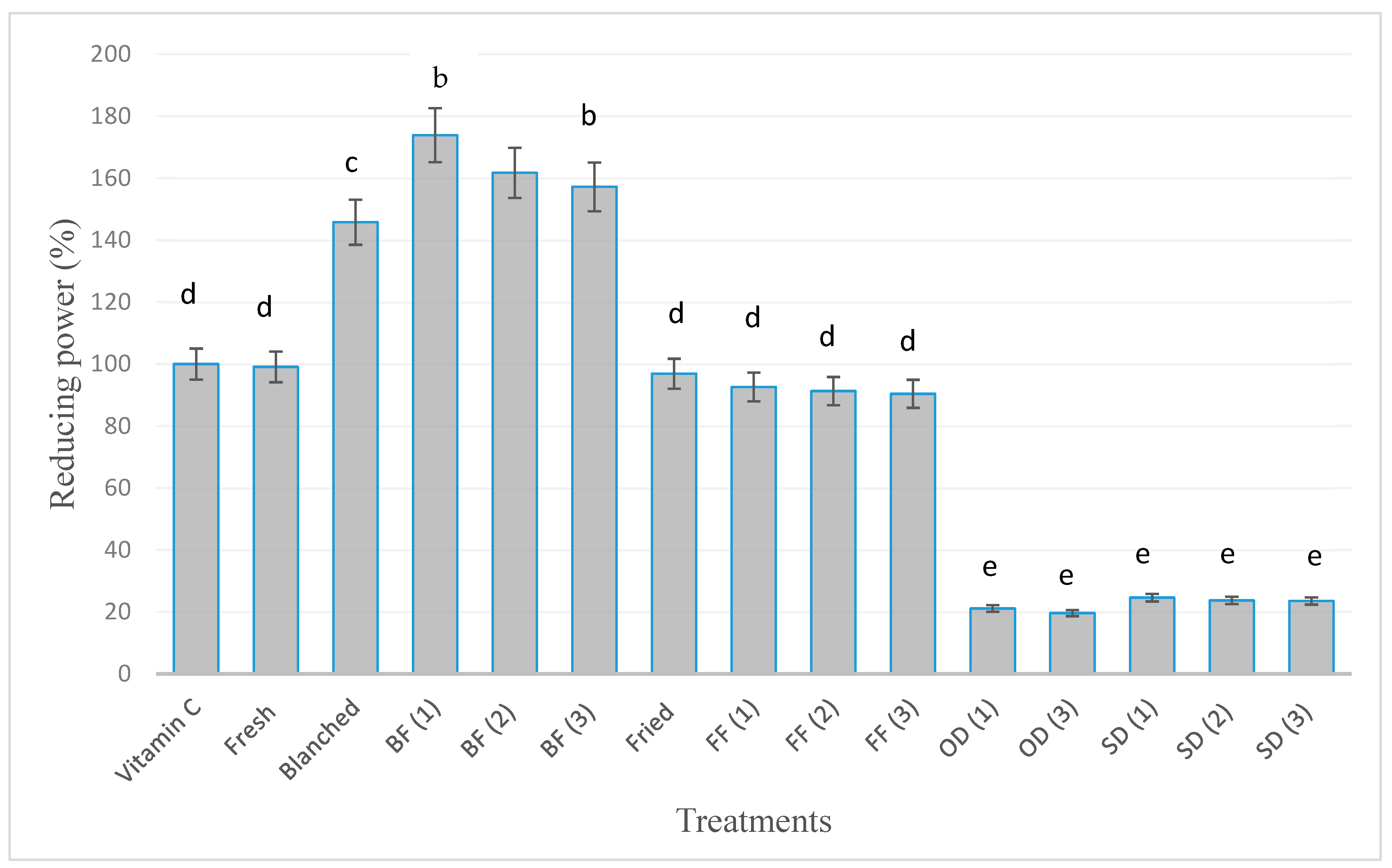
3.2.2. Reducing Power Activity (%)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arapitsas, P. Identification and Quantification of Polyphenolic Compounds from Okra Seeds and Skins. Food Chem. 2008, 110, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Badmus, U.O.; Taggart, M.A.; Boyd, K.G. The Effect of Different Drying Methods on Certain Nutritionally Important Chemical Constituents in Edible Brown Seaweeds. J. Appl. Phycol. 2019, 31, 3883–3897. [Google Scholar] [CrossRef]
- Bernhardt, S.; Schlich, E. Impact of Different Cooking Methods on Food Quality: Retention of Lipophilic Vitamins in Fresh and Frozen Vegetables. J. Food Eng. 2006, 77, 327–333. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Novellino, E.; Souto, E.B.; Daliu, P.; Santini, A. Abelmoschus esculentus (L.): Bioactive Components’ Beneficial Properties-Focused on Antidiabetic Role-for Sustainable Health Applications. Molecules 2019, 24, 38. [Google Scholar] [CrossRef] [PubMed]
- Duha, P.-D.; Yed, C. Antioxidative Activity of Three Herbal Water Extracts. Food Chem. 1997, 60, 639–645. [Google Scholar] [CrossRef]
- Gemede, H.F.; Haki, G.D.; Beyene, F.; Woldegiorgis, A.Z.; Rakshit, S.K. Proximate, Mineral, and Antinutrient Compositions of Indigenous Okra (Abelmoschus esculentus) Pod Accessions: Implications for Mineral Bioavailability. Food Sci. Nutr. 2016, 4, 223–233. [Google Scholar] [CrossRef]
- Gemede, H.F.; Haki, G.D.; Beyene, F.; Rakshit, S.K.; Woldegiorgis, A.Z. Indigenous Ethiopian Okra (Abelmoschus esculentus) Mucilage: A Novel Ingredient with Functional and Antioxidant Properties. Food Sci. Nutr. 2018, 6, 563–571. [Google Scholar] [CrossRef]
- Gemede, H.F.; Ratta, N.; Haki, G.D.; Woldegiorgis, A.Z.; Beyene, F. Nutritional Quality and Health Benefits of Okra (Abelmoschus esculentus): A Review. Pakistan J. Food Sci. 2015, 25, 16–25. [Google Scholar] [CrossRef]
- Duzyaman, E. Okra: Botany and Horticulture; Wiley: Hoboken, NJ, USA, 1997; Volume 21, ISBN 9780471189077. [Google Scholar]
- Gertz, C.; Klostermann, S.; Kochhar, S.P. Testing and Comparing Oxidative Stability of Vegetable Oils and Fats at Frying Temperature. Eur. J. Lipid Sci. Technol. 2000, 102, 543–551. [Google Scholar] [CrossRef]
- González, E.M.; De Ancos, B.; Cano, M.P. Relation between Bioactive Compounds and Free Radical-Scavenging Capacity in Berry Fruits during Frozen Storage. J. Sci. Food Agric. 2003, 83, 722–726. [Google Scholar] [CrossRef]
- Longvah, T.; Ananthan, R.; Bhaskarachary, K.; Venkaiah, K. Indian Food Composition Tables; National Institute of Nutrition, Indian Council of Medical Research: Hyderabad, India, 2017. [Google Scholar]
- Harbourne, N.; Marete, E.; Jacquier, J.C.; O’Riordan, D. Effect of Drying Methods on the Phenolic Constituents of Meadowsweet (Filipendula ulmaria) and Willow (Salix alba). LWT 2009, 42, 1468–1473. [Google Scholar] [CrossRef]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two New Flavonoids and Other Constituents in Licorice Root: Their Relative Astringency and Radical Scavenging Effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Marjan, Z.M.; Foong, C.W. Total Antioxidant Activity and Phenolic Content in Selected Vegetables. Food Chem. 2004, 87, 581–586. [Google Scholar] [CrossRef]
- Jiménez-Monreal, A.M.; García-Diz, L.; Martínez-Tomé, M.; Mariscal, M.; Murcia, M.A. Influence of Cooking Methods on Antioxidant Activity of Vegetables. J. Food Sci. 2009, 74, H97–H103. [Google Scholar] [CrossRef]
- Kahlon, T.S.; Chapman, M.H.; Smith, G.E. In Vitro Binding of Bile Acids by Okra, Beets, Asparagus, Eggplant, Turnips, Green Beans, Carrots, and Cauliflower. Food Chem. 2007, 103, 676–680. [Google Scholar] [CrossRef]
- Karakaya, S. Bioavailability of Phenolic Compounds. Crit. Rev. Food Sci. Nutr. 2004, 44, 453–464. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; Van Beek, T.A. Screening of Radical Scavenging Activity of Some Medicinal and Aromatic Plant Extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. The Effect of Cooking Methods on Total Phenolics and Antioxidant Activity of Selected Green Vegetables. Food Chem. 2005, 93, 713–718. [Google Scholar] [CrossRef]
- Yildirim, A.; Mavi, A.; Kara, A.A. Antioxidant and Antimicrobial Activities of Polygonum Cognatum Meissn Extracts. J. Sci. Food Agric. 2003, 83, 64–69. [Google Scholar] [CrossRef]
- Randhir, R.; Kwon, Y.I.; Shetty, K. Effect of Thermal Processing on Phenolics, Antioxidant Activity and Health-Relevant Functionality of Select Grain Sprouts and Seedlings. Innov. Food Sci. Emerg. Technol. 2008, 9, 355–364. [Google Scholar] [CrossRef]
- Lima, G.P.P.; Lopes, T.D.V.C.; Rossetto, M.R.M.; Vianello, F. Nutritional Composition, Phenolic Compounds, Nitrate Content in Eatable Vegetables Obtained by Conventional and Certified Organic Grown Culture Subject to Thermal Treatment. Int. J. Food Sci. Technol. 2009, 44, 1118–1124. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Iqbal, S. Effect of Different Cooking Methods on the Antioxidant Activity of Some Vegetables from Pakistan. Int. J. Food Sci. Technol. 2008, 43, 560–567. [Google Scholar] [CrossRef]
- Somsub, W.; Kongkachuichai, R.; Sungpuag, P.; Charoensiri, R. Effects of Three Conventional Cooking Methods on Vitamin C, Tannin, Myo-Inositol Phosphates Contents in Selected Thai Vegetables. J. Food Compos. Anal. 2008, 21, 187–197. [Google Scholar] [CrossRef]
- Petzold, G.; Aguilera, J.M. Ice Morphology: Fundamentals and Technological Applications in Foods. Food Biophys. 2009, 4, 378–396. [Google Scholar] [CrossRef]
- Wani, S.M.; Masoodi, F.A.; Ahmad, M.; Mir, S.A. Processing and Storage of Apricots: Effect on Physicochemical and Antioxidant Properties. J. Food Sci. Technol. 2018, 55, 4505–4514. [Google Scholar] [CrossRef]
- Korus, A. Effect of Preliminary Processing, Method of Drying and Storage Temperature on the Level of Antioxidants in Kale (Brassica oleracea L. Var. Acephala) Leaves. LWT 2011, 44, 1711–1716. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Murtijaya, J. Antioxidant Properties of Phyllanthus Amarus Extracts as Affected by Different Drying Methods. LWT 2007, 40, 1664–1669. [Google Scholar] [CrossRef]
- Mohammed, S.; Edna, M.; Siraj, K. The Effect of Traditional and Improved Solar Drying Methods on the Sensory Quality and Nutritional Composition of Fruits: A Case of Mangoes and Pineapples. Heliyon 2020, 6, e04163. [Google Scholar] [CrossRef]
- Martín-Cabrejas, M.A.; Aguilera, Y.; Pedrosa, M.M.; Cuadrado, C.; Hernández, T.; Díaz, S.; Esteban, R.M. The Impact of Dehydration Process on Antinutrients and Protein Digestibility of Some Legume Flours. Food Chem. 2009, 114, 1063–1068. [Google Scholar] [CrossRef]
- Rababah, T.M.; Al-U’ Datt, M.; Alhamad, M.; Al-Mahasneh, M.; Ereifej, K.; Andrade, J.; Altarifi, B.; Almajwal, A.; Yang, W. Effects of Drying Process on Total Phenolics, Antioxidant Activity and Flavonoid Contents of Common Mediterranean Herbs. Int. J. Agric. Biol. Eng. 2015, 8, 145–150. [Google Scholar] [CrossRef]
- Hammerstone, J.F.; Lazarus, S.A.; Schmitz, H.H. Procyanidin Content and Variation in Some Commonly Consumed Foods. J. Nutr. 2000, 130, 2086S–2092S. [Google Scholar] [CrossRef] [PubMed]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. Phytochemical Content and Antioxidant Activities of Thirteen Fruits of Assam, India. Food Biosci. 2016, 13, 15–20. [Google Scholar] [CrossRef]
- Zhang, D.; Hamauzu, Y. Phenolics, Ascorbic Acid, Carotenoids and Antioxidant Activity of Broccoli and Their Changes during Conventional and Microwave Cooking. Food Chem. 2004, 88, 503–509. [Google Scholar] [CrossRef]
| Okra Treatments Extract | Phenolics (mg GAE/100g) | Flavonoids (mg QE/100g) | DPPH IC50 (mg/mL) |
|---|---|---|---|
| Fresh okra | 86.35 ± 0.35 e | 105.10 ± 0.82 a | 3.81 ± 0.85 h |
| Blanched okra (B) | 134.10 ± 1.42 d | 60.43 ± 0.97 b,c | 3.02 ± 0.17 h |
| (BF1)-Frozen (1 month) | 167.10 ± 2.10 a | 45.73 ± 0.52 e | 2.91 ± 0.34 h |
| (BF2)-Frozen (2 months) | 156.15 ± 3.95 b | 42.21 ± 0.85 f | 3.06 ± 0.15 h |
| (BF3)-Frozen (3 months) | 148.70 ± 4.28 b,c | 40.45 ± 0.73 f | 3.65 ± 0.37 h |
| Fried okra (F) | 75.35 ± 1.05 f | 55.75 ± 0.17 d | 21.1 ± 0.46 f |
| (FF1)-Frozen (1 month) | 72.25 ± 2.65 f | 64.08 ± 1.78 b | 12.07 ± 0.59 g |
| (FF2)-Frozen (2 months) | 56.75 ± 2.65 g | 62.05 ± 1.35 b | 12.84 ± 0.64 g |
| (FF3)-Frozen (3 months) | 53.30 ± 1.28 g | 60.42 ± 0.90 b,c | 12.91 ± 0.72 g |
| (SD1)-Sun drying (1month) | 15.53 ± 1.24 j | 28.23 ± 0.64 g | 35.8 ± 0.52 e |
| (SD2)-Sun drying (2 months) | 14.70 ± 1.14 j | 13.85 ± 0.26 i | 57.8 ± 0.40 c |
| (SD3)-Sun drying (3 months) | 14.45 ± 2.36j | 13.23 ± 0.52 i | 134.0 ± 0.34 a |
| (OD1)-Oven drying (1month) | 44.60 ± 1.55 h | 39.31 ± 0.37 f | 50.4 ± 0.29 d |
| (OD3)-Oven drying (3 months) | 18.80 ± 0.99 i | 25.57 ± 0.62 h | 112.1 ± 0.62 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Dabbas, M.M.; Moumneh, M.; Hamad, H.J.; Abughoush, M.; Abuawad, B.; Al-Nawasrah, B.A.; Al-Jaloudi, R.; Iqbal, S. Impact of Processing and Preservation Methods and Storage on Total Phenolics, Flavonoids, and Antioxidant Activities of Okra (Abelmoschus esculentus L.). Foods 2023, 12, 3711. https://doi.org/10.3390/foods12193711
Al-Dabbas MM, Moumneh M, Hamad HJ, Abughoush M, Abuawad B, Al-Nawasrah BA, Al-Jaloudi R, Iqbal S. Impact of Processing and Preservation Methods and Storage on Total Phenolics, Flavonoids, and Antioxidant Activities of Okra (Abelmoschus esculentus L.). Foods. 2023; 12(19):3711. https://doi.org/10.3390/foods12193711
Chicago/Turabian StyleAl-Dabbas, Maher M., Majd Moumneh, Hani J. Hamad, Mahmoud Abughoush, Balkees Abuawad, Bha’a Aldin Al-Nawasrah, Rawan Al-Jaloudi, and Sehar Iqbal. 2023. "Impact of Processing and Preservation Methods and Storage on Total Phenolics, Flavonoids, and Antioxidant Activities of Okra (Abelmoschus esculentus L.)" Foods 12, no. 19: 3711. https://doi.org/10.3390/foods12193711
APA StyleAl-Dabbas, M. M., Moumneh, M., Hamad, H. J., Abughoush, M., Abuawad, B., Al-Nawasrah, B. A., Al-Jaloudi, R., & Iqbal, S. (2023). Impact of Processing and Preservation Methods and Storage on Total Phenolics, Flavonoids, and Antioxidant Activities of Okra (Abelmoschus esculentus L.). Foods, 12(19), 3711. https://doi.org/10.3390/foods12193711






