Modeling of Sensory Properties of Poppy Sherbet by Turkish Consumers and Changes in Quality Properties during Storage Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Thermal Pasteurization of Poppy Sherbet
2.2. Modeling Procedure for Response Surface Method
2.3. Determination of Color Parameters
2.4. Sensory Analysis
2.5. Total Phenolic Contents and Total Flavonoid Contents
2.6. Determination of Total Antioxidant Capacity by DPPH
2.7. Determination of Total Antioxidant Capacity by CUPRAC
2.8. Determination of Total Monomeric Anthocyanin
2.9. Phenolic Compounds (HPLC-DAD)
2.10. Statistical Analysis
3. Results
3.1. Sensory Properties in the Storage Process
3.2. Color Properties in the Storage Process
3.3. TPC and TFC in the Storage Process
3.4. Antioxidant Activity in the Storage Process
3.5. Total Anthocyanin Content
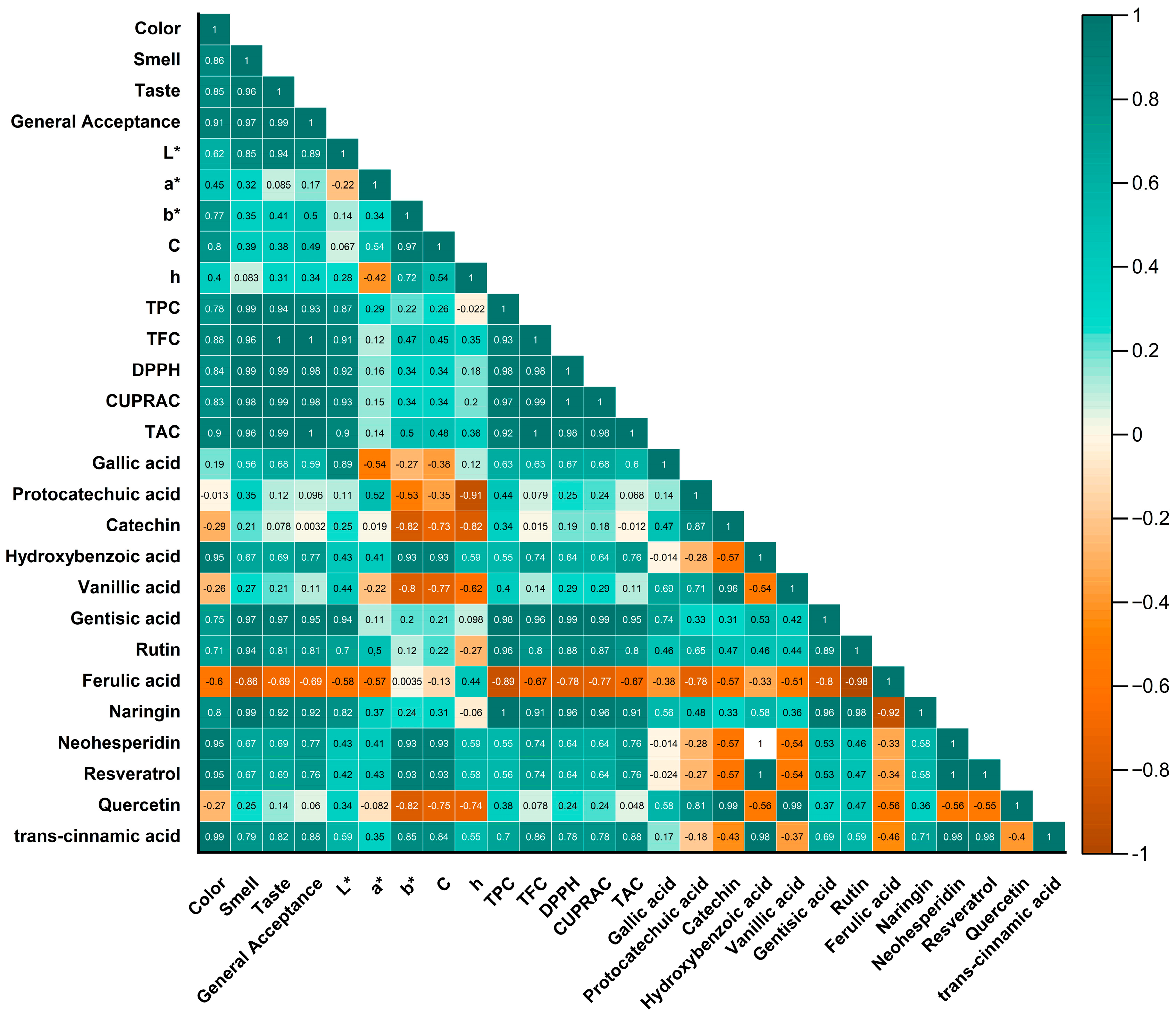
3.6. Analysis of Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, B.; Li, M.; Yin, R. Phytochemical Content, Health Benefits, and Toxicology of Common Edible Flowers: A Review (2000–2015). Crit. Rev. Food Sci. Nutr. 2016, 56, S130–S148. [Google Scholar] [CrossRef] [PubMed]
- Yücel, E.; Yücel Şengün, İ.; Çoban, Z. The Wild Plants Consumed as a Food in Afyonkarahisar/Turkey and Consumption Forms of These Plants. Biol. Divers. Conserv. 2012, 5, 95–105. [Google Scholar]
- Shahbaz, M.U.; Arshad, M.; Mukhtar, K.; Nabi, B.G.; Goksen, G.; Starowicz, M.; Nawaz, A.; Ahmad, I.; Walayat, N.; Manzoor, M.F.; et al. Natural Plant Extracts: An Update about Novel Spraying as an Alternative of Chemical Pesticides to Extend the Postharvest Shelf Life of Fruits and Vegetables. Molecules 2022, 27, 5152. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, S.; Dziągwa-becker, M.; Junka, A.; Piątczak, E.; Jezierska-domaradzka, A.; Brożyna, M.; Paleczny, J.; Sobiecka, A.; Słupski, W.; Mess, E.; et al. Screening Papaveraceae as Novel Antibiofilm Natural-Based Agents. Molecules 2021, 26, 4778. [Google Scholar] [CrossRef] [PubMed]
- Akın, B. The Effects of Different Treatments on in Vitro Seed Germination of Papaver Rhoeas. J. Sci. Technol. Dumlupınar Univ. 2011, 1, 17–24. [Google Scholar]
- Arslan, N.; Sarihan, E.O.; Ipek, A.; Gümüşçü, A. Türkiye’deki Yabani Haşhaşların Değerlendirilebilme İmkanları. Turk. J. Sci. Rev. 2012, 5, 41–44. [Google Scholar]
- Soulimani, R.; Younos, C.; Jarmouni-Idrissi, S.; Bousta, D.; Khalouki, F.; Laila, A. Behavioral and Pharmaco-Toxicological Study of Papaer rhoeas L. in Mice. J. Ethnopharmacol. 2001, 74, 265–274. [Google Scholar] [CrossRef]
- Katarzyna, J.; Karolina, J.; Patrycja, K.; Mateusz, B.; Izabela, G. Mineral Composition and Antioxidant Potential in the Common Poppy (Papaver rhoeas L.) Petal Infusions. Biol. Trace Elem. Res. 2021, 199, 371–381. [Google Scholar] [CrossRef]
- Çoban, İ.; Toplan, G.G.; Özbek, B.; Gürer, Ç.U.; Sarıyar, G. Variation of Alkaloid Contents and Antimicrobial Activities of Papaver rhoeas L. Growing in Turkey and Northern Cyprus. Pharm. Biol. 2017, 55, 1894–1898. [Google Scholar] [CrossRef] [PubMed]
- Todorova, T.; Pesheva, M.; Gregan, F.; Chankova, S. Antioxidant, Antimutagenic, and Anticarcinogenic Effects of Papaver rhoeas L. Extract on Saccharomyces Cerevisiae. J. Med. Food 2015, 18, 460–467. [Google Scholar] [CrossRef]
- Kaya, İ.; İncekara, N.; Nemli, Y. Ege Bölgesi’nde Sebze Olarak Tüketilen Yabani Kuşkonmaz, Sirken, Yabani Hindiba, Rezene, Gelincik, Çoban Değneği ve Ebegümecinin Bazı Kimyasal Analizleri. Yuz. Yıl Univ. J. Agric. Sci. 2004, 14, 1–6. [Google Scholar]
- Sirichan, T.; Kijpatanasilp, I.; Asadatorn, N.; Assatarakul, K. Optimization of Ultrasound Extraction of Functional Compound from Makiang Seed by Response Surface Methodology and Antimicrobial Activity of Optimized Extract with Its Application in Orange Juice. Ultrason. Sonochem 2022, 83, 105916. [Google Scholar] [CrossRef] [PubMed]
- Dogan, G.; Bagci, E. Essential Oil Composition of Papaver rhoeas L. Corn Poppy Papaveraceae from Turkey. Hacet. J. Biol. Chem. 2014, 42, 545–549. [Google Scholar]
- Renna, M.; Cocozza, C.; Gonnella, M.; Abdelrahman, H.; Santamaria, P. Elemental Characterization of Wild Edible Plants from Countryside and Urban Areas. Food Chem. 2015, 177, 29–36. [Google Scholar] [CrossRef]
- Hmamou, A.; Kara, M.; El Khomsi, M.; Saleh, A.; Al Kamaly, O.; Bendaoud, A.; El Ouadrhiri, F.; Adachi, A.; Tlemcani, S.; Eloutassi, N.; et al. Comparative Study on the Total Phenolics, Total Flavonoids, and Biological Activities of Papaver rhoeas L. Extracts from Different Geographical Regions of Morocco. Appl. Sci. 2023, 13, 2695. [Google Scholar] [CrossRef]
- Hijazi, M.A.; Aboul-Ela, M.; Bouhadir, K.; Fatfat, M.; Khalife, H.; Ellakany, A.; Gali-Muhtasib, H. Cytotoxic Activity of Alkaloids from Papaver rhoeas Growing in Lebanon. Nat. Prod. 2017, 11, 211–216. [Google Scholar]
- Marsoul, A.; Ijjaali, M.; Oumous, I.; Bennani, B.; Boukir, A. Determination of Polyphenol Contents in Papaver rhoeas L. Flowers Extracts (Soxhlet, Maceration), Antioxidant and Antibacterial Evaluation. Mater. Today Proc. 2020, 31, S183–S189. [Google Scholar] [CrossRef]
- Grauso, L.; de Falco, B.; Motti, R.; Lanzotti, V. Corn Poppy, Papaver rhoeas L.: A Critical Review of Its Botany, Phytochemistry and Pharmacology. Phytochem. Rev. 2021, 20, 227–248. [Google Scholar] [CrossRef]
- Günaydin, Y.K.; Dündar, Z.D.; Çekmen, B.; Akilli, N.B.; Köylü, R.; Cander, B. Intoxication Due to Papaver rhoeas (Corn Poppy): Five Case Reports. Case Rep. Med. 2015, 2015, 321360. [Google Scholar] [CrossRef]
- View of Effect of Poppy (Papaver somniferum L.) Fertilization with Potassium and Magnesium on the Seed Yield and Its Quality. Available online: http://www.acta.fapz.uniag.sk/journal/article/view/86/32 (accessed on 3 August 2023).
- Stankiewicz-Kosyl, M.; Synowiec, A.; Haliniarz, M.; Wenda-Piesik, A.; Domaradzki, K.; Parylak, D.; Wrochna, M.; Pytlarz, E.; Gala-Czekaj, D.; Marczewska-Kolasa, K.; et al. Herbicide Resistance and Management Options of Papaver rhoeas L. and Centaurea cyanus L. in Europe: A Review. Agronomy 2020, 10, 874. [Google Scholar] [CrossRef]
- Kati, V.; Scarabel, L.; Thiery-Lanfranchi, D.; Kioleoglou, V.; Liberopoulou, S.; Délye, C. Multiple Resistance of Papaver rhoeas L. to 2,4-D and Acetolactate Synthase Inhibitors in Four European Countries. Weed Res. 2019, 59, 367–376. [Google Scholar] [CrossRef]
- Linn, A.I.; Mink, R.; Peteinatos, G.G.; Gerhards, R. In-Field Classification of Herbicide-Resistant Papaver rhoeas and Stellaria Media Using an Imaging Sensor of the Maximum Quantum Efficiency of Photosystem II. Weed Res. 2019, 59, 357–366. [Google Scholar] [CrossRef]
- Recasens, J.; Royo-Esnal, A.; Valencia-Gredilla, F.; Torra, J. Efficiency, Profitability and Carbon Footprint of Different Management Programs under No-Till to Control Herbicide Resistant Papaver Rhoeas. Plants 2020, 9, 433. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz-Kosyl, M.; Haliniarz, M.; Wrochna, M.; Obrępalska-Stęplowska, A.; Kuc, P.; Łukasz, J.; Wińska-Krysiak, M.; Wrzesińska-Krupa, B.; Puła, J.; Podsiadło, C.; et al. Occurrence and Mechanism of Papaver rhoeas ALS Inhibitors Resistance in Poland. Agriculture 2022, 13, 82. [Google Scholar] [CrossRef]
- Kiettiolarn, M.; Kitsanayanyong, L.; Maneerote, J.; Unajak, S.; Tepwong, P. Optimization and Production of Protein Hydrolysate Containing Antioxidant Activity from Tuna Cooking Juice Concentrate by Response Surface Methodology. Fish. Aquat. Sci. 2022, 25, 335–349. [Google Scholar] [CrossRef]
- Aloulou, W.; Aloulou, H.; Attia, A.; Chakraborty, S.; Ben Amar, R. Treatment of Tuna Cooking Juice via Ceramic Ultrafiltration Membrane: Optimization Using Response Surface Methodology. Membranes 2022, 12, 813. [Google Scholar] [CrossRef]
- Abidoye, A.O.; Ojedokun, F.O.; Fasogbon, B.M.; Bamidele, O.P. Effects of Sweet Basil Leaves (Ocimum basilicum L) Addition on the Chemical, Antioxidant, and Storage Stability of Roselle Calyces (Hibiscus sabdariffa) Drink. Food Chem. 2022, 371, 131170. [Google Scholar] [CrossRef]
- Varela-Santos, E.; Ochoa-Martinez, A.; Tabilo-Munizaga, G.; Reyes, J.E.; Pérez-Won, M.; Briones-Labarca, V.; Morales-Castro, J. Effect of High Hydrostatic Pressure (HHP) Processing on Physicochemical Properties, Bioactive Compounds and Shelf-Life of Pomegranate Juice. Innov. Food Sci. Emerg. Technol. 2011, 13, 13–22. [Google Scholar] [CrossRef]
- Fuleki, T.; Ricardo-Da-Silva, J.M. Effects of Cultivar and Processing Method on the Contents of Catechins and Procyanidins in Grape Juice. J. Agric. Food Chem. 2003, 51, 640–646. [Google Scholar] [CrossRef]
- Park, Y.K.; Park, E.; Kim, J.S.; Kang, M.H. Daily Grape Juice Consumption Reduces Oxidative DNA Damage and Plasma Free Radical Levels in Healthy Koreans. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2003, 529, 77–86. [Google Scholar] [CrossRef]
- Petrou, S.; Tsiraki, M.; Giatrakou, V.; Savvaidis, I.N. Chitosan Dipping or Oregano Oil Treatments, Singly or Combined on Modified Atmosphere Packaged Chicken Breast Meat. Int. J. Food Microbiol. 2012, 156, 264–271. [Google Scholar] [CrossRef]
- Eren, E.; Şahingöz, S.A. Researching Consumable Potential Of Edible Insects In Everyday Diets: The Example Of Bread With Mealworm Addition. In Global & Emerging Trends in Tourism, 1st ed.; Bayrakcı, S., Aras, S., Yetimoğlu, S., Eds.; NEU Press: Konya, Turkey, 2021; pp. 208–220. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Singh, R.P.; Chidambara Murthy, K.N.; Jayaprakasha, G.K. Studies on the Antioxidant Activity of Pomegranate (Punica granatum) Peel and Seed Extracts Using In Vitro Models. J. Agric. Food Chem. 2001, 50, 81–86. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Özyürek, M.; Esin Karademir, S.; Erçǧ, E. The Cupric Ion Reducing Antioxidant Capacity and Polyphenolic Content of Some Herbal Teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Cemeroğlu, B. Gıda Analizleri, 2nd ed.; Nobel Yayıncılık: Ankara, Turkey, 2010; ISBN 9759857868. [Google Scholar]
- Portu, J.; López, R.; Santamaría, P.; Garde-Cerdán, T. Elicitation with Methyl Jasmonate Supported by Precursor Feeding with Phenylalanine: Effect on Garnacha Grape Phenolic Content. Food Chem. 2017, 237, 416–422. [Google Scholar] [CrossRef]
- Li, H.H.; Huang, R.; Li, M.C. Formula Optimization of Cordyceps Flower Beverage Based on Sensory Analysis Experiments. E3S Web Conf. 2021, 292, 03079. [Google Scholar] [CrossRef]
- Palka, A.; Wilczynska, A. Storage Quality Changes in Craft and Industrial Blueberry, Strawberry, Raspberry and Passion Fruit-Mango Sorbets. Foods 2023, 12, 2733. [Google Scholar] [CrossRef] [PubMed]
- Hipólito, C.; Ramalheira, R.; da Costa, S.B.; Moldão-Martins, M. The Effect of Fruit Cultivar/Origin and Storage Time on Sorbets Quality. LWT Food Sci. Technol. 2016, 68, 462–469. [Google Scholar] [CrossRef]
- Ekici, L.; Kafadar, A.D.; Albayrak, S. Physicochemical, Sensory, and Bioactive Properties of Some Traditional Turkish Sorbets. J. Food Process. Preserv. 2018, 42, e13664. [Google Scholar] [CrossRef]
- Yikmiş, S.; Özpancar, N.; Bozkir, Ç.; Çöl, B.G. Functional Sirkencubin Syrup with Purple Basil; Bioactive Properties, Organoleptic Acceptability, and Possible Effects on Blood Pressure. Food Sci. Technol. 2020, 40, 550–557. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Sicari, V.; Spizzirri, U.G.; Romeo, R.; Tundis, R.; Mincione, A.; Nicoletta, F.P.; Restuccia, D. Evaluation of Selected Quality Parameters of “Agristigna” Monovarietal Extra Virgin Olive Oil and Its Apple Vinegar-Based Dressing during Storage. Foods 2022, 11, 1113. [Google Scholar] [CrossRef] [PubMed]
- Matabura, V.V.; Kibazohi, O. Physicochemical and Sensory Evaluation of Mixed Juices from Banana, Pineapple and Passion Fruits during Storage. Tanzan. J. Sci. 2021, 47, 332–343. [Google Scholar]
- Ekici, L. Effects of Concentration Methods on Bioactivity and Color Properties of Poppy (Papaver rhoeas L.) Sorbet, a Traditional Turkish Beverage. LWT Food Sci. Technol. 2014, 56, 40–48. [Google Scholar] [CrossRef]
- Lekjing, S.; Venkatachalam, K. Changes in Physicochemical Characteristics, Polyphenolics, and Amino Acids of Wax Apple Cider Vinegar during Prolonged Storage. Ital. J. Food Sci. 2021, 33, 129–141. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Avoiding Non-Enzymatic Browning by High-Intensity Pulsed Electric Fields in Strawberry, Tomato and Watermelon Juices. J. Food Eng. 2009, 92, 37–43. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Özen, M.; Özdemir, N.; Ertekin Filiz, B.; Budak, N.H.; Kök-Taş, T. Sour Cherry (Prunus cerasus L.) Vinegars Produced from Fresh Fruit or Juice Concentrate: Bioactive Compounds, Volatile Aroma Compounds and Antioxidant Capacities. Food Chem. 2020, 309, 125664. [Google Scholar] [CrossRef]
- Shams Najafabadi, N.; Sahari, M.A.; Barzegar, M.; Hamidi Esfahani, Z. Effects of Concentration Method and Storage Time on Some Bioactive Compounds and Color of Jujube (Ziziphus Jujuba var Vulgaris) Concentrate. J. Food Sci. Technol. 2017, 54, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- Basyigit, B.; Hayoglu, I. Liquorice (Glycyrrhiza glabra L.) Root Sherbet (Extract): Microencapsulation and Storage Stability. Acta Aliment. 2019, 48, 76–85. [Google Scholar] [CrossRef]
- Wang, L.F.; Kim, D.M.; Lee, C.Y. Effects of Heat Processing and Storage on Flavanols and Sensory Qualities of Green Tea Beverage. J. Agric. Food Chem. 2000, 48, 4227–4232. [Google Scholar] [CrossRef] [PubMed]
- Harun-Or-Rashid, M.; Mahmuda Akter, M.; Uddin, J.; Islam, S.; Rahman, M.; Jahan, K.; Moklesur Rahman Sarker, M.; Sadik, G. Antioxidant, Cytotoxic, Antibacterial and Thrombolytic Activities of Centella asiatica L.: Possible Role of Phenolics and Flavonoids. Clin. Phytosci. 2023, 9, 1–9. [Google Scholar] [CrossRef]
- Morales, P.; Ferreira, I.C.F.R.; Carvalho, A.M.; Sánchez-Mata, M.C.; Cámara, M.; Fernández-Ruiz, V.; Pardo-de-Santayana, M.; Tardío, J. Mediterranean Non-Cultivated Vegetables as Dietary Sources of Compounds with Antioxidant and Biological Activity. LWT Food Sci. Technol. 2014, 55, 389–396. [Google Scholar] [CrossRef]
- Prohens, J.; Sánchez, M.C.; Rodríguez-Burruezo, A.; Cámara, M.; Torija, E.; Nuez, F. Morphological and Physico-Chemical Characteristics of Fruits of Pepino (Solanum muricatum), Wild Relatives (S. caripense and S. tabanoense) and Interspecific Hybrids. Implications in Pepino Breeding. Europ. J. Hort. Sci. 2005, 70, 1611–4426. [Google Scholar]
- Krošlák, E.; Maliar, T.; Nemeček, P.; Viskupičová, J.; Maliarová, M.; Havrlentová, M.; Kraic, J. Antioxidant and Proteinase Inhibitory Activities of Selected Poppy (Papaver somniferum L.) Genotypes. Chem. Biodivers. 2017, 14, e1700176. [Google Scholar] [CrossRef] [PubMed]
- Londoño, M.B.Z.; Chaparro, D.; Rojano, B.A.; Arbelaez, A.F.A.; Betancur, L.F.R.; Celis, M.E.M. Effect of storage time on physicochemical, sensorial, and antioxidant characteristics, and composition of mango (cv. Azúcar) juice. Emir. J. Food Agric. 2017, 29, 367–377. [Google Scholar] [CrossRef]
- Yıkmış, S.; Tuğgüm, S. Evaluation of Microbiological, Physicochemical and Sensorial Properties of Purple Basil Kombucha Beverage. Turk. J. Agric. Food Sci. Technol. 2019, 7, 1321–1327. [Google Scholar] [CrossRef]
- Sarabandi, K.; Akbarbaglu, Z.; Peighambardoust, S.H.; Ayaseh, A.; Jafari, S.M. Biological Stabilization of Natural Pigment-Phytochemical from Poppy-Pollen (Papaver bracteatum) Extract: Functional Food Formulation. Food Chem. 2023, 429, 136885. [Google Scholar] [CrossRef]
- Kang, M.; Ha, J.H.; Lee, Y. Physicochemical Properties, Antioxidant Activities and Sensory Characteristics of Commercial Gape Vinegars during Long-Term Storage. Food Sci. Technol. 2020, 40, 909–916. [Google Scholar] [CrossRef]
- Machado, C.C.d.S.; Fernandes, M.T.C.; Mauro, C.S.I.; Farinazzo, F.S.; Prudencio, S.H.; Garcia, S. Probiotic Juçara and Banana Sorbet: Cell Viability, Antioxidant Activity during Storage and Sensory Acceptability by Children. J. Culin. Sci. Technol. 2020, 19, 460–474. [Google Scholar] [CrossRef]
- Akdemir Evrendilek, G.; Tanriverdi, H.; Demir, I.; Uzuner, S. Shelf-Life Extension of Traditional Licorice Root “Sherbet” with a Novel Pulsed Electric Field Processing. Front. Food Sci. Technol. 2023, 3, 1157649. [Google Scholar] [CrossRef]
- Physical, Chemical and Sensory Properties of Sour Grape Based Beverages and EKUAL Keşif. Available online: https://eds.s.ebscohost.com/eds/pdfviewer/pdfviewer?vid=0&sid=36d0ac85-ac96-4150-86f0-6a4b3eb32c9f%40redis (accessed on 26 July 2023).
- Fazaeli, M.; Yousefi, S.; Emam-Djomeh, Z. Investigation on the Effects of Microwave and Conventional Heating Methods on the Phytochemicals of Pomegranate (Punica granatum L.) and Black Mulberry Juices. Food Res. Int. 2013, 50, 568–573. [Google Scholar] [CrossRef]
- Nabi, B.G.; Mukhtar, K.; Ahmed, W.; Manzoor, M.F.; Ranjha, M.M.A.N.; Kieliszek, M.; Bhat, Z.F.; Aadil, R.M. Natural Pigments: Anthocyanins, Carotenoids, Chlorophylls, and Betalains as Colorants in Food Products. Food Biosci. 2023, 52, 102403. [Google Scholar] [CrossRef]
- Bakhshizadeh, M.; Moghaddam, T.N.; Tavassoli, M.; Ayaseh, A.; Bangar, S.P. Gelatin/Chitosan Nanofibres Containing β-Cyclodextrin Complex and Corn Poppy (Papaver rhoeas L.) for Intelligent Packaging. Int. J. Food Sci. Technol. 2023, 58, 2360–2368. [Google Scholar] [CrossRef]
- Velickovic, J.; Mitic, M.; Arsic, B.; Paunovic, D.; Stojanovic, B.; Veljkovic, J.; Dimitrijevic, D.; Stevanovic, S.; Kostic, D. Hplc Analysis of Extracts of Fresh Petals of Papaver rhoeas L. Stud. Univ. Babes-Bolyai Chem. 2019, 64, 239–247. [Google Scholar] [CrossRef]
- Kostic, D.A.; Mitic, S.S.; Mitic, M.N.; Zarubica, A.R.; Velickovic, J.M.; Dordevic, A.S.; Randelovic, S.S. Phenolic Contents, Antioxidant and Antimicrobial Activity of Papaver rhoeas L. Extracts from Southeast Serbia. J. Med. Plants Res. 2010, 4, 1727–1732. [Google Scholar]
- Ergün, A.R.; Tekgül, Y.; Bozkır, H.; Baysal, T. Changes in the Quality of Black Mulberry and Blueberry Sherbets During Storage. Turk. J. Agric. Food Sci. Technol. 2017, 5, 54–59. [Google Scholar] [CrossRef]
- Ertan, K.; Türkyılmaz, M.; Özkan, M. Effect of Sweeteners on Anthocyanin Stability and Colour Properties of Sour Cherry and Strawberry Nectars during Storage. J. Food Sci. Technol. 2018, 55, 4346–4355. [Google Scholar] [CrossRef]
- Mgaya-Kilima, B.; Remberg, S.F.; Chove, B.E.; Wicklund, T. Physiochemical and Antioxidant Properties of Roselle-Mango Juice Blends; Effects of Packaging Material, Storage Temperature and Time. Food Sci. Nutr. 2015, 3, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Kafadar, A.D.; Ekici, L.; Albayrak, S. Determination of Changes in Some Biological Properties during Storage of Some Conventional Sorbets. Sigma J. Eng. Nat. Sci. 2021, 39, 351–366. [Google Scholar] [CrossRef]
- Ekici, L.; Ozaltin, B. Effects of Concentration Methods and Storage Conditions on Some Bioactive Compounds and Color of Tamarind Sorbet: A Traditional Turkish Beverage. J. Food Meas. Charact. 2018, 12, 2045–2056. [Google Scholar] [CrossRef]
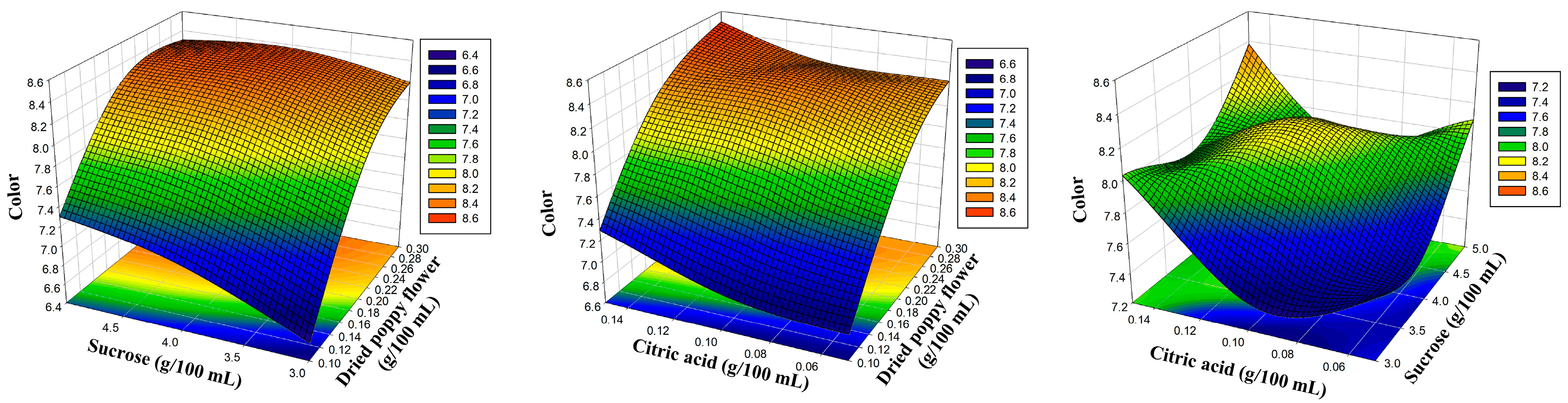
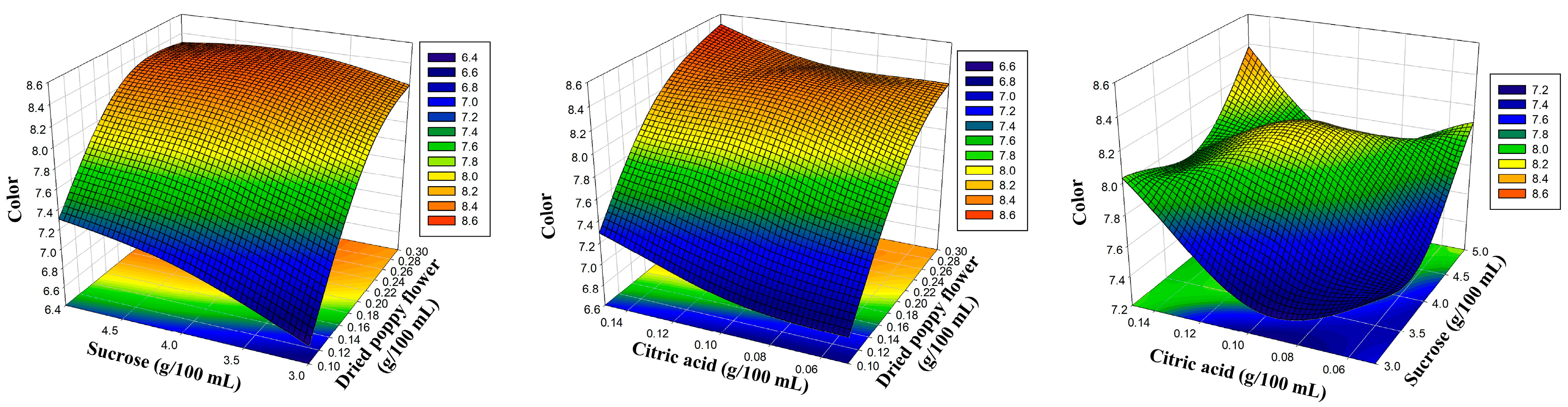
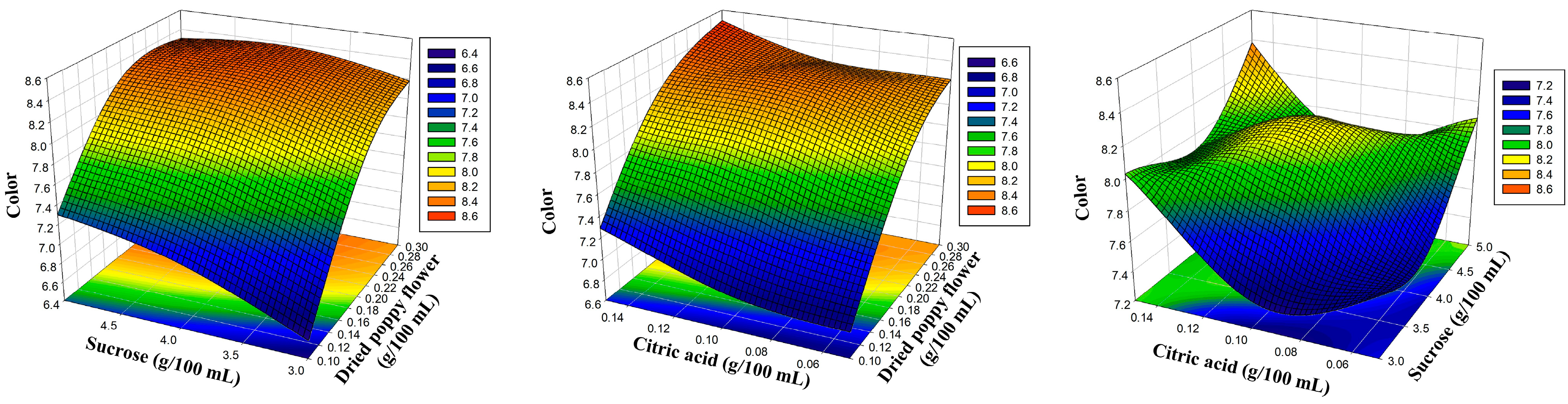
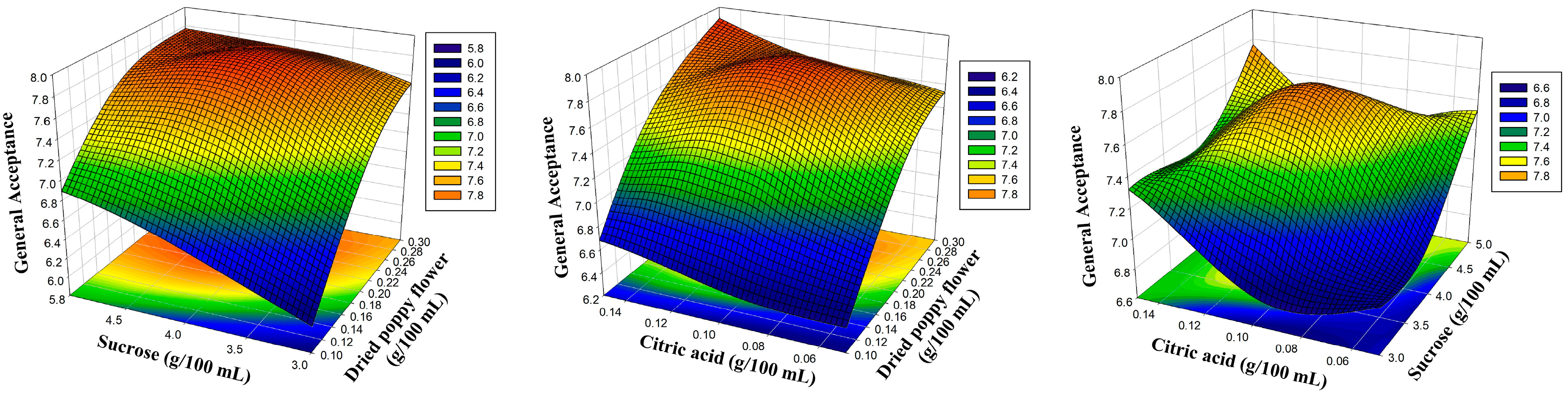
| Independent Variables | Levels | |||
|---|---|---|---|---|
| Codes | −1 | 0 | 1 | |
| Dried poppy flower (g/100 mL) | X1 | 0.1 | 0.2 | 0.3 |
| Sucrose (g/100 mL) | X2 | 3 | 4 | 5 |
| Citric acid (g/100 mL) | X3 | 0.05 | 0.10 | 0.15 |
| Sample | Encoded Independent Variables | Dependent Variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Response 1 | Response 2 | Response 3 | Response 4 | ||||||||
| Color | Smell | Taste | General Acceptance | ||||||||
| X1 | X2 | X3 | Experimental Data | RSM Predicted | Experimental Data | RSM Predicted | Experimental Data | RSM Predicted | Experimental Data | RSM Predicted | |
| 1 | 0.10 | 3.00 | 0.10 | 6.56 | 6.58 | 5.48 | 5.46 | 6.42 | 6.43 | 6.10 | 6.07 |
| 2 | 0.30 | 3.00 | 0.10 | 8.08 | 8.18 | 6.75 | 6.78 | 7.92 | 7.97 | 7.52 | 7.52 |
| 3 | 0.10 | 5.00 | 0.10 | 7.40 | 7.30 | 6.20 | 6.18 | 7.26 | 7.20 | 6.89 | 6.89 |
| 4 | 0.30 | 5.00 | 0.10 | 8.33 | 8.30 | 6.95 | 6.96 | 8.15 | 8.13 | 7.74 | 7.77 |
| 5 | 0.10 | 4.00 | 0.05 | 6.87 | 6.84 | 5.76 | 5.74 | 6.73 | 6.68 | 6.42 | 6.41 |
| 6 | 0.30 | 4.00 | 0.05 | 8.34 | 8.23 | 6.81 | 6.75 | 7.97 | 7.87 | 7.57 | 7.53 |
| 7 | 0.10 | 4.00 | 0.15 | 7.19 | 7.29 | 5.97 | 6.03 | 6.97 | 7.07 | 6.64 | 6.68 |
| 8 | 0.30 | 4.00 | 0.15 | 8.47 | 8.50 | 7.10 | 7.12 | 8.30 | 8.35 | 7.89 | 7.90 |
| 9 | 0.20 | 3.00 | 0.05 | 7.60 | 7.60 | 6.18 | 6.21 | 7.23 | 7.27 | 6.86 | 6.91 |
| 10 | 0.20 | 5.00 | 0.05 | 7.97 | 8.09 | 6.77 | 6.81 | 7.80 | 7.91 | 7.52 | 7.53 |
| 11 | 0.20 | 3.00 | 0.15 | 8.16 | 8.03 | 6.74 | 6.69 | 8.00 | 7.88 | 7.33 | 7.32 |
| 12 | 0.20 | 5.00 | 0.15 | 8.39 | 8.38 | 7.03 | 6.99 | 8.22 | 8.17 | 7.81 | 7.76 |
| 13 | 0.20 | 4.00 | 0.10 | 8.16 | 8.16 | 6.99 | 6.98 | 8.00 | 8.00 | 7.77 | 7.77 |
| 14 | 0.20 | 4.00 | 0.10 | 8.11 | 8.16 | 6.92 | 6.98 | 7.92 | 8.00 | 7.74 | 7.77 |
| 15 | 0.20 | 4.00 | 0.10 | 8.23 | 8.16 | 7.02 | 6.98 | 8.08 | 8.00 | 7.80 | 7.77 |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value | Adj SS | Adj MS | F-Value | p-Value | Adj SS | Adj MS | F-Value | p-Value | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Color | Smell | Taste | General Acceptance | ||||||||||||||
| Model | 9 | 4.83 | 0.54 | 30.88 | 0.001 | 3.76 | 0.42 | 92.02 | 0.000 | 4.76 | 0.53 | 35.13 | 0.001 | 4.63 | 0.51 | 221.58 | 0.000 |
| Linear | 3 | 3.98 | 1.33 | 76.19 | 0.000 | 2.82 | 0.94 | 207.08 | 0.000 | 3.89 | 1.30 | 86.07 | 0.000 | 3.51 | 1.17 | 503.92 | 0.000 |
| X1 | 1 | 3.37 | 3.37 | 193.52 | 0.000 | 2.20 | 2.20 | 484.41 | 0.000 | 3.07 | 3.07 | 203.71 | 0.000 | 2.72 | 2.72 | 1173.20 | 0.000 |
| X2 | 1 | 0.35 | 0.35 | 20.36 | 0.006 | 0.41 | 0.41 | 89.26 | 0.000 | 0.43 | 0.43 | 28.86 | 0.003 | 0.58 | 0.58 | 249.23 | 0.000 |
| X3 | 1 | 0.26 | 0.26 | 14.71 | 0.012 | 0.22 | 0.22 | 47.56 | 0.001 | 0.39 | 0.39 | 25.65 | 0.004 | 0.21 | 0.21 | 89.32 | 0.000 |
| Square | 3 | 0.76 | 0.25 | 14.53 | 0.007 | 0.85 | 0.28 | 62.15 | 0.000 | 0.75 | 0.25 | 16.56 | 0.005 | 1.03 | 0.34 | 147.58 | 0.000 |
| X1 × X1 | 1 | 0.72 | 0.72 | 41.35 | 0.001 | 0.75 | 0.75 | 164.44 | 0.000 | 0.72 | 0.72 | 47.54 | 0.001 | 0.85 | 0.85 | 364.53 | 0.000 |
| X2 × X2 | 1 | 0.06 | 0.06 | 3.62 | 0.116 | 0.12 | 0.12 | 26.82 | 0.004 | 0.06 | 0.06 | 3.73 | 0.111 | 0.19 | 0.19 | 82.24 | 0.000 |
| X3 × X3 | 1 | 0.00 | 0.00 | 0.01 | 0.927 | 0.05 | 0.05 | 11.24 | 0.020 | 0.02 | 0.02 | 1.05 | 0.352 | 0.10 | 0.10 | 41.89 | 0.001 |
| 2-Way Interaction | 3 | 0.10 | 0.03 | 1.92 | 0.244 | 0.09 | 0.03 | 6.84 | 0.032 | 0.12 | 0.04 | 2.76 | 0.151 | 0.09 | 0.03 | 13.25 | 0.008 |
| X1 × X2 | 1 | 0.09 | 0.09 | 4.99 | 0.076 | 0.07 | 0.07 | 15.14 | 0.012 | 0.09 | 0.09 | 6.04 | 0.057 | 0.08 | 0.08 | 35.39 | 0.002 |
| X1 × X3 | 1 | 0.01 | 0.01 | 0.50 | 0.509 | 0.00 | 0.00 | 0.29 | 0.615 | 0.00 | 0.00 | 0.15 | 0.716 | 0.00 | 0.00 | 0.94 | 0.376 |
| X2 × X3 | 1 | 0.00 | 0.00 | 0.28 | 0.618 | 0.02 | 0.02 | 5.10 | 0.073 | 0.03 | 0.03 | 2.09 | 0.208 | 0.01 | 0.01 | 3.43 | 0.123 |
| Error | 5 | 0.09 | 0.02 | 0.02 | 0.00 | 0.08 | 0.02 | 0.01 | 0.00 | ||||||||
| Lack-of-Fit | 3 | 0.08 | 0.03 | 6.86 | 0.130 | 0.02 | 0.01 | 2.16 | 0.332 | 0.06 | 0.02 | 3.26 | 0.244 | 0.01 | 0.00 | 3.63 | 0.223 |
| Pure Error | 2 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | ||||||||
| Total | 14 | 4.92 | 3.79 | 4.84 | 4.64 | ||||||||||||
| R2 | 98.23% | 99.40% | 98.44% | 99.75% | |||||||||||||
| Adj. R2 | 95.05% | 98.32% | 95.64% | 99.30% | |||||||||||||
| Pred. R2 | 73.88% | 92.34% | 78.73% | 96.53% | |||||||||||||
| Variable | Setting | |||
|---|---|---|---|---|
| X1—Dried poppy flower (gram) | 0.26 | |||
| X2—Sucrose (gram) | 4.29 | |||
| X3—Citric acid (gram) | 0.15 | |||
| Response | Fit | SE Fit | %95 CI | %95 PI |
| General acceptance | 7.98 | 0.03 | (7.8966; 8.0653) | (7.8311; 8.1308) |
| Taste | 8.38 | 0.08 | (8.1673; 8.5973) | (8.0005; 8.7641) |
| Smell | 7.19 | 0.04 | (7.0757; 7.3118) | (6.9841; 7.4034) |
| Color | 8.55 | 0.08 | (8.3216; 8.7836) | (8.1424; 8.9628) |
| Sensory Features | Storage Time | Sensory Analysis Results |
|---|---|---|
| Color | 0 day | 8.44 ± 0.64 a |
| 10 day | 8.02 ± 0.87 b | |
| 20 day | 7.96 ± 0.60 b | |
| 30 day | 7.82 ± 0.75 b | |
| Smell | 0 day | 7.14 ± 0.57 a |
| 10 day | 7.06 ± 0.55 ab | |
| 20 day | 6.92 ± 0.70 ab | |
| 30 day | 6.74 ± 0.75 b | |
| Taste | 0 day | 8.12 ± 1.10 a |
| 10 day | 8.02 ± 0.84 ab | |
| 20 day | 7.68 ± 1.00 ab | |
| 30 day | 7.58 ± 0.81 b | |
| General Acceptance | 0 day | 8.02 ± 0.98 a |
| 10 day | 7.90 ± 0.54 a | |
| 20 day | 7.70 ± 0.79 a | |
| 30 day | 7.62 ± 1.01 a |
| Color Properties | Storage Time | |||
|---|---|---|---|---|
| 0 Day | 10 Day | 20 Day | 30 Day | |
| L* | 41.93 ± 0.69 ab | 42.37 ± 1.20 a | 40.41 ± 0.22 b | 40.36 ± 0.26 b |
| a* | 7.06 ± 0.23 a | 6.75 ± 0.26 a | 7.16 ± 0.12 a | 6.75 ± 0.13 a |
| b* | 12.21 ± 0.05 a | 11.12 ± 0.20 c | 11.28 ± 0.04 bc | 11.54 ± 0.03 b |
| C | 14.10 ± 0.13 a | 13.01 ± 0.31 b | 13.36 ± 0.06 b | 13.37 ± 0.09 b |
| h | 59.95 ± 0.81 a | 58.76 ± 0.53 ab | 57.60 ± 0.48 b | 59.71 ± 0.42 a |
| ΔE | 1.91 ± 0.74 a | 1.87 ± 0.70 a | 1.78 ± 0.80 a | |
| Analyses | Storage Time | |||
|---|---|---|---|---|
| 0 Day | 10 Day | 20 Day | 30 Day | |
| TPC (mg GAE/100 mL) | 47.36 ± 1.01 a | 46.87 ± 0.48 a | 43.76 ± 0.53 b | 39.66 ± 0.67 c |
| TFC (mg CE/100 mL) | 16.69 ± 0.40 a | 15.54 ± 0.37 b | 13.01 ± 0.30 c | 12.28 ± 0.21 c |
| DPPH (% inhibition) | 56.06 ± 0.45 a | 55.66 ± 0.24 a | 54.03 ± 0.15 b | 53.05 ± 0.34 c |
| CUPRAC (% inhibition) | 58.84 ± 0.46 a | 58.44 ± 0.31 a | 56.73 ± 0.16 b | 55.78 ± 0.17 c |
| TAC (mg Cy-3-gly/100 mL) | 127.23 ± 1.57 a | 116.87 ± 2.20 b | 98.22 ± 1.14 c | 92.45 ± 1.08 d |
| Phenolic Compounds (μg/mL) | Storage Time | |||
|---|---|---|---|---|
| 0 Day | 10 Day | 20 Day | 30 Day | |
| Gallic acid | 23.886 ± 0.164 a | 25.137 ± 0.222 b | 23.191 ± 0.126 a | 23.403 ± 0.343 a |
| Protocatechuic acid | 1.146 ± 0.048 ab | 1.264 ± 0.017 bc | 1.350 ± 0.021 c | 1.047 ± 0.038 a |
| Catechin | ND | 0.289 ± 0.003 a | 0.234 ± 0 b | ND |
| Hydroxybenzoic acid | 0.024 ± 0.002 a | ND | ND | ND |
| Vanillic acid | ND | 0.041 ± 0.008 a | 0.021 ± 0.002 b | 0.001 ± 0 c |
| Gentisic acid | 0.237 ± 0.016 a | 0.240 ± 0.008 a | 0.161 ± 0.004 b | 0.114 ± 0.001 c |
| p-coumaric acid | ND | ND | ND | ND |
| Rutin | 1.350 ± 0.038 a | 1.255 ± 0.014 b | 1.073 ± 0.014 c | ND |
| Ferulic acid | ND | ND | ND | 0.217 ± 0.008 a |
| Naringin | 0.267 ± 0.008 a | 0.234 ± 0.006 b | 0.155 ± 0.004 c | ND |
| o-coumaric acid | ND | ND | ND | ND |
| Neohesperidin | 0.844 ± 0.012 a | ND | ND | ND |
| Coumarin | ND | ND | ND | ND |
| Resveratrol | 0.053 ± 0.002 a | ND | 0.001 ± 0 b | ND |
| Quercetin | ND | 1.237 ± 0.017 a | 0.828 ± 0.006 b | ND |
| trans-cinnamic acid | 3.214 ± 0.013 a | 0.758 ± 0.007 b | 0.252 ± 0.016 c | 0.079 ± 0.006 d |
| Total | 31.388 ± 0.413 a | 30.399 ± 0.380 a | 27.152 ± 0.016 b | 24.945 ± 0.247 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydoğdu, B.İ.; Tokatlı Demirok, N.; Yıkmış, S. Modeling of Sensory Properties of Poppy Sherbet by Turkish Consumers and Changes in Quality Properties during Storage Process. Foods 2023, 12, 3114. https://doi.org/10.3390/foods12163114
Aydoğdu Bİ, Tokatlı Demirok N, Yıkmış S. Modeling of Sensory Properties of Poppy Sherbet by Turkish Consumers and Changes in Quality Properties during Storage Process. Foods. 2023; 12(16):3114. https://doi.org/10.3390/foods12163114
Chicago/Turabian StyleAydoğdu, Behiye İncisu, Nazan Tokatlı Demirok, and Seydi Yıkmış. 2023. "Modeling of Sensory Properties of Poppy Sherbet by Turkish Consumers and Changes in Quality Properties during Storage Process" Foods 12, no. 16: 3114. https://doi.org/10.3390/foods12163114
APA StyleAydoğdu, B. İ., Tokatlı Demirok, N., & Yıkmış, S. (2023). Modeling of Sensory Properties of Poppy Sherbet by Turkish Consumers and Changes in Quality Properties during Storage Process. Foods, 12(16), 3114. https://doi.org/10.3390/foods12163114






