Effect of High-Pressure Processing on the Qualities of Carrot Juice during Cold Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Carrot Sample Preparation and Treatment Conditions
2.2. High-Pressure Processing
2.3. Microbial Analysis
2.4. Chemical Quality Analysis
2.5. Colour Analysis
2.6. Antioxidant Capacity Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Impact of Blanching and HPP on the Microbiological Quality of Carrot Juice
3.2. Impact of Blanching and HPP on the Chemical Quality of Carrot Juice
3.3. Impact of Blanching and HPP on the Colour of Carrot Juice
3.4. Impact of Blanching and HPP on the Antioxidant Properties of Carrot Juice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, L.; Xu, S.Y.; Zhang, W.B. Effect of enzymatic hydrolysis on the yield of cloudy carrot juice and the effects of hydrocolloids on color and cloud stability during ambient storage. J. Sci. Food Agric. 2005, 85, 505–512. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, J.I.; Park, J. Effects of a combined process of high-pressure carbon dioxide and high hydrostatic pressure on the quality of carrot juice. J. Food Sci. 2002, 67, 1827–1834. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Zhao, W.; Sun, Z.; Liao, X. Quality comparison of carrot juices processed by high-pressure processing and high-temperature short-time processing. Innov. Food Sci. Emerg. Technol. 2016, 33, 135–144. [Google Scholar] [CrossRef]
- Kim, H.; Gerber, L.H. Influence of processing on quality of carrot juice. Korean J. Food Sci. Technol. 1988, 20, 683–686. [Google Scholar]
- Jabbar, S.; Abid, M.; Hu, B.; Wu, T.; Hashim, M.M.; Lei, S.; Zhu, X.; Zeng, X. Quality of carrot juice as influenced by blanching and sonication treatments. LWT-Food Sci. Technol. 2014, 55, 16–21. [Google Scholar] [CrossRef]
- Bahceci, K.S.; Serpen, A.; Gokmen, V.; Acar, J. Study of lipoxygenase and peroxidase as indicator enzymes in green beans: Change of enzyme activity, ascorbic acid and chlorophylls during frozen storage. J. Food Eng. 2005, 66, 187–192. [Google Scholar] [CrossRef]
- Hiranvarachat, B.; Devahastin, S.; Chiewchan, N.; Raghavan, G.V. Structural modification by different pretreatment methods to enhance microwave-assisted extraction of α-carotene from carrots. J. Food Eng. 2013, 115, 190–197. [Google Scholar] [CrossRef]
- Barba, F.J.; Esteve, M.J.; Frigola, A. Physicochemical and nutritional characteristics of blueberry juice after high pressure processing. Food Res. Int. 2013, 50, 545–549. [Google Scholar] [CrossRef]
- Dede, S.; Alpas, H.; Bayýndýrlý, A. High hydrostatic pressure treatment and storage of carrot and tomato juices: Antioxidant activity and microbial safety. J. Sci. Food Agric. 2007, 87, 773–782. [Google Scholar] [CrossRef]
- Soysal, C.; Soylemez, Z.; Bozoglu, F. Effect of high hydrostatic pressure and temperature on carrot peroxidase inactivation. Eur. Food Res. Technol. 2004, 218, 152–156. [Google Scholar] [CrossRef]
- Patterson, M.F.; McKay, A.M.; Connolly, M.; Linton, M. The effect of high hydrostatic pressure on the microbiological quality and safety of carrot juice during refrigerated storage. Food Microbiol. 2012, 30, 205–212. [Google Scholar] [CrossRef]
- FDA. Bacteriological Analytical Manual; AOAC International: Arlington, VA, USA, 1998.
- Lisiewska, Z.; Kmiecik, W. Effect of storage period and temperature on the chemical composition and organoleptic quality of frozen tomato cubes. Food Chem. 2000, 70, 167–173. [Google Scholar] [CrossRef]
- Wang, H.Y.; Hu, X.S.; Chen, F.; Wu, J.H.; Zhang, Z.H.; Liao, X.J.; Wang, Z.F. Kinetic analysis of non-enzymatic browning in carrot juice concentrate during storage. Eur. Food Res. Technol. 2006, 223, 282. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.A.; Han, Z.; Sun, D.W. Effects of ultrasound treatments on quality of grapefruit juice. Food Chem. 2013, 141, 3201–3206. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sanusi, R.A.; Adebiyi, A.E. Beta carotene content of commonly consumed foods and soups in Nigeria. Pak. J. Nutr. 2009, 8, 1512–1516. [Google Scholar]
- Bhat, S.; Sharma, H.K. Combined effect of blanching and sonication on quality parameters of bottle gourd (Lagenaria siceraria) juice. Ultrason. Sonochem. 2016, 33, 182–189. [Google Scholar] [CrossRef]
- Gonçalves, E.; Pinheiro, J.; Abreu, M.; Brandão, T.; Silva, C.L. Carrot (Daucus carota L.) peroxidase inactivation, phenolic content and physical changes kinetics due to blanching. J. Food Eng. 2010, 97, 574–581. [Google Scholar] [CrossRef]
- Landl, A.; Abadias, M.; Sárraga, C.; Viñas, I.; Picouet, P. Effect of high pressure processing on the quality of acidified Granny Smith apple purée product. Innov. Food Sci. Emerg. Technol. 2010, 11, 557–564. [Google Scholar] [CrossRef]
- Puttongsiri, T.; Haruenkit, R. Changes in ascorbic acid, total polyphenol, phenolic acids and antioxidant activity in juice extracted from coated kiew wan tangerine during storage at 4, 12 and 20 °C. Nat. Sci. 2010, 44, 280–289. [Google Scholar]
- Barba, F.J.; Esteve, M.J.; Frigola, A. Ascorbic acid is the only bioactive that is better preserved by high hydrostatic pressure than by thermal treatment of a vegetable beverage. J. Agric. Food Chem. 2010, 58, 10070–10075. [Google Scholar] [CrossRef]
- Cao, J.; Yang, J.; Hou, Q.; Xu, H.; Zheng, Y.; Zhang, H.; Zhang, L. Assessment of bacterial profiles in aged, home-made Sichuan paocai brine with varying titratable acidity by PacBio SMRT sequencing technology. Food Control 2017, 78, 14–23. [Google Scholar] [CrossRef]
- Barba, F.; Cortés, C.; Esteve, M.; Frigola, A. Study of antioxidant capacity and quality parameters in an orange juice-milk beverage after high-pressure processing treatment. Food Bioproc. Tech. 2012, 5, 2222–2232. [Google Scholar] [CrossRef]
- Ma, T.; Tian, C.; Luo, J.; Zhou, R.; Sun, X.; Ma, J. Influence of technical processing units on polyphenols and antioxidant capacity of carrot (Daucus carrot L.) juice. Food Chem. 2013, 141, 1637–1644. [Google Scholar] [CrossRef]
- Xu, G.; Ye, X.; Chen, J.; Liu, D. Effect of heat treatment on the phenolic compounds and antioxidant capacity of citrus peel extract. J. Agric. Food Chem. 2007, 55, 330–335. [Google Scholar] [CrossRef]
- Sila, D.; Duvetter, T.; De Roeck, A.; Verlent, I.; Smout, C.; Van Loey, A. Texture changes of processed plant based foods: Potential role of novel technologies. Trends Food Sci. Technol. 2007, 19, 309–319. [Google Scholar] [CrossRef]
- de Ancos, B.; Sgroppo, S.; Plaza, L.; Cano, M.P. Possible nutritional and health-related value promotion in orange juice preserved by high-pressure treatment. J. Sci. Food Agric. 2002, 82, 790–796. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Barba, F.J.; Esteve, M.J.; Frígola, A. High pressure processing of fruit juice mix ture sweetened with Stevia rebaudiana Bertoni: Optimal retention of physical and nutritional quality. Innov. Food Sci. Emerg. Technol. 2013, 18, 48–56. [Google Scholar] [CrossRef]
- Huang, H.W.; Hsu, C.P.; Wang, C.Y. Healthy expectations of high hydrostatic pressure treatment in food processing industry. J. Food Drug Anal. 2020, 28, 1–13. [Google Scholar] [CrossRef]
- Nasiłowska, J.; Sokołowska, B.; Fonberg-Broczek, M. Escherichia coli and Listeria innocua stability in carrot juice preserved by high hydrostatic pressure. AIMS Agric. Food 2022, 7, 623–636. [Google Scholar] [CrossRef]
- Usaga, J.; Acosta, O.; Churey, J.J.; Padilla-Zakour, O.I.; Worobo, R.W. Evaluation of high pressure processing (HPP) inactivation of Escherichia coli O157: H7, Salmonella enterica, and Listeria monocytogenes in acid and acidified juices and beverages. Int. J. Food Microbiol. 2021, 339, 109034. [Google Scholar] [CrossRef]
- Pokhrel, P.J.; Boulet, C.; Yildiz, S.; Sablani, S.; Tang, J.; Barbosa-C´anovas, G.V. Effect of high hydrostatic pressure on microbial inactivation and quality changes in carrot-orange juice blends at varying pH. LWT-Food Sci. Technol. 2022, 159, 113219. [Google Scholar] [CrossRef]

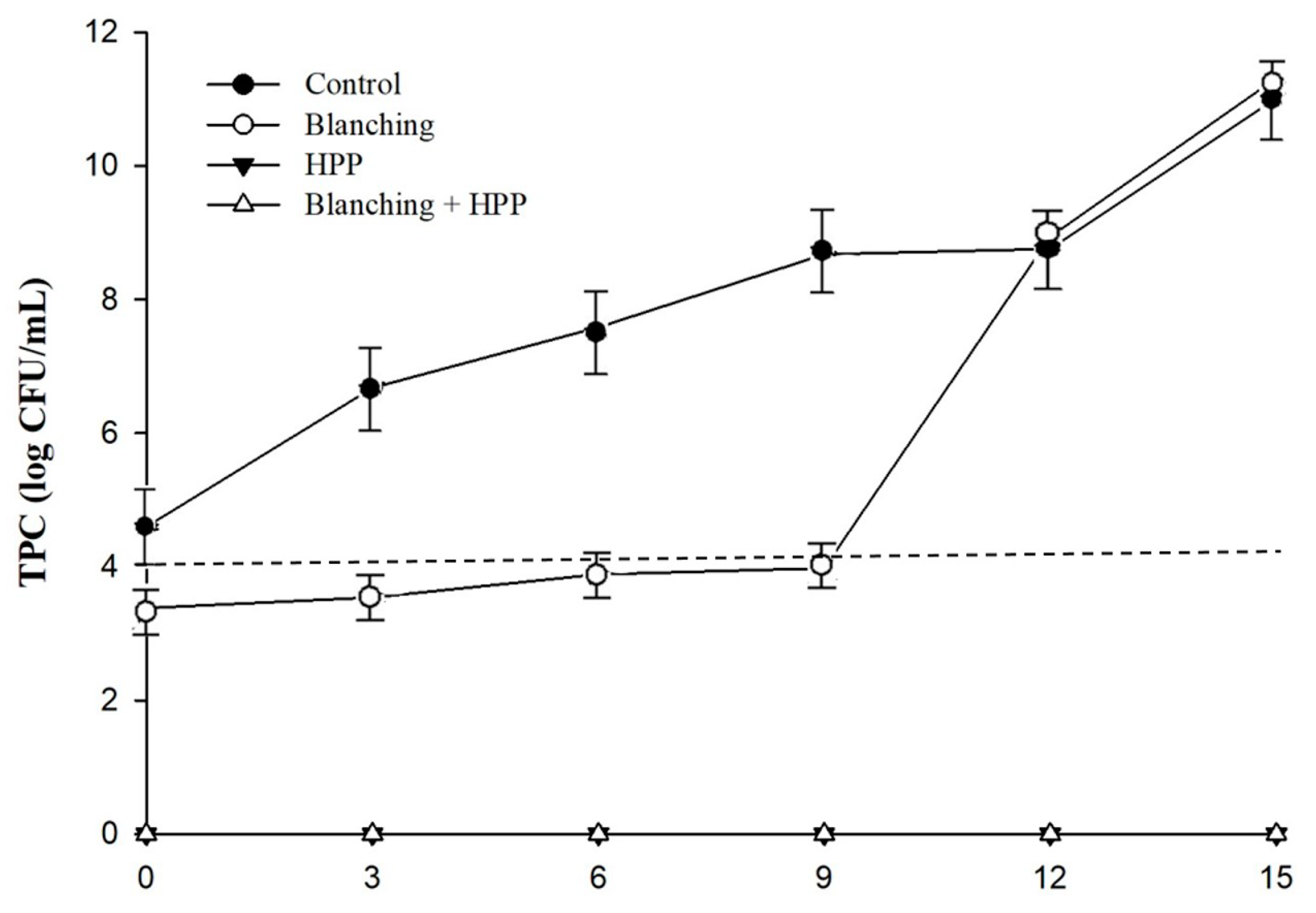

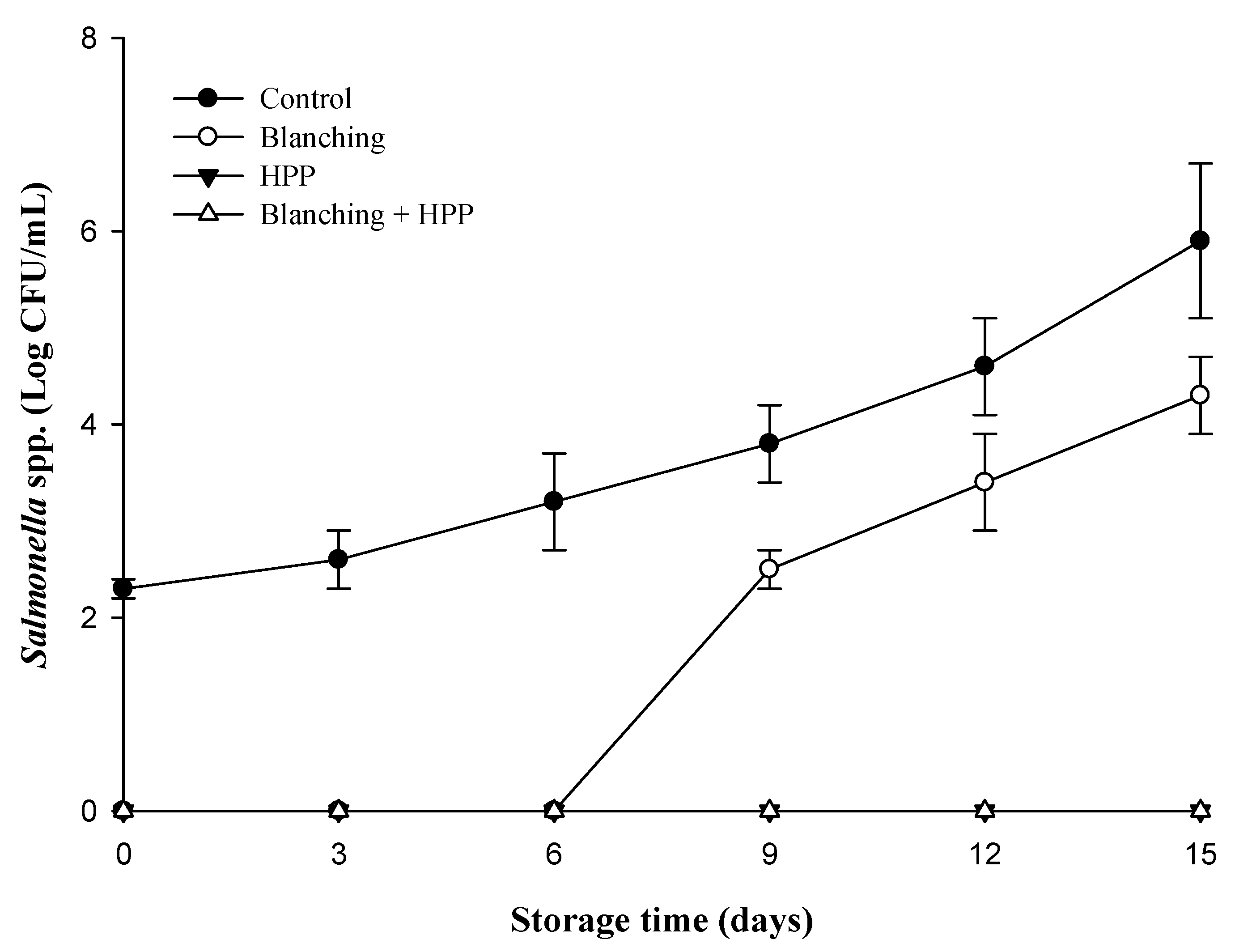
| Treatments | TPC (log CFU/mL) | Coliform (MPN/mL) | E. coli (MPN/mL) | Salmonella spp. (log CFU/mL) |
|---|---|---|---|---|
| Control | 4.60 ± 0.03 A* | 3.0 | <3.0 | 2.30 ± 0.10 |
| Blanching | 3.38 ± 0.05 B | 3.0 | <3.0 | <1.0 |
| HPP | <1.0 C | <3.0 | <3.0 | <1.0 |
| Blanching + HPP | <1.0 C | <3.0 | <3.0 | <1.0 |
| Quality Attributes | Treatments | Storage Time (Days) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | ||
| pH | Control | 6.15 ± 0.10 aA* | 6.23 ± 0.10 aA | 5.91 ± 0.10 cB | 5.82 ± 0.10 bC | 4.37 ± 0.10 dD | 4.27 ± 0.10 dE |
| Blanching | 5.84 ± 0.10 cB | 5.87 ± 0.10 cB | 6.06 ± 0.10 bA | 6.00 ± 0.13 aA | 5.60 ± 0.07 cC | 5.42 ± 0.10 cD | |
| HPP | 6.00 ± 0.10 bC | 6.12 ± 0.10 bB | 6.23 ± 0.05 aA | 6.15 ± 0.15 aAB | 6.17 ± 0.18 aAB | 5.81 ± 0.10 bC | |
| Blanching + HPP | 5.59 ± 0.10 dD | 5.76 ± 0.10 dC | 5.94 ± 0.08 cAB | 5.90 ± 0.10 bAB | 5.84 ± 0.25 bBC | 5.98 ± 0.20 aA | |
| TSS (°Brix) | Control | 2.03 ± 0.10 aA | 2.00 ± 0.10 aA | 1.97 ± 0.10 aAB | 1.83 ± 0.10 aB | 1.23 ± 0.10 bC | 1.23 ± 0.10 bC |
| Blanching | 2.07 ± 0.12 aA | 1.93 ± 0.10 abA | 1.97 ± 0.10 aA | 1.93 ± 0.10 aA | 1.57 ± 0.10 aB | 1.13 ± 0.10 bC | |
| HPP | 1.83 ± 0.10 bA | 1.88 ± 0.10 bcA | 1.93 ± 0.10 aA | 1.83 ± 0.10 aA | 1.63 ± 0.10 aB | 1.60 ± 0.10 aB | |
| Blanching + HPP | 1.73 ± 0.10 b AB | 1.82 ± 0.10 cAB | 1.90 ± 0.10 aA | 1.90 ± 0.10 aA | 1.63 ± 0.10 aB | 1.63 ± 0.10 aB | |
| TTA (%) | Control | 0.027 ± 0.001 bE | 0.025 ± 0.006 bE | 0.043 ± 0.003 aD | 0.058 ± 0.001 aC | 0.150 ± 0.011 aB | 0.162 ± 0.002 aA |
| Blanching | 0.027 ± 0.001 bC | 0.032 ± 0.002 aC | 0.032 ± 0.003 aC | 0.040 ± 0.002 bB | 0.042 ± 0.001 bB | 0.084 ± 0.001 bA | |
| HPP | 0.032 ± 0.001 aC | 0.037 ± 0.002 aB | 0.041 ± 0.003 aA | 0.040 ± 0.002 bAB | 0.038 ± 0.002 bB | 0.039 ± 0.001 cAB | |
| Blanching + HPP | 0.028 ± 0.002 bC | 0.037 ± 0.002 aB | 0.046 ± 0.003 aA | 0.041 ± 0.001 bAB | 0.040 ± 0.001 bB | 0.038 ± 0.001 cB | |
| Quality Attributes | Treatment | Storage Time (Days) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | ||
| Hunter colour | Control | 15.49 ± 0.40 bA* | 15.22 ± 0.20 aA | 11.70 ± 0.27 bB | 11.53 ± 0.07 cB | 10.04 ± 0.08 dC | 10.23 ± 0.18 cC |
| L | Blanching | 16.87 ± 0.07 aA | 14.12 ± 0.12 bB | 12.66 ± 1.02 bC | 12.72 ± 0.11 bC | 11.30 ± 0.13 cCD | 9.95 ± 0.22 cD |
| HPP | 14.70 ± 0.14 cA | 13.80 ± 0.11 bBC | 13.06 ± 0.22 bD | 13.43 ± 0.32 bCD | 13.19 ± 0.36 bD | 14.42 ± 0.06 aAB | |
| Blanching + HPP | 14.35 ± 0.35 cBC | 15.17 ± 0.13 aAB | 15.99 ± 0.23 aA | 15.08 ± 0.59 aB | 13.68 ± 0.05 aC | 13.86 ± 0.27 bC | |
| a | Control | 5.42 ± 0.71 bAB | 4.62 ± 0.07 bB | 5.85 ± 0.13 aA | 2.11 ± 0.13 dC | 0.64 ± 0.11 dD | 0.57 ± 0.31 cD |
| Blanching | 4.77 ± 0.11 bA | 3.75 ± 0.03 cB | 4.78 ± 0.14 cA | 2.87 ± 0.11 cC | 1.64 ± 0.12 cD | 0.51 ± 0.02 cE | |
| HPP | 6.22 ± 0.04 aA | 5.92 ± 0.02 aB | 5.62 ± 0.02 aC | 4.13 ± 0.13 bE | 3.40 ± 0.11 bF | 4.53 ± 0.18 aD | |
| Blanching + HPP | 6.41 ± 0.15 aA | 5.80 ± 0.14 aB | 5.20 ± 0.15 bC | 4.56 ± 0.15 aD | 4.21 ± 0.03 aDE | 3.86 ± 0.14 bE | |
| b | Control | 6.8 ± 0.22 bB | 7.73 ± 0.26 aA | 6.92 ± 0.09 aA | 4.02 ± 0.66 cB | 3.42 ± 0.34 cBC | 2.72 ± 0.18 cC |
| Blanching | 5.6 ± 0.09 cB | 7.19 ± 0.04 bA | 6.95 ± 0.31 aA | 5.22 ± 0.21 bB | 4.57 ± 0.03 bC | 2.07 ± 0.32 dD | |
| HPP | 8.56 ± 0.13 aA | 7.6 ± 0.13 aB | 7.04 ± 0.62 aBC | 6.35 ± 0.07 aC | 6.40 ± 0.17 aC | 7.06 ± 0.10 aBC | |
| Blanching + HPP | 8.44 ± 0.11 aA | 7.26 ± 0.21 bB | 5.97 ± 0.05 bD | 6.67 ± 0.36 aBC | 6.23 ± 0.35 aC | 5.96 ± 0.28 bD | |
| Browning | Control | 0.25 ± 0.01 aC | 0.27 ± 0.03 aC | 0.14 ± 0.01 cE | 0.19 ± 0.01 cD | 0.76 ± 0.01 aB | 1.20 ± 0.02 aA |
| degree | Blanching | 0.27 ± 0.01 aCD | 0.17 ± 0.01 bE | 0.22 ± 0.01 aD | 0.29 ± 0.01 aC | 0.37 ± 0.03 bB | 0.42 ± 0.01 bA |
| HPP | 0.08 ± 0.01 cB | 0.08 ± 0.01 cBC | 0.08 ± 0.01 dC | 0.07 ± 0.01 dD | 0.09 ± 0.02 dA | 0.08 ± 0.01 dBC | |
| Blanching + HPP | 0.12 ± 0.01 bD | 0.15 ± 0.01 bC | 0.18 ± 0.01 bB | 0.24 ± 0.01 bA | 0.20 ± 0.01 cA | 0.16 ± 0.01 cBC | |
| Quality Attributes | Treatment | Storage Time (Days) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | ||
| Total phenols (mg/100 mL) | Control | 1.37 ± 0.03 abE* | 1.83 ± 0.03 aD | 5.89 ± 0.29 aB | 7.53 ± 0.04 aA | 5.61 ± 0.01 aB | 4.61 ± 0.07 bC |
| Blanching | 0.94 ± 0.03 cE | 1.23 ± 0.06 bE | 2.16 ± 0.21 bD | 3.51 ± 0.40 bC | 5.13 ± 0.01 bB | 6.71 ± 0.06 aA | |
| HPP | 1.63 ± 0.03 aD | 1.77 ± 0.03 aC | 1.83 ± 0.03 cC | 2.23 ± 0.03 cA | 1.93 ± 0.01 dB | 2.17 ± 0.03 cA | |
| Blanching + HPP | 1.30 ± 0.24 bD | 1.34 ± 0.11 bD | 2.37 ± 0.03 bC | 3.11 ± 0.03 bB | 4.24 ± 0.01 cA | 4.59 ± 0.04 bA | |
| Scavenging DPPH radicals (%) | Control | 17.99 ± 0.88 bD | 23.04 ± 0.42 cC | 27.05 ± 0.96 bA | 27.16 ± 0.55 bA | 27.28 ± 0.16 bA | 25.05 ± 0.32 bB |
| Blanching | 29.69 ± 0.42 aC | 30.87 ± 0.27 aBC | 30.97 ± 0.42 aBC | 32.08 ± 0.16 aAB | 32.92 ± 0.82 aA | 30.46 ± 0.47 aC | |
| HPP | 17.03 ± 0.42 bA | 18.44 ± 1.25 dA | 18.31 ± 0.99 cA | 16.85 ± 1.40 cA | 15.94 ± 0.42 cA | 15.94 ± 0.42 cA | |
| Blanching + HPP | 28.69 ± 0.95 aA | 28.23 ± 0.83 bA | 27.78 ± 0.83 bA | 27.32 ± 0.71 bA | 26.87 ± 0.69 bA | 24.00 ± 0.69 bB | |
| β-carotene (μg/mL) | Control | 0.551 ± 0.008 bC | 0.592 ± 0.029 aB | 0.613 ± 0.017 abB | 0.667 ± 0.014 aA | 0.659 ± 0.004 bA | 0.647 ± 0.011 cA |
| Blanching | 0.563 ± 0.010 bC | 0.576 ± 0.002 abCD | 0.591 ± 0.003 bC | 0.583 ± 0.001 cD | 0.611 ± 0.005 dB | 0.731 ± 0.010 bA | |
| HPP | 0.605 ± 0.006 aD | 0.608 ± 0.009 aD | 0.642 ± 0.017 abC | 0.644 ± 0.001 bC | 0.683 ± 0.008 aB | 0.764 ± 0.009 aA | |
| Blanching + HPP | 0.556 ± 0.009 bD | 0.538 ± 0.009 bD | 0.611 ± 0.010 aC | 0.596 ± 0.004 cC | 0.642 ± 0.001 cB | 0.751 ± 0.005 abA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, C.-C.; Chien, H.-I.; Lee, Y.-C.; Lin, C.-S.; Hsiao, Y.-T.; Kuo, C.-H.; Yen, F.-L.; Tsai, Y.-H. Effect of High-Pressure Processing on the Qualities of Carrot Juice during Cold Storage. Foods 2023, 12, 3107. https://doi.org/10.3390/foods12163107
Hwang C-C, Chien H-I, Lee Y-C, Lin C-S, Hsiao Y-T, Kuo C-H, Yen F-L, Tsai Y-H. Effect of High-Pressure Processing on the Qualities of Carrot Juice during Cold Storage. Foods. 2023; 12(16):3107. https://doi.org/10.3390/foods12163107
Chicago/Turabian StyleHwang, Chiu-Chu, Hung-I Chien, Yi-Chen Lee, Chung-Saint Lin, Yun-Ting Hsiao, Chia-Hung Kuo, Feng-Lin Yen, and Yung-Hsiang Tsai. 2023. "Effect of High-Pressure Processing on the Qualities of Carrot Juice during Cold Storage" Foods 12, no. 16: 3107. https://doi.org/10.3390/foods12163107
APA StyleHwang, C.-C., Chien, H.-I., Lee, Y.-C., Lin, C.-S., Hsiao, Y.-T., Kuo, C.-H., Yen, F.-L., & Tsai, Y.-H. (2023). Effect of High-Pressure Processing on the Qualities of Carrot Juice during Cold Storage. Foods, 12(16), 3107. https://doi.org/10.3390/foods12163107









