Effect of Encapsulation Techniques on Aroma Retention of Pistacia terebinthus L. Fruit Oil: Spray Drying, Spray Freeze Drying, and Freeze Drying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Methods
2.2.1. Oil Extraction
2.2.2. Determination of Oil Characteristics
2.2.3. Preparation of Emulsion
2.2.4. Emulsion Characteristic Analysis
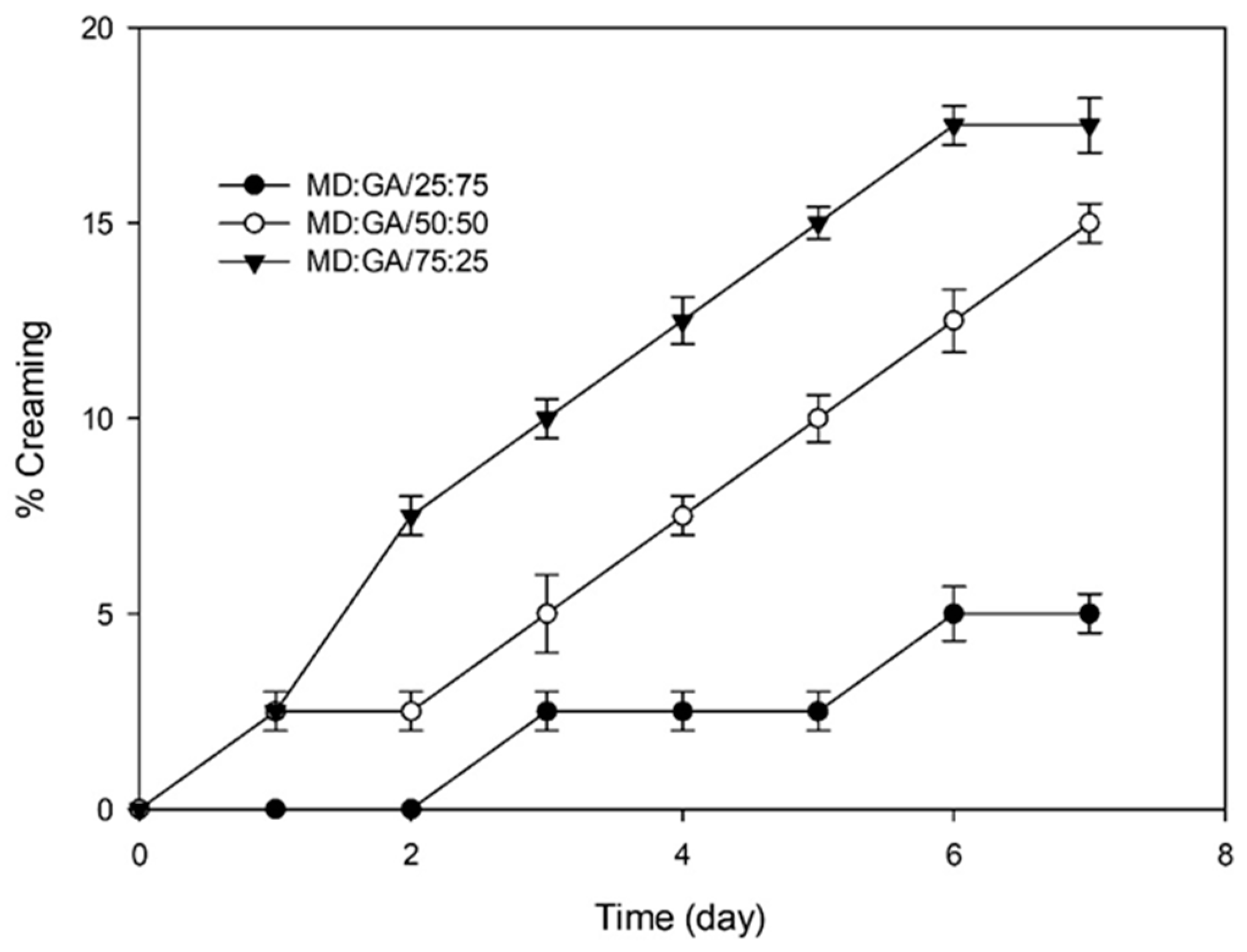
Creaming Stability
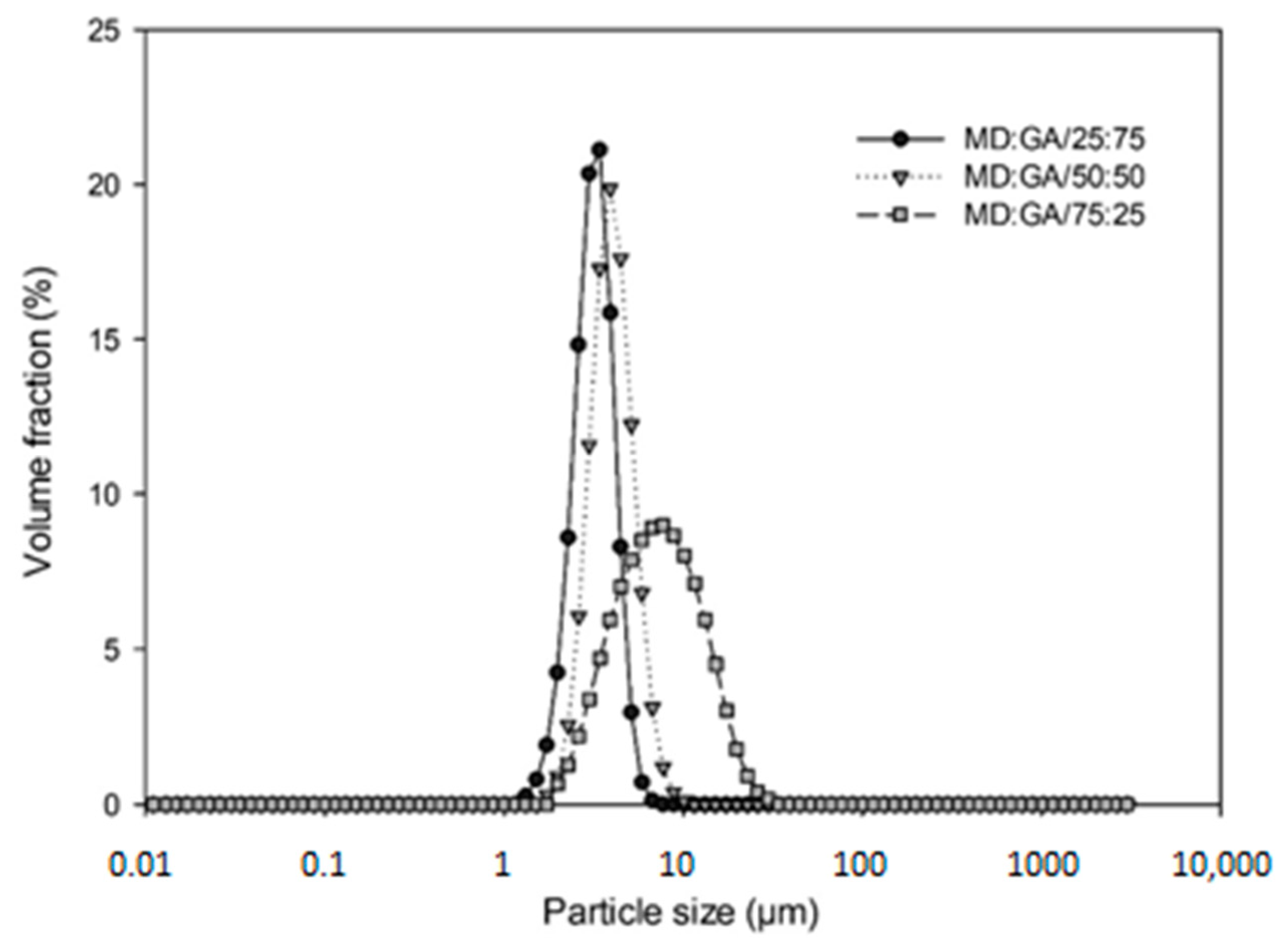
Droplet Size Measurement
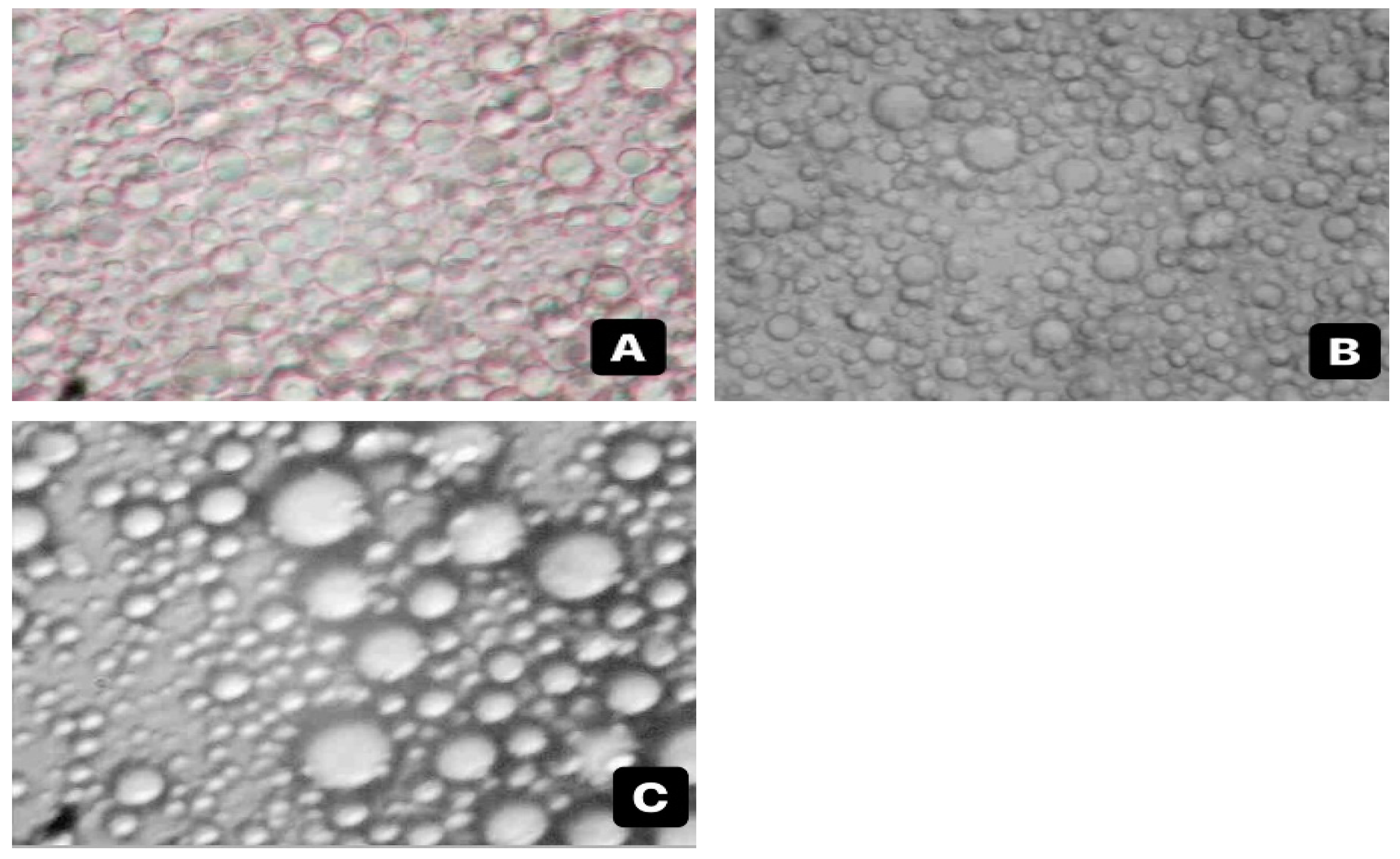
Microstructure Observation
2.2.5. Encapsulation Methods
Spray Drying
Spray Freeze-Drying
Freeze-Drying
2.2.6. Encapsulation Efficiency and Drying Yield
2.2.7. Determination of Bulk and Tapped Density and Flowability Characteristics
2.2.8. Moisture Content
2.2.9. Analysis of Aroma Compounds
Headspace Solid-Phase Microextraction (HS-SPME) for Oil
Headspace Solid-Phase Microextraction (HS-SPME) for Powder
GC–MS Analysis of Aroma Compounds
2.3. Statistical Analysis
3. Results and Discussion
3.1. Pistacia terebinth L. Fruit Oil Quality Parameters and Aroma Composition
3.2. Comparison of Emulsion Characteristics
3.3. Comparison of Encapsulation Methods in Terms of Efficiency, Yield and Powder Characteristics
3.4. Comparison of Retention of the Aroma Compounds during the Different Encapsulation Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Özcan, M. Characteristics of fruit and oil of terebinth (Pistacia terebinthus L) growing wild in Turkey. J. Sci. Food Agric. 2004, 84, 517–520. [Google Scholar] [CrossRef]
- Piras, A.; Marzouki, H.; Maxia, A.; Marengo, A.; Porcedda, S.; Falconieri, D.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L. Chemical characterisation and biological activity of leaf essential oils obtained from Pistacia terebinthus growing wild in Tunisia and Sardinia Island. Nat. Prod. Res. 2017, 31, 2684–2689. [Google Scholar] [CrossRef]
- Aydın, C.; Özcan, M. Some physico-mechanic properties of terebinth (Pistacia terebinthus L.) fruits. J. Food Eng. 2002, 53, 97–101. [Google Scholar] [CrossRef]
- Topçu, G.; Ay, M.; Bilici, A.; Sarıkürkcü, C.; Öztürk, M.; Ulubelen, A. A new flavone from antioxidant extracts of Pistacia terebinthus. Food Chem. 2007, 103, 816–822. [Google Scholar] [CrossRef]
- Koçak, D.; Keskin, H.; Fadıloğlu, S.; Kowalski, B.; Göğüş, F. Characterization of terebinth fruit oil and optimization of acidolysis reaction with caprylic and stearic acids. J. Am. Oil Chem. Soc. 2011, 88, 1531–1538. [Google Scholar] [CrossRef]
- Gogus, F.; Ozel, M.; Kocak, D.; Hamilton, J.; Lewis, A. Analysis of roasted and unroasted Pistacia terebinthus volatiles using direct thermal desorption-GCxGC–TOF/MS. Food Chem. 2011, 129, 1258–1264. [Google Scholar] [CrossRef]
- Amanpour, A.; Guclu, G.; Kelebek, H.; Selli, S. Characterization of key aroma compounds in fresh and roasted terebinth fruits using aroma extract dilution analysis and GC–MS-Olfactometry. Microchem. J. 2019, 145, 96–104. [Google Scholar] [CrossRef]
- Küsmenoglu, S.; Baser, K.; Özek, T. Constituents of the Essential Oil from the Hulls of Pistacia vera L. J. Essent. Oil Res. 1995, 7, 441–442. [Google Scholar] [CrossRef]
- Penci, M.C.; Martinez, M.L.; Fabani, M.P.; Feresin, G.E.; Tapia, A.; Ighani, M.; Ribotta, P.D.; Wunderlin, D.A. Matching changes in sensory evaluation with physical and chemical parameters: A case study: Argentinean pistachio nuts (Pistachia vera L. cv Kerman). Food Bioprocess Technol. 2013, 6, 3305–3316. [Google Scholar] [CrossRef]
- Sonmezdag, A.S.; Kelebek, H.; Selli, S. Characterization and comparative evaluation of volatile, phenolic and antioxidant properties of pistachio (Pistacia vera L.) hull. J. Essent. Oil Res. 2017, 29, 262–270. [Google Scholar] [CrossRef]
- Bahena-Garrido, S.M.; Ohama, T.; Suehiro, Y.; Hata, Y.; Isogai, A.; Iwashita, K.; Goto-Yamamoto, N.; Koyama, K. The potential aroma and flavor compounds in Vitis sp. cv. Koshu and V. vinifera L. cv. Chardonnay under different environmental conditions. J. Sci. Food Agric. 2019, 99, 1926–1937. [Google Scholar] [CrossRef] [PubMed]
- de Lacy Costello, B.; Wieczorek, M.N.; Drabinska, N. The use of volatile compounds analysis for the assessment of food and beverage quality. Front. Nutr. 2023, 10, 1250634. [Google Scholar] [CrossRef] [PubMed]
- Anandaraman, S.; Reineccius, G. Stability of encapsulated orange peel oil. Food Technol. 1986, 40, 88–93. [Google Scholar]
- Anandharamakrishnan, C.; Rielly, C.; Stapley, A. Effects of process variables on the denaturation of whey proteins during spray drying. Dry. Technol. 2007, 25, 799–807. [Google Scholar] [CrossRef]
- Can Karaca, A.; Low, N.; Nickerson, M. Encapsulation of flaxseed oil using a benchtop spray dryer for legume protein–maltodextrin microcapsule preparation. J. Agric. Food Chem. 2013, 61, 5148–5155. [Google Scholar] [CrossRef]
- Elik, A.; Yanık, D.K.; Göğüş, F. A comparative study of encapsulation of carotenoid enriched-flaxseed oil and flaxseed oil by spray freeze-drying and spray drying techniques. LWT 2021, 143, 111153. [Google Scholar] [CrossRef]
- Finney, J.; Buffo, R.; Reineccius, G. Effects of type of atomization and processing temperatures on the physical properties and stability of spray-dried flavors. J. Food Sci. 2002, 67, 1108–1114. [Google Scholar] [CrossRef]
- Pellicer, J.A.; Fortea, M.I.; Trabal, J.; Rodríguez-López, M.I.; Gabaldón, J.A.; Núñez-Delicado, E. Stability of microencapsulated strawberry flavour by spray drying, freeze drying and fluid bed. Powder Technol. 2019, 347, 179–185. [Google Scholar] [CrossRef]
- Ishwarya, S.P.; Anandharamakrishnan, C.; Stapley, A.G. Spray-freeze-drying: A novel process for the drying of foods and bioproducts. Trends Food Sci. Technol. 2015, 41, 161–181. [Google Scholar] [CrossRef]
- Turan, F.T.; Cengiz, A.; Kahyaoglu, T. Evaluation of ultrasonic nozzle with spray-drying as a novel method for the microencapsulation of blueberry’s bioactive compounds. Innov. Food Sci. Emerg. Technol. 2015, 32, 136–145. [Google Scholar] [CrossRef]
- Cheow, W.S.; Ng, M.L.L.; Kho, K.; Hadinoto, K. Spray-freeze-drying production of thermally sensitive polymeric nanoparticle aggregates for inhaled drug delivery: Effect of freeze-drying adjuvants. Int. J. Pharm. 2011, 404, 289–300. [Google Scholar] [CrossRef]
- Karthik, P.; Anandharamakrishnan, C. Microencapsulation of docosahexaenoic acid by spray-freeze-drying method and comparison of its stability with spray-drying and freeze-drying methods. Food Bioprocess Technol. 2013, 6, 2780–2790. [Google Scholar] [CrossRef]
- Wanning, S.; Süverkrüp, R.; Lamprecht, A. Pharmaceutical spray freeze drying. Int. J. Pharm. 2015, 488, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Mohri, K.; Okuda, T.; Mori, A.; Danjo, K.; Okamoto, H. Optimized pulmonary gene transfection in mice by spray–freeze dried powder inhalation. J. Control. Release 2010, 144, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Fernandes, L.; Oliveira, W.; Sztatisz, J.; Novák, C. Thermal properties and release of Lippia sidoides essential oil from gum arabic/maltodextrin microparticles. J. Therm. Anal. Calorim. 2008, 94, 461–467. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation efficiency of food flavours and oils during spray drying. Dry. Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Apintanapong, M.; Noomhorm, A. The use of spray drying to microencapsulate 2-acetyl-1-pyrroline, a major flavour component of aromatic rice. Int. J. Food Sci. Technol. 2003, 38, 95–102. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, Y.-B.; Hong, J.-H.; Chung, J.-H.; Kim, S.-S.; Lee, W.-J.; Yoon, J. Optimization of pine flavor microencapsulation by spray drying. Food Sci. Biotechnol. 2005, 14, 747–751. [Google Scholar]
- Prince, M.; Thangavel, K.; Meda, V.; Visvanathan, R.; Ananthakrishnan, D. Effect of carrier blend proportion and flavor load on physical characteristics of nutmeg (Myristica frangrans Houtt.) oleoresin microencapsulated by spray drying. Int. Food Res. J. 2014, 21, 2039. [Google Scholar]
- Felix, P.H.C.; Birchal, V.S.; Botrel, D.A.; Marques, G.R.; Borges, S.V. Physicochemical and thermal stability of microcapsules of cinnamon essential oil by spray drying. J. Food Process. Preserv. 2017, 41, e12919. [Google Scholar] [CrossRef]
- Alves, S.F.; Borges, L.L.; dos Santos, T.O.; de Paula, J.R.; Conceição, E.C.; Bara, M.T. Microencapsulation of essential oil from fruits of Pterodon emarginatus using gum arabic and maltodextrin as wall materials: Composition and stability. Dry. Technol. 2014, 32, 96–105. [Google Scholar] [CrossRef]
- Yoshii, H.; Soottitantawat, A.; Liu, X.-D.; Atarashi, T.; Furuta, T.; Aishima, S.; Ohgawara, M.; Linko, P. Flavor release from spray-dried maltodextrin/gum arabic or soy matrices as a function of storage relative humidity. Innov. Food Sci. Emerg. Technol. 2001, 2, 55–61. [Google Scholar] [CrossRef]
- Uekane, T.M.; Costa, A.C.P.; Pierucci, A.P.T.; da Rocha-Leão, M.H.M.; Rezende, C.M. Sulfur aroma compounds in gum Arabic/maltodextrin microparticles. LWT 2016, 70, 342–348. [Google Scholar] [CrossRef]
- Poyrazoglu, E.S.; Ozat, E.T.; Coksari, G.; Ozat, E.; Konar, N. Effect of various process conditions on efficiency and colour properties of Pistacia terebinthus oil encapsulated by spray drying. Int. J. Food Eng 2017, 3, 132–135. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society; AOCS: Champaign, IL, USA, 1998; pp. 2389–2395. [Google Scholar]
- Baştürk, A.; Ceylan, M.M.; Çavuş, M.; Boran, G.; Javidipour, I. Effects of some herbal extracts on oxidative stability of corn oil under accelerated oxidation conditions in comparison with some commonly used antioxidants. LWT 2018, 89, 358–364. [Google Scholar] [CrossRef]
- Zarena, A.; Bhattacharya, S.; Kadimi, U.S. Mangosteen oil-in-water emulsions: Rheology, creaming, and microstructural characteristics during storage. Food Bioprocess Technol. 2012, 5, 3007–3013. [Google Scholar] [CrossRef]
- Quispe-Condori, S.; Saldaña, M.D.; Temelli, F. Microencapsulation of flax oil with zein using spray and freeze drying. LWT-Food Sci. Technol. 2011, 44, 1880–1887. [Google Scholar] [CrossRef]
- Carneiro, H.C.; Tonon, R.V.; Grosso, C.R.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef]
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J. Food Eng. 2008, 84, 194–205. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Obuchowska, M.; Zawirska-Wojtasiak, R.; Wasowicz, E. Headspace solid-phase microextraction use for the characterization of volatile compounds in vegetable oils of different sensory quality. J. Agric. Food Chem. 2000, 48, 2360–2367. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Healy, L.E.; Sevindik, O.; Sun, D.-W.; Selli, S.; Kelebek, H.; Tiwari, B.K. Impacts of novel blanching treatments combined with commercial drying methods on the physicochemical properties of Irish brown seaweed Alaria esculenta. Food Chem. 2022, 369, 130949. [Google Scholar] [CrossRef] [PubMed]
- Vukoja, J.; Pichler, A.; Ivić, I.; Šimunović, J.; Kopjar, M. Cellulose as a delivery system of raspberry juice volatiles and their stability. Molecules 2020, 25, 2624. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Tzakou, O.; Couladis, M. Essential oil composition of the turpentine tree (Pistacia terebinthus L.) fruits growing wild in Turkey. Food Chem. 2009, 114, 282–285. [Google Scholar] [CrossRef]
- Hao, D.C.; Gu, X.-J.; Xiao, P.G. Phytochemical and biological research of cannabis pharmaceutical resources. In Medicinal Plants; Elsevier: Amsterdam, The Netherlands, 2015; pp. 431–464. [Google Scholar]
- Caputi, L.; Aprea, E. Use of terpenoids as natural flavouring compounds in food industry. Recent Pat. Food Nutr. Agric. 2011, 3, 9–16. [Google Scholar] [CrossRef]
- Fan, M.; Yuan, S.; Li, L.; Zheng, J.; Zhao, D.; Wang, C.; Wang, H.; Liu, X.; Liu, J. Application of terpenoid compounds in food and pharmaceutical products. Fermentation 2023, 9, 119. [Google Scholar] [CrossRef]
- Risner, D.; Marco, M.L.; Pace, S.A.; Spang, E.S. The potential production of the bioactive compound pinene using whey permeate. Processes 2020, 8, 263. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene—What are the potential health benefits of this flavouring and aroma agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef]
- Couladis, M.; Özcan, M.; Tzakou, O.; Akgül, A. Comparative essential oil composition of various parts of the turpentine tree (Pistacia terebinthus L) growing wild in Turkey. J. Sci. Food Agric. 2003, 83, 136–138. [Google Scholar] [CrossRef]
- Pulaj, B.; Mustafa, B.; Nelson, K.; Quave, C.L.; Hajdari, A. Chemical composition and in vitro antibacterial activity of Pistacia terebinthus essential oils derived from wild populations in Kosovo. BMC Complement. Altern. Med. 2016, 16, 147. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Mnif, W.; Ouerhani, B.; Ghrissi, K. Chemical composition and antibacterial activity of essential oil from the seeds of Pistacia terebinthus grown in Tunisia. J. Essent. Oil Bear. Plants 2012, 15, 582–588. [Google Scholar] [CrossRef]
- Ozel, M.Z.; Yanık, D.K.; Gogus, F.; Hamilton, J.F.; Lewis, A.C. Effect of roasting method and oil reduction on volatiles of roasted Pistacia terebinthus using direct thermal desorption-GCxGC-TOF/MS. LWT-Food Sci. Technol. 2014, 59, 283–288. [Google Scholar] [CrossRef]
- Dickinson, E.; Golding, M. Depletion flocculation of emulsions containing unadsorbed sodium caseinate. Food Hydrocoll. 1997, 11, 13–18. [Google Scholar] [CrossRef]
- Silva, K.A.; Rocha-Leão, M.H.; Coelho, M.A.Z. Evaluation of aging mechanisms of olive oil–lemon juice emulsion through digital image analysis. J. Food Eng. 2010, 97, 335–340. [Google Scholar] [CrossRef]
- Minemoto, Y.; Hakamata, K.; Adachi, S.; Matsuno, R. Oxidation of linoleic acid encapsulated with gum arabic or maltodextrin by spray-drying. J. Microencapsul. 2002, 19, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Turchiuli, C.; Fuchs, M.; Bohin, M.; Cuvelier, M.-E.; Ordonnaud, C.; Peyrat-Maillard, M.; Dumoulin, E. Oil encapsulation by spray drying and fluidised bed agglomeration. Innov. Food Sci. Emerg. Technol. 2005, 6, 29–35. [Google Scholar] [CrossRef]
- Bae, E.; Lee, S.J. Microencapsulation of avocado oil by spray drying using whey protein and maltodextrin. J. Microencapsul. 2008, 25, 549–560. [Google Scholar] [CrossRef]
- Wedegaertner, O.A. Handling Characteristic Evaluation and Broiler Growth and Production Trait Effects of Free and Lipid Matrix Encapsulated Vitamin and Trace Mineral Premixes. Masters’ Thesis, North Carolina State University, Raleigh, NC, USA, 2019. [Google Scholar]
- Shah, R.B.; Tawakkul, M.A.; Khan, M.A. Comparative evaluation of flow for pharmaceutical powders and granules. Aaps Pharmscitech 2008, 9, 250–258. [Google Scholar] [CrossRef]
- Brishti, F.H.; Chay, S.Y.; Muhammad, K.; Ismail-Fitry, M.R.; Zarei, M.; Karthikeyan, S.; Saari, N. Effects of drying techniques on the physicochemical, functional, thermal, structural and rheological properties of mung bean (Vigna radiata) protein isolate powder. Food Res. Int. 2020, 138, 109783. [Google Scholar] [CrossRef]
- Goubet, I.; Le Quere, J.-L.; Voilley, A. Retention of aroma compounds by carbohydrates: Influence of their physicochemical characteristics and of their physical state. A review. J. Agric. Food Chem. 1998, 46, 1981–1990. [Google Scholar] [CrossRef]
- Reineccius, G.A. The spray drying of food flavors. Dry. Technol. 2004, 22, 1289–1324. [Google Scholar] [CrossRef]
- Frascareli, E.; Silva, V.; Tonon, R.; Hubinger, M. Effect of process conditions on the microencapsulation of coffee oil by spray drying. Food Bioprod. Process. 2012, 90, 413–424. [Google Scholar] [CrossRef]
- Badee, A.; Amal, E.; El-Kader, A.; Hanan, M. Microencapsulation of peppermint oil by spray drying. Aust. J. Basic Appl. Sci. 2012, 6, 499–504. [Google Scholar]
- Chen, Q.; Zhong, F.; Wen, J.; McGillivray, D.; Quek, S.Y. Properties and stability of spray-dried and freeze-dried microcapsules co-encapsulated with fish oil, phytosterol esters, and limonene. Dry. Technol. 2013, 31, 707–716. [Google Scholar] [CrossRef]
- Enciso-Sáenz, S.; Borrás-Enriquez, A.; Ventura-Canseco, L.; Gutiérrez-Miceli, F.; Dendooven, L.; Grajales-Lagunes, A.; Ruiz-Cabrera, M.; Ruíz-Valdiviezo, V.; Abud-Archila, M. Lemongrass (Cymbopogon citratus (DC) Stapf) essential oil encapsulation by freeze-drying. Rev. Mex. De Ing. Química 2018, 17, 407–420. [Google Scholar] [CrossRef]
- Bertolini, A.; Siani, A.; Grosso, C. Stability of monoterpenes encapsulated in gum arabic by spray-drying. J. Agric. Food Chem. 2001, 49, 780–785. [Google Scholar] [CrossRef] [PubMed]
| MD:GA Ratio | Maltodextrin (g) | Gum Acacia (g) | Water (g) | Oil (g) |
|---|---|---|---|---|
| 25:75 | 5 | 15 | 70 | 10 |
| 50:50 | 10 | 10 | 70 | 10 |
| 75:25 | 15 | 5 | 70 | 10 |
| Physical-Chemical Parameters | Value |
|---|---|
| Acidity (oleic %) | 1.11 ± 0.01 |
| Peroxide (meq/kg oil) | 2.52 ± 0.07 |
| p-Anisidine value | not detected |
| TBARS | not detected |
| Color | |
| L* | 77.33 ± 0.08 |
| a* | 16.71 ± 0.10 |
| b* | 97.74 ± 0.25 |
| Fatty acid composition (%) | |
| Myristic acid (C14:0) | 0.05 ± 0.03 |
| Palmitic Acid (C16:0) | 25.04 ± 0.21 |
| Palmitoleic acid (C16:1) | 5.12 ± 0.10 |
| Heptadecanoic acid (C17:0) | 0.01 ± 0.01 |
| Margaric acid (C17:1) | 0.1 ± 0.02 |
| Stearic acid (C18:0) | 1.58 ± 0.32 |
| Oleic acid (C18:1) | 51.77 ± 0.17 |
| Linoleic acid (C18:2) | 15.17 ± 0.40 |
| Eicosanoic acid (C20:0) | 0.11 ± 0.02 |
| Linolenic acid (C18:3) | 0.92 ± 0.03 |
| Docosahexaenoic acid (C22:0) | 0.02 ± 0.03 |
| Eicosadienoic acid (C20:2) | 0.01 ± 0.02 |
| Lignoceric acid (C24:0) | 0.04 ± 0.01 |
| LRI a | Compounds | Concentration (μg·kg−1) b | Identification c |
|---|---|---|---|
| 1023 | α-Pinene | 8588 ± 54.0 | LRI, MS, Std |
| 1112 | β-Pinene | 7778 ± 2.1 | LRI, MS, Std |
| 1119 | Sabinene | 4427 ± 21.8 | LRI, MS, tent |
| 1132 | p-Xylene | 15,321 ± 41.1 | LRI, MS, Std |
| 1144 | δ-3-Carene | 5241 ± 44.0 | LRI, MS, tent |
| 1160 | β-Myrcene | 1278 ± 19.0 | LRI, MS, Std |
| 1179 | α-Terpinene | 18,937 ± 27.6 | LRI, MS, Std |
| 1197 | D-Limonene | 29,626 ± 26.6 | LRI, MS, Std |
| 1210 | β-Phellandrene | 7220 ± 23.6 | LRI, MS, Std |
| 1254 | (Z)-β-Ocimene | 8685 ± 225.0 | LRI, MS, Std |
| 1236 | Ocimene | 20,808 ± 146.0 | LRI, MS, Std |
| 1268 | p-Cymene | 17,140 ± 11.4 | LRI, MS, Std |
| 1286 | α-Terpinolene | 14,880 ± 77.2 | LRI, MS, tent |
| 1366 | Alloocimene | 11,545 ± 46.1 | LRI, MS, tent |
| 1441 | Acetic acid | 2678 ± 2.1 | LRI, MS, Std |
| 1486 | α-Copaene | 1567 ± 47.0 | LRI, MS, Std |
| 1545 | Linalool | 1984 ± 69.2 | LRI, MS, Std |
| 1574 | Bornyl acetate | 1287 ± 57.7 | LRI, MS, Std |
| 1596 | 4-Terpineol | 1352 ± 56.7 | LRI, MS, Std |
| 1668 | α-Terpineol | 1562 ± 41.9 | LRI, MS, Std |
| 1711 | Piperitone | 2232 ± 51.5 | LRI, MS, tent |
| 1749 | δ-Cadinene | 1884 ± 22.7 | LRI, MS, Std |
| 1832 | (-)-Calamenene | 3123 ± 37.2 | LRI, MS, tent |
| 2116 | Thymol | 644 ± 20.0 | LRI, MS, Std |
| General Total | 189,785 ± 248.0 |
| Encapsulation Methods | ||||
|---|---|---|---|---|
| SFD | SD-2FN | SD-UN | FD | |
| Encapsulation efficiency (%) | 51.66 ±4.16 a | 96.33 ±2.08 b | 93.33 ±2.30 b | 63.00 ± 3.61 c |
| Drying yield (%) | 84.40 ± 1.75 a | 74.27 ± 1.05 b | 45.27 ± 1.80 c | 95.39 ±0.82 d |
| Powder Properties | ||||
| Moisture content (%) | 0.23 ± 0.01 a | 1.27 ± 0.59 b | 1.31 ± 0.27 b | 0.57 ± 0.22 a |
| Bulk density (g·mL−1) | 0.19 ± 0.01 a | 0.29 ± 0.01 b | 0.27 ± 0.02 b | 0.20 ± 0.01 a |
| Tapped density (g·mL−1) | 0.24 ± 0.01 a | 0.35 ± 0.01 b | 0.33 ± 0.02 b | 0.24 ± 0.01 a |
| Carr index | 20.58 ± 1.00 a | 18.68 ± 4.28 ab | 17.87 ± 3.13 ab | 14.33 ± 1.52 b |
| Hausner ratio | 1.25 ± 0.01 a | 1.22 ± 0.06 ab | 1.21 ± 0.05 ab | 1.16 ± 0.02 b |
| Particle size D43 (μm) | 3.18 ± 0.18 a | 11.62 ± 0.35 b | 6.64 ± 0.35 c | 14.22 ± 0.79 d |
| Compounds | Retention (%) | |||
|---|---|---|---|---|
| SFD | FD | SD-2FN | SD-UN | |
| α-Pinene | 42.42 ± 0.37 a | 57.64 ± 1.24 b | 90.31 ± 2.19 c | 104.06 ± 3.47 d |
| Sabinene | 8.90 ± 0.29 a | 11.75 ± 0.32 b | 39.58 ± 1.27 c | 46.07 ± 1.49 d |
| β-Myrcene | 43.14 ± 0.39 a | 96.30 ± 3.51 b | 44.19 ± 1.34 a | 45.14 ± 0.89 a |
| (Z)-β-Ocimene | 5.87 ± 0.21 a | 14.08 ± 0.90 b | 61.13 ± 0,57 c | 31.95 ± 0.41 d |
| Ocimene | 5.32 ± 0.12 a | 13.84 ± 0.76 b | 52.58 ± 0.45 c | 82.28 ± 1.14 d |
| Linalool | 13.80 ± 0.46 a | 22.17 ± 0.66 b | 97.32 ± 0,8 c | 99.71 ± 1.86 d |
| Total | 14.16 ± 0.13 a | 24.52 ± 0.17 b | 62.28 ± 0.1 c | 73.52 ± 0.63 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaman, D.M.; Koçak Yanık, D.; Elik Demir, A.; Uzun Karka, H.; Güçlü, G.; Selli, S.; Kelebek, H.; Göğüş, F. Effect of Encapsulation Techniques on Aroma Retention of Pistacia terebinthus L. Fruit Oil: Spray Drying, Spray Freeze Drying, and Freeze Drying. Foods 2023, 12, 3244. https://doi.org/10.3390/foods12173244
Yaman DM, Koçak Yanık D, Elik Demir A, Uzun Karka H, Güçlü G, Selli S, Kelebek H, Göğüş F. Effect of Encapsulation Techniques on Aroma Retention of Pistacia terebinthus L. Fruit Oil: Spray Drying, Spray Freeze Drying, and Freeze Drying. Foods. 2023; 12(17):3244. https://doi.org/10.3390/foods12173244
Chicago/Turabian StyleYaman, Delal Meryem, Derya Koçak Yanık, Aysel Elik Demir, Hicran Uzun Karka, Gamze Güçlü, Serkan Selli, Haşim Kelebek, and Fahrettin Göğüş. 2023. "Effect of Encapsulation Techniques on Aroma Retention of Pistacia terebinthus L. Fruit Oil: Spray Drying, Spray Freeze Drying, and Freeze Drying" Foods 12, no. 17: 3244. https://doi.org/10.3390/foods12173244
APA StyleYaman, D. M., Koçak Yanık, D., Elik Demir, A., Uzun Karka, H., Güçlü, G., Selli, S., Kelebek, H., & Göğüş, F. (2023). Effect of Encapsulation Techniques on Aroma Retention of Pistacia terebinthus L. Fruit Oil: Spray Drying, Spray Freeze Drying, and Freeze Drying. Foods, 12(17), 3244. https://doi.org/10.3390/foods12173244






