Main Challenges Expected from the Impact of Climate Change on Microbial Biodiversity of Table Olives: Current Status and Trends
Abstract
:1. Climate Changes and the Climate Emergency
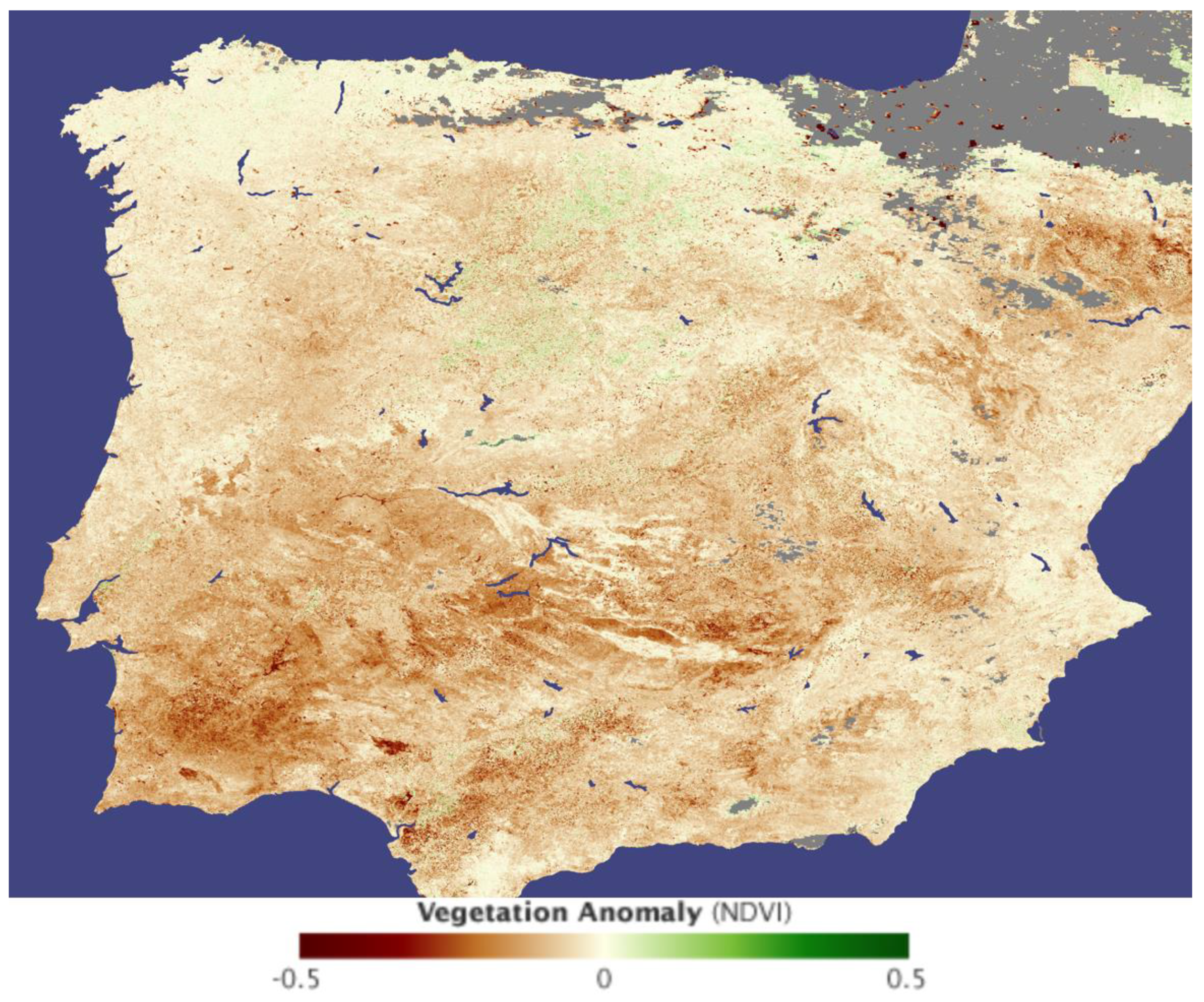
Overview of Climate Change on the Iberian Peninsula
2. Influence of Climate Change on Olive Cultivars
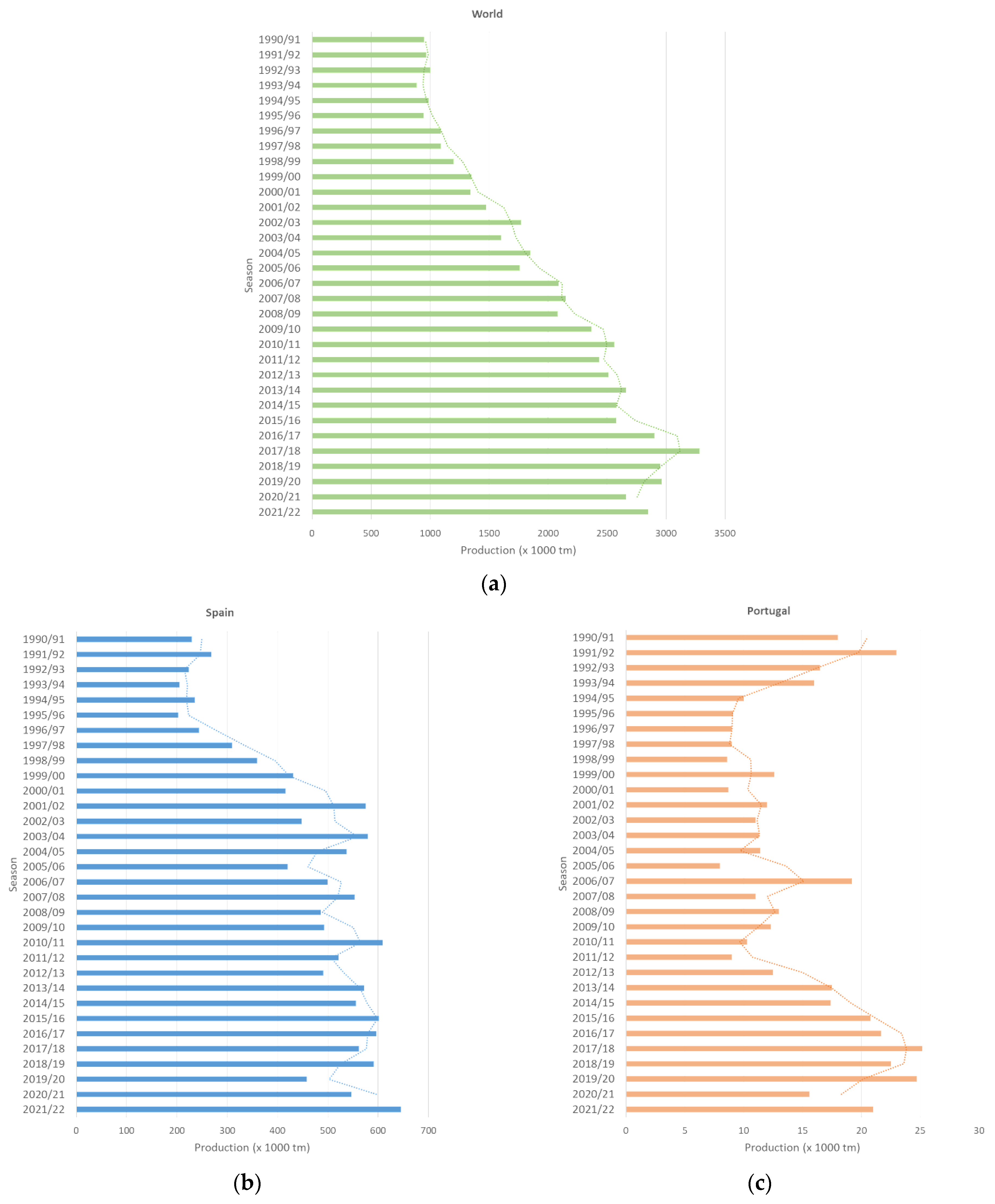
2.1. Table Olives—Trends in Production and Consumption and Preparation Styles
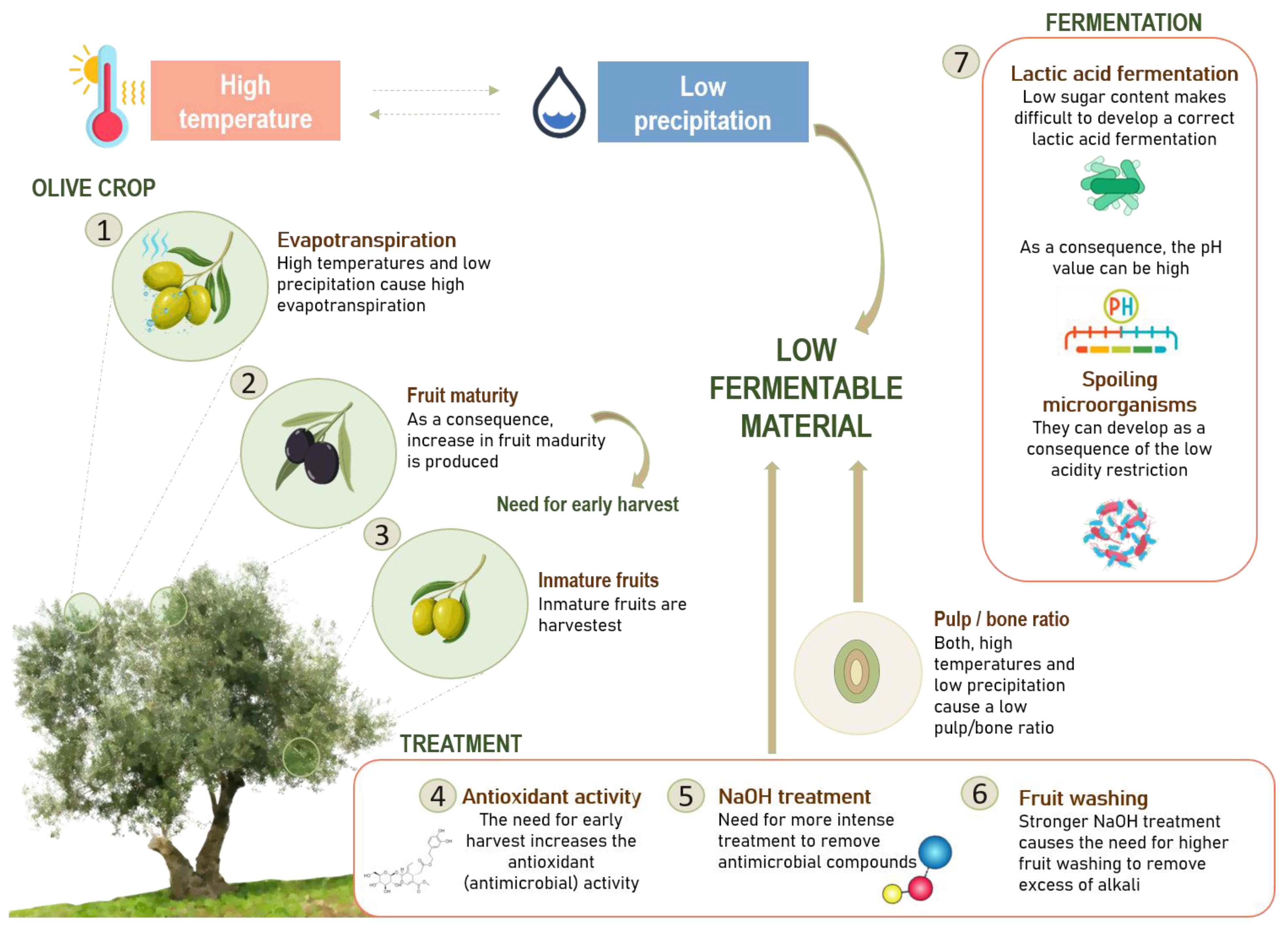
2.2. Impact of Climate Change on Biological Conditions of Olive Cultivars—Consequences on Olive Trees and Crop Yields
2.3. Impact of Climate Changes on the Main Chemical Compounds of Olives
3. Impact of Biological Changes on Table Olive Microbiological Populations
3.1. Microorganisms Involved in Table Olive Fermentations
3.2. Impact of Climate Change on Fruit Composition, Fermentation, Microbial Population, and Product Safety
3.2.1. Microbial Origin Alterations in Table Olives
3.2.2. Effect of Temperature during the Fermentation
| Microbial and Physicochemical Characteristics | Total Microbiota Log CFU/mL | Yeasts Log CFU/mL | Enterobacteria Log CFU/mL | pH | Reducing Sugars g/L | Total Phenolic Compounds g Gallic Acid/L | |
|---|---|---|---|---|---|---|---|
| Fermentation Process | Unprocessed fruits | 4.08 ± 0.30 | 3.33 ± 0.18 | <Detection limit | ND | ND | ND |
| Process A | 25 °C | 7.16 ± 0.65 | 6.47 ± 0.17 | 6.49 ± 0.21 | 4.31 ± 0.03 | 0.76 ± 0.07 | 0.28 ± 0.01 |
| Process B | 6.46 ± 0.06 | 6.15 ± 0.11 | <Detection limit | 4.54 ± 0.00 | 2.89 ± 0.41 | 0.89 ± 0.00 | |
| Process A | 18 °C | 4.93 ± 0.02 | 4.97 ± 0.19 | <Detection limit | 4.31 ± 0.10 | 1.97 ± 0.33 | 0.29 ± 0.02 |
| Process B | 5.15 ± 0.11 | 5.16 ± 0.31 | <Detection limit | 4.51 ± 0.04 | 12.31 ± 1.37 | 1.09 ± 0.07 | |
4. Effect of Climate Change on Plagues and Diseases
5. Economic Impact of Climate Change on the Olive Sector
6. Climate Adaptation Measures
6.1. Selection of Resilient Varieties
6.2. Measures during Fermentation
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. AR6 Synthesis Report: Climate Change 2023. Available online: https://www.ipcc.ch/report/sixth-assessment-report-cycle/ (accessed on 1 September 2023).
- WWF. Report Wildlife in a Warming World. Available online: https://wwfint.awsassets.panda.org/downloads/FOCUS_Mediterranean_14032018.pdf (accessed on 1 September 2023).
- IUCN. Invasive Alien Species and Climate Change. Available online: https://www.iucn.org/resources/issues-brief/invasive-alien-species-and-climate-change (accessed on 1 September 2023).
- European Parliament. Climate Change in Europe: Facts and Figures. Available online: https://www.europarl.europa.eu/news/en/headlines/society/20180703STO07123/climate-change-in-europe-facts-and-figures (accessed on 1 September 2023).
- NASA. Earth Observatory Drought on the Iberian Peninsula. Available online: https://earthobservatory.nasa.gov/images/14719/drought-on-the-iberian-peninsula (accessed on 1 September 2023).
- Garrido, J.L.; González-Rouco, J.F.; Vivanco, M.G.; Navarro, J. Regional surface temperature simulations over the Iberian Peninsula: Evaluation and climate projections. Clim. Dyn. 2020, 55, 3445–3468. [Google Scholar] [CrossRef]
- European Environment Agency. Drought Impact on Ecosystems in Europe (8th EAP). Available online: https://www.eea.europa.eu/ims/drought-impact-on-ecosystems-in-europe (accessed on 1 September 2023).
- Food and Agriculture Organization of the United Nations Statistics. 2023. Available online: https://www.fao.org/statistics/en/ (accessed on 3 October 2023).
- Hernández, J.V.; Pereira, J.E.; Urieta, D.; Menor, A.; Caño, S.; Barreal, J.; del Mar Velasco, M.; Poyatos, R.P. International Olive Growing: Worldwide Analysis and Summary; Fundación Caja Rural Jaén: Jaén, Spain, 2018. [Google Scholar]
- International Olive Oil Council Economic Affairs & Promotion Unit. International Olive Council. 2020. Available online: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/#economy-production-unit (accessed on 3 October 2023).
- Junta de Andalucía Aforo Producción de Olivar en Andalucía, 2021–2022. Available online: https://www.juntadeandalucia.es/export/drupaljda/Estimacion_ACEITE_MESA_2021_2022.pdf (accessed on 1 September 2023).
- Lopes, M.S.; Mendonça, D.; Sefc, K.M.; Gil, F.S.; Machado, A.d.C. Genetic Evidence of Intra-cultivar Variability within Iberian Olive Cultivars. HortScience 2004, 39, 1562–1565. [Google Scholar] [CrossRef]
- Fernández, A.G.; Adams, M.R.; Fernández-Díez, M.J. Table Olives: Production and Processing; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Gómez, A.H.S.; García, P.G.; Navarro, L.R. Elaboration of table olives. Grasas Aceites 2006, 57, 86–94. [Google Scholar]
- Pires-Cabral, P.; Barros, T.; Nunes, P.; Quintas, C. Physicochemical, nutritional and microbiological characteristics of traditional table olives from Southern Portugal. Emir. J. Food Agric. 2018, 30, 611–620. [Google Scholar]
- Arnfield, A.J. Köppen Climate Classification. Encyclopedia Britannica, 8 September 2023. Available online: https://www.britannica.com/science/Koppen-climate-classification (accessed on 3 October 2023).
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Ulpiani, G. On the linkage between urban heat island and urban pollution island: Three-decade literature review towards a conceptual framework. Sci. Total Environ. 2021, 751, 141727. [Google Scholar] [CrossRef]
- Fraga, H.; Moriondo, M.; Leolini, L.; Santos, J.A. Mediterranean olive orchards under climate change: A review of future impacts and adaptation strategies. Agronomy 2020, 11, 56. [Google Scholar] [CrossRef]
- Fraga, H.; Pinto, J.G.; Santos, J.A. Climate change projections for chilling and heat forcing conditions in European vineyards and olive orchards: A multi-model assessment. Clim. Change 2019, 152, 179–193. [Google Scholar] [CrossRef]
- Gabaldón-Leal, C.; Ruiz-Ramos, M.; de la Rosa, R.; León, L.; Belaj, A.; Rodríguez, A.; Santos, C.; Lorite, I.J. Impact of changes in mean and extreme temperatures caused by climate change on olive flowering in southern Spain. Int. J. Climatol. 2017, 37, 940–957. [Google Scholar] [CrossRef]
- De Melo-Abreu, J.P.; Barranco, D.; Cordeiro, A.M.; Tous, J.; Rogado, B.M.; Villalobos, F.J. Modelling olive flowering date using chilling for dormancy release and thermal time. Agric. For. Meteorol. 2004, 125, 117–127. [Google Scholar] [CrossRef]
- Torres, M.; Pierantozzi, P.; Searles, P.; Rousseaux, M.C.; García-Inza, G.; Miserere, A.; Bodoira, R.; Contreras, C.; Maestri, D. Olive cultivation in the southern hemisphere: Flowering, water requirements and oil quality responses to new crop environments. Front. Plant Sci. 2017, 8, 1830. [Google Scholar] [CrossRef] [PubMed]
- Dag, A.; Harlev, G.; Lavee, S.; Zipori, I.; Kerem, Z. Optimizing olive harvest time under hot climatic conditions of Jordan Valley, Israel. Eur. J. Lipid Sci. Technol. 2014, 116, 169–176. [Google Scholar] [CrossRef]
- Benlloch-González, M.; Sánchez-Lucas, R.; Bejaoui, M.A.; Benlloch, M.; Fernández-Escobar, R. Global warming effects on yield and fruit maturation of olive trees growing under field conditions. Sci. Hortic. 2019, 249, 162–167. [Google Scholar] [CrossRef]
- Morales, A.; Leffelaar, P.A.; Testi, L.; Orgaz, F.; Villalobos, F.J. A dynamic model of potential growth of olive (Olea europaea L.) orchards. Eur. J. Agron. 2016, 74, 93–102. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, M.; Gebrekirstos, A.; Bräuning, A. Trends in tree growth and intrinsic water-use efficiency in the tropics under elevated CO2 and climate change. Trees 2019, 33, 623–640. [Google Scholar] [CrossRef]
- Agencia Estatal de Meteorología (AEMET). Base de Datos Meteorológica. Available online: https://datosclima.es/ (accessed on 1 September 2023).
- Ministerio de Agricultura. Pesca y Alimentación Encuesta sobre Superficies y Rendimientos Cultivos (ESYRCE). Encuesta de Marco de Áreas de España. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/esyrce/ (accessed on 1 September 2023).
- Mateus, T.; Santo, D.; Saúde, C.; Pires-Cabral, P.; Quintas, C. The effect of NaCl reduction in the microbiological quality of cracked green table olives of the Maçanilha Algarvia cultivar. Int. J. Food Microbiol. 2016, 218, 57–65. [Google Scholar] [CrossRef]
- Pires-Cabral, P.; Barros, T.; Mateus, T.; Prata, J.; Quintas, C. The effect of seasoning with herbs on the nutritional, safety and sensory properties of reduced-sodium fermented Cobrançosa cv. Table olives. AIMS Agric. Food 2018, 3, 521–534. [Google Scholar] [CrossRef]
- Saúde, C.; Barros, T.; Mateus, T.; Quintas, C.; Pires-Cabral, P. Effect of chloride salts on the sensory and nutritional properties of cracked table olives of the Maçanilha Algarvia cultivar. Food Biosci. 2017, 19, 73–79. [Google Scholar] [CrossRef]
- Valente, S.; Machado, B.; Pinto, D.C.; Santos, C.; Silva, A.M.; Dias, M.C. Modulation of phenolic and lipophilic compounds of olive fruits in response to combined drought and heat. Food Chem. 2020, 329, 127191. [Google Scholar] [CrossRef]
- Martinelli, F.; Remorini, D.; Saia, S.; Massai, R.; Tonutti, P. Metabolic profiling of ripe olive fruit in response to moderate water stress. Sci. Hortic. 2013, 159, 52–58. [Google Scholar] [CrossRef]
- Tognetti, R.; d’Andria, R.; Sacchi, R.; Lavini, A.; Morelli, G.; Alvino, A. Deficit irrigation affects seasonal changes in leaf physiology and oil quality of Olea europaea (cultivars Frantoio and Leccino). Ann. Appl. Biol. 2007, 150, 169–186. [Google Scholar] [CrossRef]
- Dias, M.C.; Correia, S.; Serôdio, J.; Silva, A.M.S.; Freitas, H.; Santos, C. Chlorophyll fluorescence and oxidative stress endpoints to discriminate olive cultivars tolerance to drought and heat episodes. Sci. Hortic. 2018, 231, 31–35. [Google Scholar] [CrossRef]
- Marsilio, V.; Campestre, C.; Lanza, B. Phenolic compounds change during California-style ripe olive processing. Food Chem. 2001, 74, 55–60. [Google Scholar] [CrossRef]
- Tovar, M.J.; Motilva, M.J.; Romero, M.P. Changes in the phenolic composition of virgin olive oil from young trees (Olea europaea L. cv. Arbequina) grown under linear irrigation strategies. J. Agric. Food Chem. 2001, 49, 5502–5508. [Google Scholar] [CrossRef]
- Arroyo-López, F.N.; Bautista-Gallego, J.; Durán-Quintana, M.C.; Garrido-Fernández, A. Modelling the inhibition of sorbic and benzoic acids on a native yeast cocktail from table olives. Food Microbiol. 2008, 25, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-López, F.N.; Romero-Gil, V.; Bautista-Gallego, J.; Rodríguez-Gómez, F.; Jiménez-Díaz, R.; García-García, P.; Querol, A.; Garrido-Fernández, A. Yeasts in table olive processing: Desirable or spoilage microorganisms? Int. J. Food Microbiol. 2012, 160, 42–49. [Google Scholar] [CrossRef]
- Hurtado, A.; Reguant, C.; Bordons, A.; Rozès, N. Lactic acid bacteria from fermented table olives. Food Microbiol. 2012, 31, 1–8. [Google Scholar] [CrossRef]
- Randazzo, C.L.; Fava, G.; Tomaselli, F.; Romeo, F.V.; Pennino, G.; Vitello, E.; Caggia, C. Effect of kaolin and copper based products and of starter cultures on green table olive fermentation. Food Microbiol. 2011, 28, 910–919. [Google Scholar] [CrossRef]
- Alves, M.; Gonçalves, T.; Quintas, C. Microbial quality and yeast population dynamics in cracked green table olives’ fermentations. Food Control 2012, 23, 363–368. [Google Scholar] [CrossRef]
- De Castro, A.; Montaño, A.; Casado, F.; Sánchez, A.; Rejano, L. Utilization of Enterococcus casseliflavus and Lactobacillus pentosus as starter cultures for Spanish-style green olive fermentation. Food Microbiol. 2002, 19, 637–644. [Google Scholar] [CrossRef]
- Lanza, B. Abnormal fermentations in table-olive processing: Microbial origin and sensory evaluation. Front. Microbiol. 2013, 4, 91. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Rejano, L.; Montaño, A.; de Castro, A. Utilization at high pH of starter cultures of lactobacilli for Spanish-style green olive fermentation. Int. J. Food Microbiol. 2001, 67, 115–122. [Google Scholar] [CrossRef]
- Hsiao, C.; Siebert, K.J. Modeling the inhibitory effects of organic acids on bacteria. Int. J. Food Microbiol. 1999, 47, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Nakai, S.A.; Siebert, K.J. Validation of bacterial growth inhibition models based on molecular properties of organic acids. Int. J. Food Microbiol. 2003, 86, 249–255. [Google Scholar] [CrossRef]
- Delgado, A.; Brito, D.; Peres, C.; Noe-Arroyo, F.; Garrido-Fernández, A. Bacteriocin production by Lactobacillus pentosus B96 can be expressed as a function of temperature and NaCl concentration. Food Microbiol. 2005, 22, 521–528. [Google Scholar] [CrossRef]
- Delgado, A.; López, F.N.A.; Brito, D.; Peres, C.; Fevereiro, P.; Garrido-Fernández, A. Optimum bacteriocin production by Lactobacillus plantarum 17.2b requires absence of NaCl and apparently follows a mixed metabolite kinetics. J. Biotechnol. 2007, 130, 193–201. [Google Scholar] [CrossRef]
- Hurtado, A.; Othman, N.B.; Chammem, N.; Hamdi, M.; Ferrer, S.; Reguant, C.; Bordons, A.; Rozès, N. Characterization of Lactobacillus isolates from fermented olives and their bacteriocin gene profiles. Food Microbiol. 2011, 28, 1514–1518. [Google Scholar] [CrossRef]
- López-García, E.; Benítez-Cabello, A.; Rodríguez-Gómez, F.; Romero-Gil, V.; Garrido-Fernández, A.; Jiménez-Díaz, R.; Arroyo-López, F.N. Bacterial metataxonomic analysis of industrial Spanish-style green table olive fermentations. Food Control 2022, 137, 108969. [Google Scholar] [CrossRef]
- Arroyo-López, F.N.; Benítez-Cabello, A.; Romero-Gil, V.; Rodríguez-Gómez, F.; Garrido-Fernández, A. Delving into the bacterial diversity of spoiled green Manzanilla Spanish-style table olive fermentations. Int. J. Food Microbiol. 2021, 359, 109415. [Google Scholar] [CrossRef]
- Kazou, M.; Tzamourani, A.; Panagou, E.Z.; Tsakalidou, E. Unraveling the microbiota of natural black cv. Kalamata fermented olives through 16S and ITS metataxonomic analysis. Microorganisms 2020, 8, 672. [Google Scholar] [CrossRef]
- Benítez-Cabello, A.; Calero-Delgado, B.; Rodríguez-Gómez, F.; Bautista-Gallego, J.; Garrido-Fernández, A.; Jiménez-Díaz, R.; Arroyo-López, F.N. The use of multifunctional yeast-lactobacilli starter cultures improves fermentation performance of Spanish-style green table olives. Food Microbiol. 2020, 91, 103497. [Google Scholar]
- Panagou, E.Z.; Tassou, C.C. Changes in volatile compounds and related biochemical profile during controlled fermentation of cv. Conservolea green olives. Food Microbiol. 2006, 23, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Quintana, M.D.; García, P.G.; Fernández, A.G. Establishment of conditions for green table olive fermentation at low temperature. Int. J. Food Microbiol. 1999, 51, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Rejano, N.; Cancho, G.; De la Bborbolla, Y.J.M.R. Estudios sobre el aderezo de aceitunas verdes. XXIV Nuevos ensayos sobre el control de la fermentación. Grasas Aceites 1977, 28, 255–265. [Google Scholar]
- Spyropoulou, K.E.; Nychas, G. Addition of fermentable substrates and thiamine during the fermentation of green olives with or without starter cultures. In Proceedings of the 17th International Symposium of the International Committee on Food Microbiology and Hygiene (ICFMH), Veldhoven, The Netherlands, 13–17 September 1999; Foundation Food Micro’99. pp. 685–689. [Google Scholar]
- Spyropoulou, K.E.; Chorianopoulos, N.G.; Skandamis, P.N.; Nychas, G. Survival of Escherichia coli O157: H7 during the fermentation of Spanish-style green table olives (conservolea variety) supplemented with different carbon sources. Int. J. Food Microbiol. 2001, 66, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Tassou, C.C.; Panagou, E.Z.; Katsaboxakis, K.Z. Microbiological and physicochemical changes of naturally black olives fermented at different temperatures and NaCl levels in the brines. Food Microbiol. 2002, 19, 605–615. Available online: http://www.scopus.com/inward/record.url?eid=2-s2.0-0036896938&partnerID=40&md5=330e4e9382e1bec9a1477b6927e4db18 (accessed on 25 June 2014). [CrossRef]
- International Olive Council Table Olives. Available online: https://www.internationaloliveoil.org/ (accessed on 1 September 2023).
- Bencresciuto, G.F.; Mandalà, C.; Migliori, C.A.; Cortellino, G.; Vanoli, M.; Bardi, L. Assessment of Starters of Lactic Acid Bacteria and Killer Yeasts: Selected Strains in Lab-Scale Fermentations of Table Olives (Olea europaea L.) cv. Leccino. Fermentation 2023, 9, 182. [Google Scholar] [CrossRef]
- Erten, H.; Ağirman, B.; Gündüz, C.P.B.; Çarşanba, E.; Sert, S.; Bircan, S.; Tangüler, H. Importance of yeasts and lactic acid bacteria in food processing. In Food Processing: Strategies for Quality Assessment; Springer: New York, NY, USA, 2014; pp. 351–378. [Google Scholar]
- Giavalisco, M.; Zotta, T.; Parente, E.; Siesto, G.; Capece, A.; Ricciardi, A. Effect of oil-born yeasts on the quality of extra-virgin olive oils of Basilicata region. Int. J. Food Microbiol. 2023, 386, 110041. [Google Scholar] [CrossRef]
- Marsilio, V.; Campestre, C.; Lanza, B.; De Angelis, M. Sugar and polyol compositions of some European olive fruit varieties (Olea europaea L.) suitable for table olive purposes. Food Chem. 2001, 72, 485–490. [Google Scholar] [CrossRef]
- Ramírez, E.; Brenes, M.; de Castro, A.; Romero, C.; Medina, E. Oleuropein hydrolysis by lactic acid bacteria in natural green olives. LWT 2017, 78, 165–171. [Google Scholar] [CrossRef]
- De Castro, A.; Ruiz-Barba, J.L.; Romero, C.; Sánchez, A.H.; García, P.; Brenes, M. Formation of gas pocket defect in Spanish-style green olives by the halophile Celerinatantimonas sp. Food Control 2022, 136, 108868. [Google Scholar] [CrossRef]
- Ruiz-Barba, J.L.; de Castro, A.; Romero, C.; Sánchez, A.H.; García, P.; Brenes, M. Study of the factors affecting growth of Celerinatantimonas sp. and gas pocket formation in Spanish-style green olives. Food Control 2022, 141, 109208. [Google Scholar] [CrossRef]
- IOC. Trade Standard Applying to Table Olives COI/OT/NC No. 1; IOC: Madrid, Spain, 2004. [Google Scholar]
- Alves, M.; Esteves, E.; Quintas, C. Effect of preservatives and acidifying agents on the shelf life of packed cracked green table olives from Maçanilha cultivar. Food Packag. Shelf Life 2015, 5, 32–40. [Google Scholar] [CrossRef]
- Arroyo-López, F.N.; Bautista-Gallego, J.; Segovia-Bravo, K.A.; García-García, P.; Durán-Quintana, M.C.; Romero, C.; Rodríguez-Gómez, F.; Garrido-Fernández, A. Instability profile of fresh packed “seasoned” Manzanilla-Aloreña table olives. LWT Food Sci. Technol. 2009, 42, 1629–1639. [Google Scholar] [CrossRef]
- Arroyo-López, F.N.; Querol, A.; Bautista-Gallego, J.; Garrido-Fernández, A. Role of yeasts in table olive production. Int. J. Food Microbiol. 2008, 128, 189–196. [Google Scholar] [CrossRef]
- Soleas, G.J.; Yan, J.; Goldberg, D.M. Assay of ochratoxin A in wine and beer by high-pressure liquid chromatography photodiode array and gas chromatography mass selective detection. J. Agric. Food Chem. 2001, 49, 2733–2740. [Google Scholar] [CrossRef]
- Gautier, C.; Pinson-Gadais, L.; Richard-Forget, F. Fusarium mycotoxins enniatins: An updated review of their occurrence, the producing Fusarium species, and the abiotic determinants of their accumulation in crop harvests. J. Agric. Food Chem. 2020, 68, 4788–4798. [Google Scholar] [CrossRef]
- Ji, X.; Deng, T.; Xiao, Y.; Jin, C.; Lyu, W.; Wang, W.; Tang, B.; Wu, Z.; Yang, H. Evaluation of Alternaria toxins in fruits, vegetables and their derivatives marketed in China using a QuEChERS method coupled with ultra-high performance liquid chromatography-tandem mass spectrometry: Analytical methods and occurrence. Food Control 2023, 147, 109563. [Google Scholar] [CrossRef]
- Kolawole, O.; Meneely, J.; Petchkongkaew, A.; Elliott, C. A review of mycotoxin biosynthetic pathways: Associated genes and their expressions under the influence of climatic factors. Fungal Biol. Rev. 2021, 37, 8–26. Available online: https://www.sciencedirect.com/science/article/pii/S1749461321000208 (accessed on 1 September 2023). [CrossRef]
- Mattoon, E.R.; Casadevall, A.; Cordero, R.J. Beat the heat: Correlates, compounds, and mechanisms involved in fungal thermotolerance. Fungal Biol. Rev. 2021, 36, 60–75. Available online: https://www.sciencedirect.com/science/article/pii/S1749461321000117 (accessed on 1 September 2023). [CrossRef]
- Medina-Pradas, E.; Arroyo-López, F.N. Presence of toxic microbial metabolites in table olives. Front. Microbiol. 2015, 6, 873. [Google Scholar] [CrossRef] [PubMed]
- García, P.G.; Barranco, C.R.; Durán Quintana, M.C.; Fernández, A.G. Biogenic Amine Formation and “Zapatera” Spoilage of Fermented Green Olives: Effect of Storage Temperature and Debittering Process. J. Food Prot. 2004, 67, 117–123. Available online: https://www.sciencedirect.com/science/article/pii/S0362028X22033725 (accessed on 1 September 2023). [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef]
- Rodrigues, N.; Cavaco, T.; Quintas, C. Cracked green table olive from the south of Portugal: The influence of different brining conditions. In Proceedings of the International Conference “Environmentally and Safe Technologies for Quality of Fruit and Vegetables, Faro, Portugal, 14–16 January 2010; pp. 223–229. [Google Scholar]
- Amelio, M.; De Muro, E. Naturally fermented black olives of Taggiasca variety (Olea europaea L.). Grasas Aceites 2000, 51, 429–439. [Google Scholar] [CrossRef]
- Panagou, E.Z.; Tassou, C.C.; Vamvakoula, P.; Saravanos, E.K.; Nychas, G.E. Survival of Bacillus cereus vegetative cells during Spanish-style fermentation of conservolea green olives. J. Food Prot. 2008, 71, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Tuipulotu, D.E.; Mathur, A.; Ngo, C.; Man, S.M. Bacillus cereus: Epidemiology, virulence factors, and host–pathogen interactions. Trends Microbiol. 2021, 29, 458–471. [Google Scholar] [CrossRef]
- Benítez-Cabello, A.; Romero-Gil, V.; Medina-Pradas, E.; Garrido-Fernández, A.; Arroyo-López, F.N. Exploring bacteria diversity in commercialized table olive biofilms by metataxonomic and compositional data analysis. Sci. Rep. 2020, 10, 11381. [Google Scholar] [CrossRef]
- Heperkan, D. Microbiota of table olive fermentations and criteria of selection for their use as starters. Front. Microbiol. 2013, 4, 143. [Google Scholar] [CrossRef]
- Cawthorne, A.; Celentano, L.P.; D’Ancona, F.; Bella, A.; Massari, M.; Anniballi, F.; Fenicia, L.; Aureli, P.; Salmaso, S. Botulism and preserved green olives. Emerg. Infect. Dis. 2005, 11, 781. [Google Scholar] [CrossRef]
- De Angelis, M.; Campanella, D.; Cosmai, L.; Summo, C.; Rizzello, C.G.; Caponio, F. Microbiota and metabolome of un-started and started Greek-type fermentation of Bella di Cerignola table olives. Food Microbiol. 2015, 52, 18–30. [Google Scholar] [CrossRef]
- Peck, M.W.; Stringer, S.C.; Carter, A.T. Clostridium botulinum in the post-genomic era. Food Microbiol. 2011, 28, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Lindström, M.; Heikinheimo, A.; Lahti, P.; Korkeala, H. Novel insights into the epidemiology of Clostridium perfringens type A food poisoning. Food Microbiol. 2011, 28, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Argyri, A.A.; Lyra, E.; Panagou, E.Z.; Tassou, C.C. Fate of Escherichia coli O157: H7, Salmonella Enteritidis and Listeria monocytogenes during storage of fermented green table olives in brine. Food Microbiol. 2013, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Brenes, M.; Romero, C.; Ramírez, E.; de Castro, A. Survival of foodborne pathogenic bacteria in table olive brines. Food Control 2013, 34, 719–724. [Google Scholar] [CrossRef]
- Touchon, M.; Hoede, C.; Tenaillon, O.; Barbe, V.; Baeriswyl, S.; Bidet, P.; Bingen, E.; Bonacorsi, S.; Bouchier, C.; Bouvet, O. Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet. 2009, 5, e1000344. [Google Scholar] [CrossRef]
- Solomon, E.B.; Potenski, C.J.; Matthews, K.R. Effect of irrigation method on transmission to and persistence of Escherichia coli O157: H7 on lettuce. J. Food Prot. 2002, 65, 673–676. [Google Scholar] [CrossRef]
- Natvig, E.E.; Ingham, S.C.; Ingham, B.H.; Cooperband, L.R.; Roper, T.R. Salmonella enterica serovar Typhimurium and Escherichia coli contamination of root and leaf vegetables grown in soils with incorporated bovine manure. Appl. Environ. Microbiol. 2002, 68, 2737–2744. [Google Scholar] [CrossRef]
- López-García, E.; Benítez-Cabello, A.; Ramiro-García, J.; Romero-Gil, V.; Rodríguez-Gómez, F.; Arroyo-López, F.N. New insights into microbial diversity of the traditional packed table olives Aloreña de Málaga through metataxonomic analysis. Microorganisms 2021, 9, 561. [Google Scholar] [CrossRef]
- Romero-Gil, V.; Medina, E.; Garrido-Fernández, A.; Arroyo-López, F.N. Foodborne pathogen survival in commercial Aloreña de Málaga table olive packaging. Front. Microbiol. 2018, 9, 2471. [Google Scholar] [CrossRef]
- Randazzo, C.L.; Ribbera, A.; Pitino, I.; Romeo, F.V.; Caggia, C. Diversity of bacterial population of table olives assessed by PCR-DGGE analysis. Food Microbiol. 2012, 32, 87–96. [Google Scholar] [CrossRef]
- Caggia, C.; Randazzo, C.L.; Di Salvo, M.; Romeo, F.; Giudici, P. Occurrence of Listeria monocytogenes in green table olives. J. Food Prot. 2004, 67, 2189–2194. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Wiedmann, M.; Teixeira, P.; Stasiewicz, M.J. Listeria monocytogenes persistence in food-associated environments: Epidemiology, strain characteristics, and implications for public health. J. Food Prot. 2014, 77, 150–170. [Google Scholar] [CrossRef]
- Melo, J.; Andrew, P.W.; Faleiro, M.L. Listeria monocytogenes in cheese and the dairy environment remains a food safety challenge: The role of stress responses. Food Res. Int. 2015, 67, 75–90. [Google Scholar] [CrossRef]
- Panagou, E.Z.; Tassou, C.C.; Manitsa, C.; Mallidis, C. Modelling the effect of high pressure on the inactivation kinetics of a pressure-resistant strain of Pediococcus damnosus in phosphate buffer and gilt-head seabream (Sparus aurata). J. Appl. Microbiol. 2007, 102, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Podolak, R.; Enache, E.; Stone, W.; Black, D.G.; Elliott, P.H. Sources and risk factors for contamination, survival, persistence, and heat resistance of Salmonella in low-moisture foods. J. Food Prot. 2010, 73, 1919–1936. [Google Scholar] [CrossRef]
- Nascimento, M.d.S.D.; Brum, D.M.; Pena, P.O.; Berto, M.I.; Efraim, P. Inactivation of Salmonella during cocoa roasting and chocolate conching. Int. J. Food Microbiol. 2012, 159, 225–229. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Altieri, C.; Corbo, M.R.; Sinigaglia, M.; Ouoba, L.I.I. Characterization of lactic acid bacteria isolated from Italian Bella di Cerignola table olives: Selection of potential multifunctional starter cultures. J. Food Sci. 2010, 75, M536–M544. [Google Scholar] [CrossRef]
- Pereira, A.P.; Pereira, J.A.; Bento, A.; Estevinho, M.L. Microbiological characterization of table olives commercialized in Portugal in respect to safety aspects. Food Chem. Toxicol. 2008, 46, 2895–2902. [Google Scholar] [CrossRef]
- Medina, E.; Ruiz-Bellido, M.A.; Romero-Gil, V.; Rodríguez-Gómez, F.; Montes-Borrego, M.; Landa, B.B.; Arroyo-López, F.N. Assessment of the bacterial community in directly brined Aloreña de Málaga table olive fermentations by metagenetic analysis. Int. J. Food Microbiol. 2016, 236, 47–55. [Google Scholar] [CrossRef]
- Danielsson-Tham, M. Staphylococcal food poisoning. In Food Associated Pathogens; CRC Press: Boca Raton, FL, USA, 2013; Volume 250. [Google Scholar]
- Lanza, B.; Zago, M.; Carminati, D.; Rossetti, L.; Meucci, A.; Marfisi, P.; Russi, F.; Iannucci, E.; Di Serio, M.G.; Giraffa, G. Isolation and preliminary characterization of Lactobacillus plantarum bacteriophages from table olive fermentation. Ann. Microbiol. 2012, 62, 1467–1472. [Google Scholar] [CrossRef]
- Friman, V.; Hiltunen, T.; Jalasvuori, M.; Lindstedt, C.; Laanto, E.; Örmälä, A.; Laakso, J.; Mappes, J.; Bamford, J.K. High temperature and bacteriophages can indirectly select for bacterial pathogenicity in environmental reservoirs. PLoS ONE 2011, 6, e17651. [Google Scholar] [CrossRef] [PubMed]
- Bonatsou, S.; Panagou, E.Z. Fermentation of cv. kalamata natural black olives with potential multifunctional yeast starters. Foods 2022, 11, 3106. [Google Scholar] [CrossRef]
- European Comission RASFF Window. Available online: https://webgate.ec.europa.eu/rasff-window/screen/search. (accessed on 3 October 2023).
- Bavaro, S.L.; Susca, A.; Frisvad, J.C.; Tufariello, M.; Chytiri, A.; Perrone, G.; Mita, G.; Logrieco, A.F.; Bleve, G. Isolation, characterization, and selection of molds associated to fermented black table olives. Front. Microbiol. 2017, 8, 1356. [Google Scholar] [CrossRef] [PubMed]
- Mougiou, N.; Tsoureki, A.; Didos, S.; Bouzouka, I.; Michailidou, S.; Argiriou, A. Microbial and Biochemical Profile of Different Types of Greek Table Olives. Foods 2023, 12, 1527. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Cabello, A.; Ramiro-García, J.; Romero-Gil, V.; Medina, E.; Arroyo-López, F.N. Fungal biodiversity in commercial table olive packages. Food Microbiol. 2022, 107, 104082. [Google Scholar] [CrossRef]
- Ghitakou, S.; Koutras, K.; Kanellou, E.; Markaki, P. Study of aflatoxin B1 and ochratoxin A production by natural microflora and Aspergillus parasiticus in black and green olives of Greek origin. Food Microbiol. 2006, 23, 612–621. [Google Scholar] [CrossRef]
- Franzetti, L.; Scarpellini, M.; Vecchio, A.; Planeta, D. Microbiological and safety evaluation of green table olives marketed in Italy. Ann. Microbiol. 2011, 61, 843–851. [Google Scholar] [CrossRef]
- El Adlouni, C.; Tozlovanu, M.; Naman, F.; Faid, M.; Pfohl-Leszkowicz, A. Preliminary data on the presence of mycotoxins (ochratoxin A, citrinin and aflatoxin B1) in black table olives “Greek style” of Moroccan origin. Mol. Nutr. Food Res. 2006, 50, 507–512. [Google Scholar] [CrossRef]
- Abriouel, H.; Benomar, N.; Lucas, R.; Gálvez, A. Culture-independent study of the diversity of microbial populations in brines during fermentation of naturally-fermented Aloreña green table olives. Int. J. Food Microbiol. 2011, 144, 487–496. [Google Scholar] [CrossRef]
- Benítez-Cabello, A.; Romero-Gil, V.; Medina, E.; Sanchez, B.; Calero-Delgado, B.; Bautista-Gallego, J.; Jimenez-Diaz, R.; Arroyo-López, F.N. Metataxonomic analysis of the bacterial diversity in table olive dressing components. Food Control 2019, 105, 190–197. [Google Scholar] [CrossRef]
- Tribelli, P.M.; López, N.I. Insights into the temperature responses of Pseudomonas species in beneficial and pathogenic host interactions. Appl. Microbiol. Biotechnol. 2022, 106, 7699–7709. [Google Scholar] [CrossRef] [PubMed]
- Raiger Iustman, L.J.; Tribelli, P.M.; Ibarra, J.G.; Catone, M.V.; Solar Venero, E.C.; López, N.I. Genome sequence analysis of Pseudomonas extremaustralis provides new insights into environmental adaptability and extreme conditions resistance. Extremophiles 2015, 19, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Ormsby, M.J.; Akinbobola, A.; Quilliam, R.S. Plastic pollution and fungal, protozoan, and helminth pathogens—A neglected environmental and public health issue? Sci. Total Environ. 2023, 882, 163093. Available online: https://www.sciencedirect.com/science/article/pii/S0048969723017126 (accessed on 3 October 2023). [CrossRef]
- Li, C.; Wang, L.; Ji, S.; Chang, M.; Wang, L.; Gan, Y.; Liu, J. The ecology of the plastisphere: Microbial composition, function, assembly, and network in the freshwater and seawater ecosystems. Water Res. 2021, 202, 117428. Available online: https://www.sciencedirect.com/science/article/pii/S0043135421006266 (accessed on 3 October 2023). [CrossRef] [PubMed]
- Wang, R.; Li, Q.; He, S.; Liu, Y.; Wang, M.; Jiang, G. Modeling and mapping the current and future distribution of Pseudomonas syringae pv. actinidiae under climate change in China. PLoS ONE 2018, 13, e0192153. [Google Scholar] [CrossRef]
- Calero Preciado, C.; Boxall, J.; Soria-Carrasco, V.; Martínez, S.; Douterelo, I. Implications of climate change: How does increased water temperature influence biofilm and water quality of chlorinated drinking water distribution systems? Front. Microbiol. 2021, 12, 658927. [Google Scholar] [CrossRef]
- Junta de Andalucía Impacto del Cambio Climático Sobre la Agricultura Andaluza: Olivar. Available online: https://www.juntadeandalucia.es/export/drupaljda/CC_Olivar_Divulgacion_v8.pdf (accessed on 1 September 2023).
- Cano-Ortiz, A.; Fuentes, J.C.P.; Gea, F.L.; Ighbareyeh, J.M.H.; Quinto Canas, R.J.; Meireles, C.I.R.; Raposo, M.; Gomes, C.J.P.; Spampinato, G.; del Río González, S. Climatology, bioclimatology and vegetation cover: Tools to mitigate climate change in olive groves. Agronomy 2022, 12, 2707. [Google Scholar] [CrossRef]
- Xylella, E. EPPO/OEPP, 2004. Diagnostic protocol. Xylella fastidiosa. Bull. OEPP/EPPO Bull. 2004, 34, 187–192. [Google Scholar]
- Oleo Estudio de Contaminación Cruzada de Productos Fitosanitarios que Afectan al Olivar Ecológico de La Rioja. Available online: https://www.oleorevista.com/texto-diario/mostrar/3654881/estudio-contaminacion-cruzada-productos-fitosanitarios-afectan-olivar-ecologico-rioja (accessed on 1 September 2023).
- Resco, P. Empieza la Cuenta Atrás. Impactos del Cambio Climático en la Agricultura Española; Coordinadora de Organizaciones de Agricultores y Ganaderos (COAG): Madrid, Spain, 2022. [Google Scholar]
- FinancialTimes Record olive oil prices keep climbing after Spanish drought. 2023. Available online: https://www.ft.com/content/5f0b2e0b-8100-42d0-9a87-fef4a57addeb (accessed on 3 October 2023).
- Wheeler, T.; Von Braun, J. Climate change impacts on global food security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef]
- Cabezas, J.M.; Ruiz-Ramos, M.; Soriano, M.A.; Gabaldón-Leal, C.; Santos, C.; Lorite, I.J. Identifying adaptation strategies to climate change for Mediterranean olive orchards using impact response surfaces. Agric. Syst. 2020, 185, 102937. [Google Scholar] [CrossRef]
- Zaied, Y.B.; Zouabi, O. Impacts of climate change on Tunisian olive oil output. Clim. Change 2016, 139, 535–549. [Google Scholar] [CrossRef]
- Arenas-Castro, S.; Gonçalves, J.F.; Moreno, M.; Villar, R. Projected climate changes are expected to decrease the suitability and production of olive varieties in southern Spain. Sci. Total Environ. 2020, 709, 136161. [Google Scholar] [CrossRef] [PubMed]
- Vaccalluzzo, A.; Celano, G.; Pino, A.; Calabrese, F.M.; Foti, P.; Caggia, C.; Randazzo, C. Metagenetic and volatilomic approaches to elucidate the effect of Lactiplantibacillus plantarum starter cultures on Sicilian table olives. Front. Microbiol. 2022, 12, 771636. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, F.; Romero-Gil, V.; Bautista-Gallego, J.; García-García, P.; Garrido-Fernández, A.; Arroyo-López, F.N. Production of potential probiotic Spanish-style green table olives at pilot plant scale using multifunctional starters. Food Microbiol. 2014, 44, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Bonatsou, S.; Benítez, A.; Rodríguez-Gómez, F.; Panagou, E.Z.; Arroyo-López, F.N. Selection of yeasts with multifunctional features for application as starters in natural black table olive processing. Food Microbiol. 2015, 46, 66–73. [Google Scholar] [CrossRef]
- Bonatsou, S.; Tassou, C.C.; Panagou, E.Z.; Nychas, G.E. Table olive fermentation using starter cultures with multifunctional potential. Microorganisms 2017, 5, 30. [Google Scholar] [CrossRef]
- Simões, D.; Carbas, B.; Soares, A.; Freitas, A.; Silva, A.S.; Brites, C.; Andrade, E.d. Assessment of Agricultural Practices for Controlling Fusarium and Mycotoxins Contamination on Maize Grains: Exploratory Study in Maize Farms. Toxins 2023, 15, 136. [Google Scholar] [CrossRef]
- Piga, A.; Agabbio, M.C.S. Quality improvement of naturally green table olives by controlling some processing parameters. Ital. J. Food Sci. 2003, 15, 259–268. [Google Scholar]
- Ünal, K.; Nergiz, C. The effect of table olive preparing methods and storage on the composition and nutritive value of olives. Grasas Aceites 2003, 54, 71–76. [Google Scholar] [CrossRef]
- Perricone, M.; Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Use of Lactobacillus plantarum and glucose to control the fermentation of “Bella di Cerignola” table olives, a traditional variety of Apulian region (Southern Italy). J. Food Sci. 2010, 75, M430–M436. [Google Scholar] [CrossRef]
- Khayyal, M.T.; El-Ghazaly, M.A.; Abdallah, D.M.; Nassar, N.N.; Okpanyi, S.N.; Kreuter, M. Blood pressure lowering effect of an olive leaf extract (Olea europaed) in L-NAME induced hypertension in rats. Arzneimittelforschung 2002, 52, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Miro-Casas, E.; Covas, M.; Farre, M.; Fito, M.; Ortuno, J.; Weinbrenner, T.; Roset, P.; De La Torre, R. Hydroxytyrosol disposition in humans. Clin. Chem. 2003, 49, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Covas, M. Olive oil and the cardiovascular system. Pharmacol. Res. 2007, 55, 175–186. [Google Scholar] [CrossRef] [PubMed]
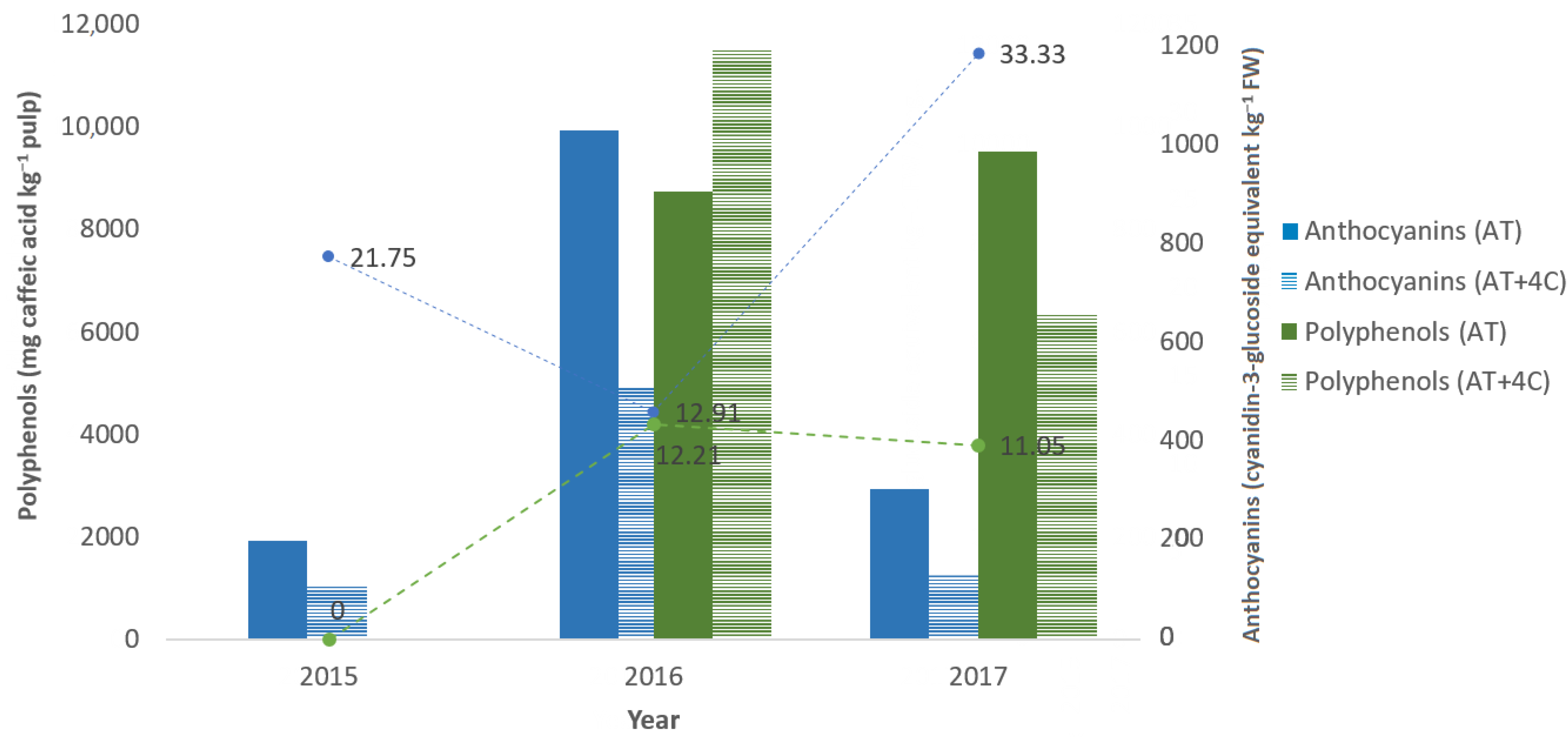
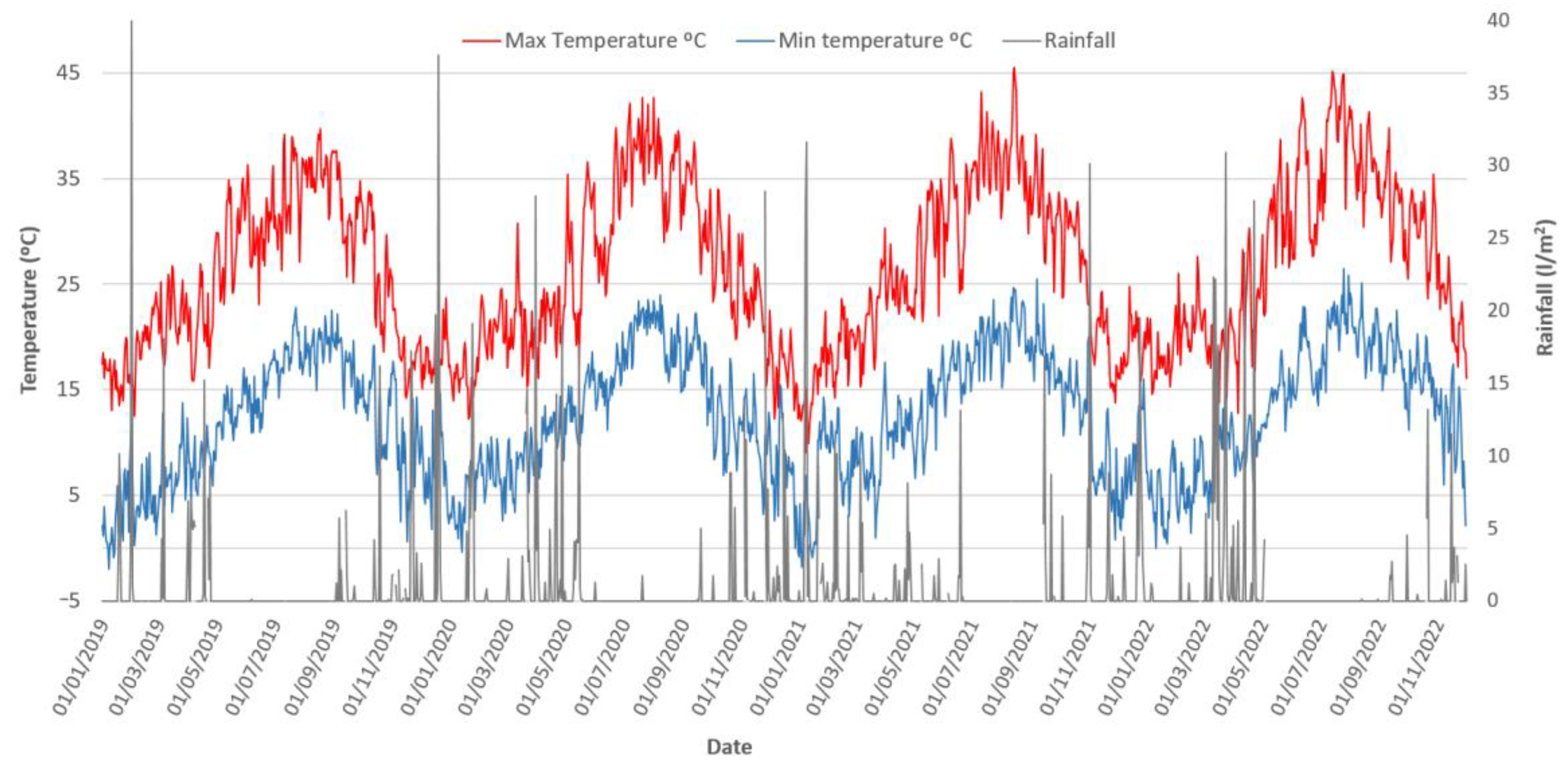
| Year | Temperature (°C) | Average Rainfall (L/m2/Year) | Fermentation Control Parameters | |||
|---|---|---|---|---|---|---|
| Maximum | Minimum | Average | Rainfall | pH | Free Acidity (%) | |
| 2019 | 25.40 ± 7.14 | 11.30 ± 5.87 | 18.35 ± 9.61 | 0.93 ± 3.99 | 4.16 ± 0.19 | 0.90 ± 0.25 |
| 2020 | 25.97 ± 7.76 | 12.36 ± 5.66 | 19.16 ± 9.61 | 1.04 ± 3.52 | 4.25 ± 0.07 | 1.01 ± 0.05 |
| 2021 | 26.33 ± 7.86 | 12.69 ± 5.89 | 19.51 ± 9.73 | 1.03 ± 3.64 | 4.44 ± 0.21 | 0.85 ± 0.15 |
| 2022 | 27.96 ± 8.01 | 13.46 ± 5.94 | 20.71 ± 10.12 | 0.90 ± 3.61 | 4.36 ± 0.08 | 0.76 ± 0.04 |
| Microbial Groups of Interest | Occurrence or Survival in Table Olives | Relevant Characteristics That Increase Microbial Risks in the Scenario of Climate Issues |
|---|---|---|
| Bacillus cereus (Main source/origin: soil, dust, vegetation) | Survival in Spanish-style Conservolea olives [84]. | Heterogeneous species comprising psychrotrophic and mesophilic strains (which grow at 37 °C) that can survive at temperatures below 10 °C. Producer of endospores and biofilms. Spores survive in severe conditions (heating, freezing, drying, and ultraviolet) that generally destroy vegetative cells [85]. |
| Campylobacter Main source/origin: soil, dust, vegetation | Detected in fruit biofilms from commercialized table olive packaging by metataxonomic analysis [86]. | Considered thermophilic, Campylobacter grows better at high temperatures. It can grow and multiply more rapidly in temperatures between 20 °C and 45 °C. Prolonged storage of unpasteurized foods at inappropriate temperatures, such as within the mentioned range of temperate temperatures, can allow the growth of Campylobacter in the food. |
| Clostridium sp. (C. botulinum, C. perfringens) Main source/origin: soil, animals, dust, air, water | Part of fruit microbiota at the beginning of fermentation [87]. Associated with table olives and recalls of the suspected products have been reported [88]. Sulphite-reducing Clostridium spores were detected in numerous samples of commercialized table olives [88]. Clostridium (species not specified) were found in the fermentation of Bella di Cerignola table olives [89]. | C. botulinum: Physiologically heterogeneous species. Producer of endospores and neurotoxins. Proteolytic strains (PS): Optimum growth temperature 37 °C-42 °C. Minimum growth pH: 4.6 Non-proteolytic strains (NPS): Optimum growth temperature 25 °C. Minimum growth pH: 5 NaCl concentration preventing growth: PS: 10%; NPS 5% PS: Inadequate heat treatment will permit the survival of spores that will germinate/grow and produce neurotoxin during ambient storage. NPS: time and/or temperature abuse of commercial or home-made refrigerated foods [90]. C. perfringens: Producer of endospores and endotoxins. Optimal conditions for food poisonings arise when food contaminated with spores is slowly chilled or held in a temperature range of 10–54 °C, allowing germination and rapid growth of C. perfringens. Upon ingestion of large numbers of vegetative C. perfringens cells, they sporulate in the intestinal lumen and produce endotoxins [91]. |
| Pathogenic Enterobacteria, e.g., Escherichia coli O157:O7 Main source/origin: water, animal stools. Fecal matter can contaminate food and water, including irrigation water and recreational water. | Survival in table olives of Halkidiki variety [92]. Survival in table olives of different varieties [93]. Survival in Spanish-style Conservolea olives [60]. | E. coli grow from 4 °C to 50 °C, with an optimum at 37 °C. The pathogenicity of E. coli has been explained by the acquisition of a series of virulence genes [94]. The ability of E. coli strains to survive and grow in environments other than the gastrointestinal tract poses a serious public health problem. E. coli was isolated from soil, manure, and irrigation water, and demonstrated the ability to colonize the internal compartments of plants [95] and plant roots [96]. Vegetal material can contribute to the dissemination of bacteria in industrial food processing and packaging environments, contributing to their dissemination in the food chain if good manufacturing practices are not respected. E. coli O157:H7 has the ability to survive and multiply outside the intestine. In table olives, the elimination of pathogenic E. coli is facilitated by a synergy of factors (lactate, phenolics, aW, bacteriocins) related to the overgrowth of preselected starter cultures [97,98]. |
| Listeria monocytogenes Main source/origin: soil, animals, insects, compost, decaying vegetation, processing environments. Persist in mammalian and avian feces | Has been found in marketed table olives in Europe, such as Spanish-style process green Nocellara dell’Etna olives [99,100]. Associated with fermented olives with and without starter cultures from different Greek, Italian, and Spanish varieties and fermentation styles [92,99,100]. Survival in table olives of different varieties [94,95,100]. | Psychrotrophic growing at temperatures less than 1 °C to 37 °C. Tolerates NaCl concentration up to 10%, pH < 4, as well as phenolic compounds to a certain extent. Producer of biofilms. Data from Caggia (2004) [100] suggest that L. monocytogenes grow in fermenting brines and can be more probable when the pH is higher (>4.5) and total phenolic and LAB counts are low. Survival under stressful conditions for long periods (+7 months) was reported by several authors. The ability to survive and grow in multiple habitats is supported by a competent system of adaptation to various stresses [101,102]. The ability to grow at refrigerated temperatures makes this bacteria an actual problem for the food industry and consumers during long storage, even at refrigeration temperatures. |
| Salmonella sp. Main source/origin: animal stools, human carriers | Survival in black table olive varieties [103]; survival in table olives of different varieties [92,93]. | Salmonella growth ranges from 5 °C to 47 °C, with an optimum at 37 °C. Numerous studies have demonstrated the survival and growth of Salmonella spp. in foods of vegetal origin. Salmonella sp. survives well on low aW foods, such as spices and aromatic herbs, which may eventually be used to season table olives. High lipid concentrations seem to have a protective effect on Salmonella in low aW conditions [104]. Nascimento et al. [105] warned that high levels of lipids protect Salmonella from stomach acidity. Temperature abuses of food products containing Salmonella sp. as a result of contamination or cross-contamination may promote its proliferation, especially during storage at inadequate temperatures. |
| Enterobacter cloacae | Isolated from Italian table olives “Bella di Cerignola” [106]. | High temperatures, particularly above 30 °C, favored the proliferation of Enterobacter cloacae. |
| Staphylococcus sp. and coagulase positive (S. aureus) Main source/origin: human skin, nasal passages, injuries, environment, surfaces | Part of fruit microbiota at the beginning of fermentation. Enumerated in table olive samples from Portuguese open-air markets [107] and Aloreña de Málaga [108]. Detected in the microbial biofilm that covers the fruit, in marketed samples from different varieties and geographical origins of commercialized table olives [86]. Survival in table olives of different varieties [93]. Survival in commercial Aloreña de Málaga table olives [98]. | S. aureus is one of the most resistant non-spore-forming human pathogens, and can survive for long periods in stressful conditions. Staphylococci are mesophilic. S. aureus growth ranges from 7 °C to 47.8 °C, with an optimum at 35 °C. The growth pH range is between 4.5 and 9.3 (optimum 7.0–7.5). Staphylococci are able to grow at low levels of aW (0.83). Strains of S. aureus are highly tolerant to salts and sugars. Some strains are resistant to multiple antibiotics and may produce (and attach to) biofilms. Temperature abuses of food products containing S. aureus may be responsible for its growth and subsequent production of enterotoxin which can be involved in staphylococcal food poisoning [109]. |
| Vibrio sp. Main source/origin: contaminated waters, salt | Part of microbiota at the beginning of fermentation. Detected in the microbial biofilm that covers the fruit, in marketed samples from different varieties and geographical origins [86]. | Vibrio growth ranges from 4 °C to 40 °C, with an optimal of 20–30 °C. Growth occurs at NaCl concentrations of 0–10%, with minimum requirements between 1 and 3.5% (halophilic species). |
| Bacteriophages Main source/origin: water, air, vegetables | Viruses that infect strains of L. plantarum species from table olive fermentation were isolated from natural green olives [110]. | High temperatures and bacteriophages can indirectly select pathogenic bacteria in environmental reservoirs [111]. |
| Microbial Groups of Interest | Occurrence or Survival in Table Olives | Relevant Characteristics That Increase Microbial Risks in the Scenario of Climate Issues |
|---|---|---|
| Celerinatantimonas sp. Main source/origin: salt, salty waters | Associated with the gas pocket formation and quality of Spanish-style green olives [69]. Detected in Aloreña de Málaga table olives [97,108]. | Growth ranges from 17 to 49 °C, with optimal at 31 °C, and occurs at NaCl concentrations of 2.5–8.0%, with optimal growth at 7.0–7.5% (halophilic species) [69]. |
| Enterobacteriaceae E. coli (non-pathogenic strains) Main source/origin: water, animal stools | Part of fruit microbiota at the beginning of fermentation. Detected at the beginning of fermentation [71,112]. Coliforms and E. coli were enumerated in a few samples of commercialized table olives [107]. | Facultative anaerobic group of microorganisms. Enterobacteriaceae are a good indicator of compliance with good hygiene practices. Its presence in fermented/processed olives means that the fermentation occurred inadequately or that contamination occurred after processing. They are also indicators of adequate washing and sanitization in products of vegetable origin and ready-to-eat foods, such as table olives. High numbers of Enterobacteriaceae represent a risk of deterioration due to the production of off-flavors and gas pocket spoilage on the olives’ surface. Some members of this group may contribute to biogenic amine formation [13]. Their survival decreases when starters are used [112]. Some strains may acquire pathogenic genes (pathogenic E. coli) |
| Fungi (e.g., mycotoxins producers Aspergillus sp., Penicillium sp., Alternaria) Main source/origin: soil, dust, air, water | Part of fruit microbiota at the beginning of fermentation. Filamentous fungi are found in bulk and packed table olives. They are easily detectable at the surface and/or by the sensorial changes they cause. Its presence can result in product recalls even if the risk ends up being low [113]. Penicillium sp. in black table olives [114]. Alternaria sp. and Penicillium sp. in Greek cultivars [115]. Penicillium sp. in packed table olives Aloreña de Málaga [97]. Alternaria sp. in brined olives from different countries [116]. Mycotoxins: Aflatoxin B1 was reported in black and green olives of Greek origin [117]; Aflatoxin B1 and ochratoxin A were detected in green Italian table olives [118]. Ochratoxin A, citrinin and aflatoxin B1 were detected in natural black table olives of Moroccan origin [119]. | Physiologically heterogeneous group globally and ubiquitously distributed, isolated from various habitats. They prefer acidic media (able to grow in pH 2.0) and aerobic conditions, but can also grow in the absence of oxygen. As mesophilic organisms (10–40 °C), however, there are thermotolerant species capable of growing at 50 °C. Climate change is contributing to modifying the geographical distribution of fungi, providing new biotic interactions, food contamination pathways, and production of mycotoxins (neurotoxic and carcinogenic) and infections. |
| Pseudomonas sp. Main source/origin: ubiquitous, including soil and water. | Detected at different fermentation times in Aloreña de Málaga table olives [108,120] and other varieties [86]. Detected in table olive dressing and seasoning material [121]. Detected in commercialized table olive packages [86]. | Pseudomonas–host interaction could be affected by climate change [122]. Under laboratory conditions, most Pseudomonas species grow at an optimal range from 25 to 30 °C. However, some strains can develop in both warm and cold environments, at temperatures between 0 and 45 °C, depending on the species [123]. |
| Other Emergent risks Main source/origin: microorganisms that would adapt to climate changes as eukaryotes, prokaryotes, and viruses in biofilms attached to plastic debris/microplastics (MP) impacting ecosystems | [124,125] | Plastic debris is becoming ubiquitous, and certain species are more likely to integrate biofilms in this so-called “plastisphere”. These include eukaryotic (protozoan and helminth) pathogens that may be associated with bacteria, in which there seems to be a tendency for the predominance of human pathogens. The plastisphere can constitute a new niche with a particular microbial ecology that may cause systemic changes [125]. |
| Preventive/Mitigation Measures | Operations/Actions |
|---|---|
| Raw materials | Hygienic quality of olives and their microenvironment |
| Hygienic quality seasonings (herbs and spices, among others) | |
| Hygienic quality of salt | |
| Use of good quality water | Debittering |
| Washing | |
| Brining | |
| Preparation of authorized additives and technological auxiliaries | |
| Disinfection | |
| Fermentation | Select adequate starter mixtures (including LAB and yeasts) to better control fermentation |
| Monitor fermentation process: pH, acidity, salt, and microbial hygiene parameters | |
| Apply corrective measures when needed (brine changes, acidification, salt increase, etc.) | |
| Avoid temperature abuse in the supply chain | Storing |
| Transport | |
| Distribution | |
| Commercialization | |
| In restaurants, hotels, and at home | |
| Selection of hygienic-designed equipment and infrastructures | Processing plants with a hygienic architecture, |
| Choose hygiene-designed equipment (fermenters, contains, and conveyor belts) | |
| Fermenters and containers without corners, joints, or right angles | |
| Filters/incoming air | |
| Selection of cleaning and disinfection plans with the appropriate frequency | Avoid microbial biofilms and limit aerosols development in the industries, equipment, and working surfaces |
| Adoption of good manufacturing procedures | Prevent contamination and cross-contamination |
| Stimulate the adoption of strict personal hygiene | |
| Provide education for food handlers | |
| Implement or adapt HACCP principles | |
| Review/adaptation of HACCP principles | Verify critical points |
| Review/adapt critical limits | |
| Check conformity regularly by measuring adequate parameters (pH, microbial, and physicochemical limits) | |
| Implement corrective measures when a specific critical control point is uncontrolled | |
| Regular verification | |
| Maintain updated reports | |
| Fermentative or beneficial microorganisms capable of | Fermentation abilities that produce acids, contribute to the lowering of pH and produce flavor compounds |
| Producing antimicrobial compounds (bacteriocins) | |
| Establishing new biotic interactions | |
| Internalizing the mesocarp/pulp of the table olives | |
| Improving the nutritional properties of table olives, notably their vitamin content | |
| Contributing to the decrease in occurrence of toxins | |
| Giving origin to probiotic and postbiotic in the final products |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benítez-Cabello, A.; Delgado, A.M.; Quintas, C. Main Challenges Expected from the Impact of Climate Change on Microbial Biodiversity of Table Olives: Current Status and Trends. Foods 2023, 12, 3712. https://doi.org/10.3390/foods12193712
Benítez-Cabello A, Delgado AM, Quintas C. Main Challenges Expected from the Impact of Climate Change on Microbial Biodiversity of Table Olives: Current Status and Trends. Foods. 2023; 12(19):3712. https://doi.org/10.3390/foods12193712
Chicago/Turabian StyleBenítez-Cabello, Antonio, Amélia M. Delgado, and Célia Quintas. 2023. "Main Challenges Expected from the Impact of Climate Change on Microbial Biodiversity of Table Olives: Current Status and Trends" Foods 12, no. 19: 3712. https://doi.org/10.3390/foods12193712
APA StyleBenítez-Cabello, A., Delgado, A. M., & Quintas, C. (2023). Main Challenges Expected from the Impact of Climate Change on Microbial Biodiversity of Table Olives: Current Status and Trends. Foods, 12(19), 3712. https://doi.org/10.3390/foods12193712








