Challenges in the Application of Circular Economy Models to Agricultural By-Products: Pesticides in Spain as a Case Study
Abstract
1. Introduction
Theoretical Background and Literature Review
2. Methods
2.1. Determination of Pesticide Presence in Major Crops in Spain
2.2. Determination of Adverse Effects of Pesticides
2.3. Safety Assessment of Citrus By-Product Revalorization
2.3.1. Selection of By-Products and Major Biomolecules
2.3.2. Harmonization of AA Results: ‘Water Content’ Factor
2.3.3. Biomass Weight Required to Fulfill Recommended Dietary Allowance of AA
2.3.4. Selection of Pesticides
2.3.5. Application of Processing Factor
3. Results and Discussion
3.1. Pesticide Content in the Most Abundant Agricultural Crops in Spain
3.2. Potatoes
| Crops | Pesticide Class | Pesticide Detected | MRLs (mg/kg) | Highest Detected Concentration (mg/kg) | Ref. |
|---|---|---|---|---|---|
| Potato | Insecticide | Deltamethrin | 0.3 | - | [88] |
| Chlorpyrifos | 0.01 | 0.170 | [92] | ||
| Fenoxycarb | 0.01 | 0.050 | [92] | ||
| Herbicide | Rimsulfuron | 0.01 | - | [89] | |
| Chlorpropham | 10 | 3.600 | [92] | ||
| Fungicide | Metalaxyl | 0.02 | 0.022 | [88] | |
| Carbendazim * | 0.1 | 0.010 | [92] | ||
| Tomato | Insecticide | Fenitrothion | 0.01 ** | - | |
| Chlorpyrifos | 0.1 | 0.730 | [94] | ||
| Metidathion * | 0.02 ** | - | [94] | ||
| Diazinon | 0.01 ** | - | [94] | ||
| Dimethoate | 0.01 ** | 0.130 | [94] | ||
| Fungicide | Carbendazim * | 0.3 | 0.400 | [92] | |
| Grapes | Herbicide | Fluometuron | 0.01 ** | 0.174 | [95] |
| Terbutylazine | 0.1 | 0.403 | [95] | ||
| Fungicide | Metalaxyl | 2 | 0.011 | [95] | |
| Triadimenol | 0.3 | 0.026 | [95] | ||
| Carbendazim * | 0.3 | 0.290 | [92] | ||
| Insecticide | Bifenthrin | 0.3 | 0.080 | [92] | |
| λ-cyhalothrin | 0.2 | 0.07 | [92] | ||
| Chlorpyrifos | 0.01 | 0.300 | [92] | ||
| Oranges | Fungicide | Carbendazim * | 0.2 | - | |
| Thiabendazole | 7.0 | 14.1 | [73] | ||
| Imazalil | 4.0 | 12.8 | [73] | ||
| Insecticide | λ-cyhalothrin | 0.2 | - | ||
| Carbofuran * | 0.01 ** | - | |||
| Chlorpyrifos | 1.5 | - | |||
| Strawberries | Fungicide | Carbendazim * | 0.1 ** | 0.100 | [92] |
| Tiabendazol | 0.05 ** | - | |||
| Imazalil | 0.05 ** | - | |||
| Thiophanate-methyl | 0.1 ** | 0.100 | [92] | ||
| Insecticide | λ-cyhalotrin | 0.01 ** | - | ||
| Carbofuran | 0.05 ** | - | |||
| Formethanate | 0.05 ** | 0.470 | [92] | ||
| Fenoxicarbp | 0.05 ** | 0.150 | [92] | ||
| Peppers | Insecticide | Bifentrin | 0.5 | 0.190 | [92] |
| λ-cyhalothrin | 0.1 | 0.080 | [96] | ||
| Cypermethrin | 0.5 | 0.400 | [96] | ||
| Acrinathrin | 0.02 | 0.600 | [96] | ||
| Fungicide | Thiophanate-methyl | 0.1 | 0.360 | [92] | |
| Olive | Fungicide | Chlorpyrifos | 0.01 ** | - | [73] |
| Oil | Iprodione | 0.01 ** | - | ||
| Chlorothalonil | 0.01 ** | - | |||
| Insecticide | Cypermethrin | 0.05 ** | - | ||
| Cereals | Insecticide | Deltametrin | 1.00 | 2.000 | [73] |
| (rice) | Fungicide | Isoprothiolane | 6.00 | - | |
| Carbendazim * | 0.01 ** | - |
3.3. Tomatoes
3.4. Grapes
3.5. Oranges
3.6. Strawberry and Other Berries
3.7. Peppers
3.8. Olives
| Crops | EFSA Report | Pesticide | Concentration/Status | Notes | Refs. |
|---|---|---|---|---|---|
| Potato | 2020 | Dithiocarbamate (thiram) | Approved isomers: alpha and zeta | [74] | |
| Dimethoate | Not approved | Grace period: June 2020 | |||
| Cypermethrin * | 0.039 mg/kg | ||||
| Fipronil, chlorpyrifos | Not approved, | Non-compliant sample | |||
| Tomato | 2019 | Chlorfenapy | >MRL. Not approved | Origin: EU and non-EU counties | [102] |
| Triadimefon | >MRL. Not approved | Origin: EU countries | |||
| Acephate, fipronil, permethrin | Not approved | Origin: non-EU counties | |||
| Dimethoate | Still approved (8 samples) | ||||
| Dithiocarbamates (ziram, maneb, propineb and thiram) | <MRL | ||||
| Chlorpyrifos | ≤MRL | ARfD exceedance 115% | |||
| Acetamiprid | ARfD exceedance | ||||
| 2020 | Chlorates | >MRL (10 samples) Not approved as pesticide | Decreasing tendency | ||
| 2020/2021 | Chlorfenapyr | Not approved | No import tolerance | [74,93] | |
| Bromide ion | Total chronic exposure: 8.1% ADI | ||||
| Spinosad | High frequency of detection (5.6%) | ||||
| 2021 | Abamectin, oxamyl, phosmet and dithiocarbamates (thiram) | Not targeted as food commodity | [93] | ||
| Grapes | 2021 | Cyhalothrin ** | Grace period: October 2022 Non-compliant: 2 samples (Cyprus) | [93] | |
| Acetamiprid | >MRL: 0.34–0.81 mg/kg | 19 samples | |||
| Indoxacarb | <MRL Approval not renewed | Grace period September 2022 | |||
| Omethoate | Never approved | Non-compliant sample (Cyprus) Mutagenic | |||
| Oranges | 2020 | Dimethoate, chlorpropham and linuron * | Not approved. | Non-compliant samples. Origin: EU countries | [74] |
| Bromopropylate, fenbutatin oxide, carbendazim, profenofos | Not approved. | Non-compliant samples. Origin: non-EU countries | |||
| Cypermethrin * | 0.12 mg/kg Not approved | ARfD and low toxicology: consideration for processing factor application | |||
| Dimethoate | >MRL (13 samples) | Grace period: 30 June 2020. Total chronic exposure: 19% ADI EFSA’s suggestion: keep monitoring | |||
| Dithiocarbamates | Exceedances rates: when illegal use | ||||
| Omeoathe | Never approved (3 samples) | Mutagenic | |||
| Thiabendazole | >MRL (3 samples) >ARfD (9 samples) | Applied a peeling factor of 0.17 | |||
| Berries | 2018 | 29 pesticides (1 Goji sample) | Highest frequency of multiple residues | [109] | |
| 2019 | Carbofuran | >MRL Non approved. | Origin: EU countries | [102] | |
| Dichlorvos | >MRL Non approved | Origin: other counties | |||
| Peppers | 2021 | Dithiocarbamates | >ARfD | Presence of precursors (ziram, propineb or thiram) | [93] |
| Cyhalothrin ** | >ARfD (8 samples) | Gamma isomer not authorized Approval expiration October 2022 | |||
| Acetamiprid | >ARfD 0.56 mg/kg 0.61 mg/kg | >ARfD (3 samples) >MRL (2 samples) | |||
| Indoxacarb | >ARfD/<MRL | Grace period: September 2022 | |||
| Bromide ion | 119 positive samples | ||||
| Omethoate | Never approved | Non-compliant samples (Uganda, Morocco) Mutagenic | |||
| Chlorfenapyr | Not approved | Origin: non-EU countries (Cambodia, Albania) | |||
| Ethephon | 5 positive samples (3 Poland, 2 Spain, 1 The Netherlands) | ||||
| Olives | 2012 | Chlorpyrifos and terbuthylazine | Chlorpyrifos in 14% of samples, terbuthylazine 12% | [113] | |
| Endosulfan, famoxadone, pendimethalin, fenthion, and terbuthylazine | >MRL | Highly frequent: fenthion and terbuthylazine | |||
| 2015 | Bromopropylate, chlorpyrifos, methyl-chlorpyrifos, iprodione, and fenthion | - | [109] | ||
| 2018 | Cypermethrin *, iprodione, chlorpyrifos, and chlorothalonil | - | [109] | ||
| Chlorothalonil | - | ||||
| Cypermethrin * | - | ||||
| Cereals | 2017 | Deltametrin | >ARfD (1.7 mg/kg) | 2017 MRL 2 mg/kg Current MRL 1 mg/kg | [114] |
| (rice) | Isoprothiolane, carbendazim | >MRL | Origin: EU countries Carbendazim: not approved | ||
| Acephate, hexaconazole, methamidophos, triazophos | >MRL | Origin: non-EU countries | |||
| 2020 | One sample: 15 pesticides | 134 multiresidue positive simples | [74] | ||
| Thiamethoxam | Not approved | Origin: EU countries | |||
| Tricyclazole, hexaconazole, thiamethoxam and chlorpyrifos | Not approved | Origin: non-EU countries | |||
| Bromide ion | >ARfD | Total chronic exposure: 5.8% ADI |
3.9. Cereals
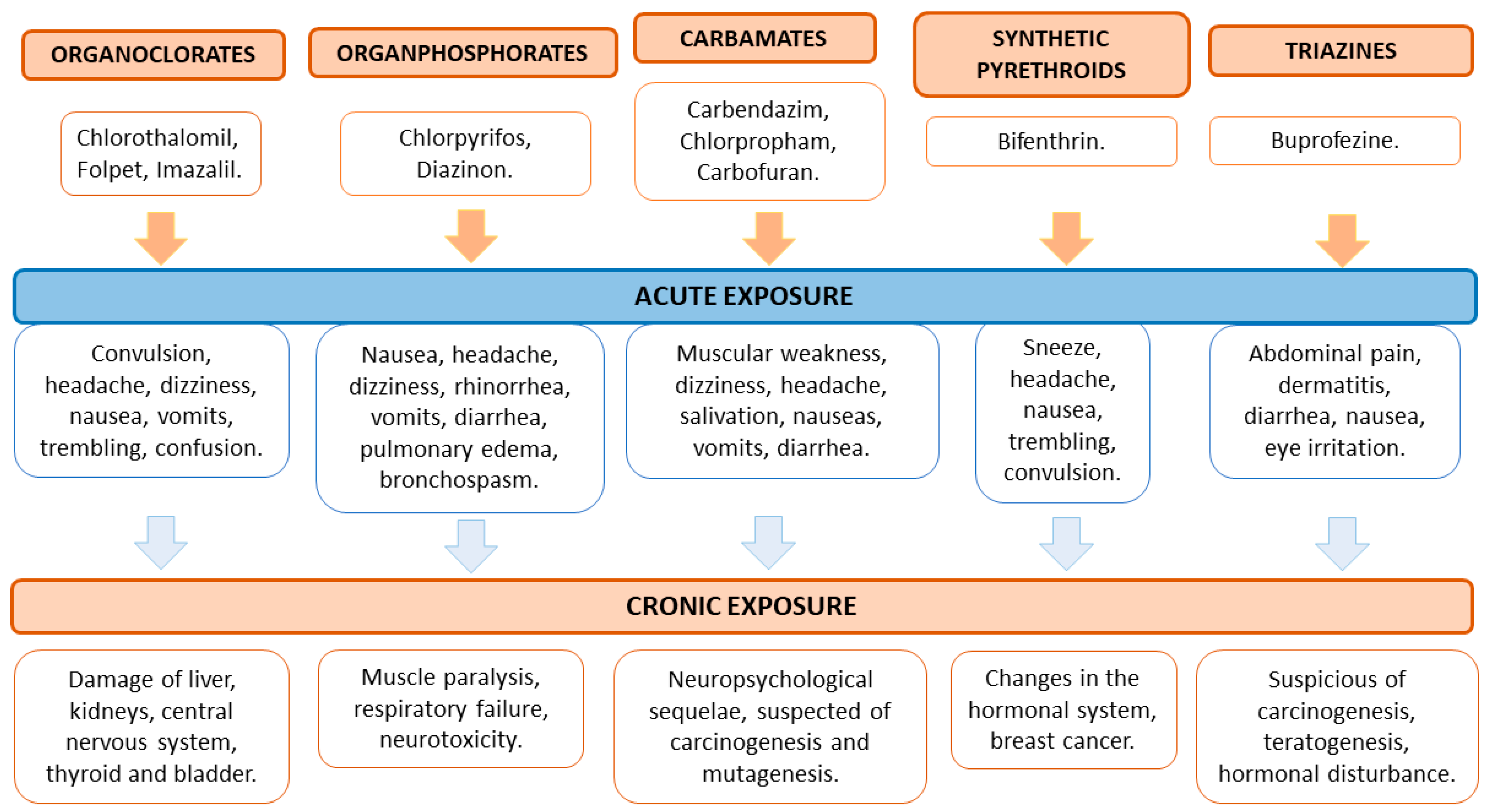
4. Adverse Effects of Pesticides on Human and Animal Health
5. Safety Assessment of Citrus Peels as a Matrix to Recover Ascorbic Acid: Case Study
| Citrus Peel (Species) | AA (mg/g) | AA (mg/g of fw) | Biomass (g) for 45 mg AA | Biomass (g) for 200 mg AA | Thiabendazole (mg/kg) Exposure | Imazalil (mg/kg) Exposure | Refs. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 45 mg AA/Day | PF | 200 mg AA/Day | PF | 45 mg AA/Day | PF | 200 mg AA/Day | PF | ||||||
| C. latifolia | 0.07 | 0.45 | 99.68 | 443.03 | 0.70 | 0.31 | 3.1 | 1.40 | 0.50 | 0.27 | 2.22 | 1.20 | [136] |
| 0.23 | 1.49 | 30.17 | 134.08 | 0.21 | 0.10 | 0.9 | 0.42 | 0.15 | 0.08 | 0.67 | 0.36 | [136] | |
| C. limon | 0.59 | 3.87 | 11.64 | 51.72 | 0.08 | 0.04 | 0.4 | 0.16 | 0.06 | 0.03 | 0.26 | 0.14 | [49] |
| 0.26 * | 1.75 | 25.77 | 114.52 | 0.18 | 0.08 | 0.8 | 0.36 | 0.13 | 0.07 | 0.57 | 0.31 | [137] | |
| C. máxima | 0.19 * | 1.28 | 35.25 | 156.69 | 0.25 | 0.11 | 1.1 | 0.49 | 0.14 | 0.08 | 0.63 | 0.34 | [138] |
| C. paradisi | 1.13 | 7.48 | 6.02 | 26.75 | 0.04 | 0.02 | 0.2 | 0.08 | 0.02 | 0.01 | 0.11 | 0.06 | [49] |
| C. reticulata | 0.48 | 3.14 | 14.32 | 63.66 | 0.10 | 0.05 | 0.4 | 0.20 | 0.07 | 0.04 | 0.32 | 0.17 | [136] |
| C. sinensis | 0.09 * | 0.59 | 76.69 | 340.87 | 0.54 | 0.24 | 2.4 | 1.07 | 0.31 | 0.17 | 1.36 | 0.74 | [50] |
| 0.93 * | 6.16 | 7.31 | 32.47 | 0.05 | 0.02 | 0.2 | 0.10 | 0.03 | 0.02 | 0.13 | 0.07 | [50] | |
| 0.43 | 2.85 | 15.78 | 70.15 | 0.11 | 0.05 | 0.5 | 0.22 | 0.06 | 0.03 | 0.28 | 0.15 | [136] | |
| 0.24 | 1.60 | 28.06 | 124.70 | 0.20 | 0.09 | 0.9 | 0.39 | 0.11 | 0.06 | 0.50 | 0.27 | [136] | |
| 1.10 | 7.29 | 6.18 | 27.45 | 0.04 | 0.02 | 0.2 | 0.09 | 0.02 | 0.01 | 0.11 | 0.06 | [49] | |
| 1.36 | 8.98 | 5.01 | 22.28 | 0.04 | 0.02 | 0.2 | 0.07 | 0.02 | 0.01 | 0.09 | 0.05 | [139] | |
| 0.01 | 0.09 | 503.19 | 2236.39 | 3.52 | 1.59 | 15.7 | 7.04 | 2.01 | 1.09 | 8.95 | 4.83 | [48] | |
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). The Future of Food and Agriculture-Alternative Pathways to 2050; FAO: Rome, Italy, 2018. [Google Scholar]
- FAO The Future of Food and Agriculture—Drivers and Triggers for Transformation. Futur. Food Agric. 2022.
- Almond, R.E.; Grooten, M.; Peterson, T. Living Planet Report 2020. Bending the Curve of Biodiversity Loss; Almond, R.E.A., Grooten, M., Petersen, T., Eds.; World Wildlife Fund: Gland, Switzerland, 2020; ISBN 978-2-940529-99-5. [Google Scholar]
- García-Oliveira, P.; Fraga-Corral, M.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Solutions for the Sustainability of the Food Production and Consumption System. Crit. Rev. Food Sci. Nutr. 2020, 62, 1765–1781. [Google Scholar] [CrossRef]
- Poponi, S.; Ruggieri, A.; Pacchera, F.; Arcese, G. The Circular Potential of a Bio-District: Indicators for Waste Management. Br. Food J. 2023; ahead-of-page. [Google Scholar] [CrossRef]
- European Commission; Directorate-General for Research and Innovation. Innovating for Sustainable Growth: A Bioeconomy for Europe; Publications Office: Brussels, Belgium, 2012.
- European Commission. Report from the Commission Supporting the Sustainable Development Goals across the World: The 2019 Joint Synthesis Report of the European Union and Its Member States; European Commission: Brussels, Belgium, 2019.
- United Nations. The Sustainable Development Agenda—United Nations Sustainable Development. Available online: https://www.un.org/sustainabledevelopment/development-agenda/ (accessed on 2 August 2023).
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions Closing the Loop—An EU Action Plan for the Circular Economy; European Commission: Brussels, Belgium, 2015.
- European Commision. Communication from the Commission: The European Green Deal; European Commission: Brussels, Belgium, 2019.
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions a Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System; European Commission: Brussels, Belgium, 2020.
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the Circular Economy: An Analysis of 114 Definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Kardung, M.; Cingiz, K.; Costenoble, O.; Delahaye, R.; Heijman, W.; Lovrić, M.; van Leeuwen, M.; M’Barek, R.; van Meijl, H.; Piotrowski, S.; et al. Development of the Circular Bioeconomy: Drivers and Indicators. Sustainability 2021, 13, 413. [Google Scholar] [CrossRef]
- D’Amato, D.; Korhonen, J. Integrating the Green Economy, Circular Economy and Bioeconomy in a Strategic Sustainability Framework. Ecol. Econ. 2021, 188, 107143. [Google Scholar] [CrossRef]
- Rana, R.L.; Bux, C.; Lombardi, M. Trends in Scientific Literature on the Environmental Sustainability of the Artichoke (Cynara cardunculus L. Spp.) Supply Chain. Br. Food J. 2023, 125, 2315–2332. [Google Scholar] [CrossRef]
- FAO. FAOSTAT: Crops and Livestock Products; FAO: Rome, Italy, 2022. [Google Scholar]
- FAO. FAOSTAT: Land Use; FAO: Rome, Italy, 2022. [Google Scholar]
- Valerio, A.; Edoardo, B.; Bettina, B.; Giovanni, B.; Kirsten, B.-U.; Carla, C.; Andrea, C.; Noemi, C.; Guido, C.; Valeria, D.E.L. Biomass Production, Supply, Uses and Flows in the European Union; Publications Office of the European Union: Luxembourg, 2023.
- del Estado, J. Ley 22/2011, de 28 de Julio, de Residuos y Suelos Contaminados; BOE: Madrid, Spain, 2011. [Google Scholar]
- del Estado, J. Ley 7/2022, de 8 de Abril, de Residuos y Suelos Contaminados Para Una Economía Circular; BOE: Madrid, Spain, 2022. [Google Scholar]
- Bandara, N.; Chalamaiah, M. Bioactives from Agricultural Processing By-Products. Encycl. Food Chem. 2018, 3, 472–480. [Google Scholar]
- Ramírez-García, R.; Gohil, N.; Singh, V. Recent Advances, Challenges, and Opportunities in Bioremediation of Hazardous Materials; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128139134. [Google Scholar]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Liu, H.; Zhao, T.; Meng, C.; Liu, Z.; Liu, X. Bioactive Compounds and in Vitro Antioxidant Activities of Peel, Flesh and Seed Powder of Kiwi Fruit. Int. J. Food Sci. Technol. 2018, 53, 2239–2245. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects - A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Rudra, S.G.; Nath, P.; Kaur, C.; Basu, S. Rheological, Storage Stability and Sensory Profiling of Low-Fat Yoghurt Fortified with Red Capsicum Carotenoids and Inulin. J. Food Process. Preserv. 2017, 41, e13067. [Google Scholar] [CrossRef]
- Güzel, M.; Akpınar, Ö. Valorisation of Fruit By-Products: Production Characterization of Pectins from Fruit Peels. Food Bioprod. Process. 2019, 115, 126–133. [Google Scholar] [CrossRef]
- Padalino, L.; Conte, A.; Lecce, L.; Likyova, D.; Sicari, V.; Pellicanò, T.M.; Poiana, M.; Del Nobile, M.A. Functional Pasta with Tomato By-Product as a Source of Antioxidant Compounds and Dietary Fibre. Czech J. Food Sci. 2017, 35, 48–56. [Google Scholar] [CrossRef]
- Pathania, S.; Kaur, N. Utilization of Fruits and Vegetable By-Products for Isolation of Dietary Fibres and Its Potential Application as Functional Ingredients. Bioact. Carbohydrates Diet. Fibre 2022, 27, 100295. [Google Scholar] [CrossRef]
- Comunian, T.A.; Silva, M.P.; Souza, C.J.F. The Use of Food By-Products as a Novel for Functional Foods: Their Use as Ingredients and for the Encapsulation Process. Trends Food Sci. Technol. 2021, 108, 269–280. [Google Scholar] [CrossRef]
- Manrique, A.G. Visión General Del Aprovechamiento de Residuos Cítricos Como Materia Prima de Biorrefinerías. Cuad. Del Tomás 2018, 10, 153–168. [Google Scholar]
- Superficies y Producciones Anuales de Cultivos; Minister of Agriculture, Fishing and Alimentation: Madrid, Spain, 2009.
- Ventosa, E.; Clemente, R.; Pereda, L. Gestión Integral de Residuos y Análisis Del Ciclo de Vida Del Sector Vinícola. De Residuos a Productos de Alto Valor Añadido; Natural Heritage Foundation: Madrid, Spain, 2011. [Google Scholar]
- Romero-Gámez, M.; Suárez-Rey, E.M. Environmental Footprint of Cultivating Strawberry in Spain. Int. J. Life Cycle Assess. 2020, 25, 719–732. [Google Scholar] [CrossRef]
- Hui, Y.H.; Chen, F.; Nollet, L.M.L.; Guiné, R.P.F.; Le Quéré, J.L.; Martín-Belloso, O.; Mínguez-Mosquera, M.I.; Paliyath, G.; Pessoa, F.L.P.; Sidhu, J.S.; et al. Handbook of Fruit and Vegetable Flavors; Wiley: Hoboken, NJ, USA, 2010; ISBN 9780470227213. [Google Scholar]
- Sansoucy, R. Los Subproductos del Olivar en la Alimentación Animal en la Cuenca del Mediterráneo; FAO: Rome, Italy, 1985. [Google Scholar]
- Moya López, A.J.; Mateo Quero, S. Aprovechamiento de Los Residuos Del Olivar. In El Olivar y Su Aceite; Editorial Agrícola: Madrid, Spain, 2013; pp. 336–341. [Google Scholar]
- Dieme, M.M.; Villot, A.; Gerente, C.; Andres, Y.; Diop, S.N.; Diawara, C.K. Sustainable Conversion of Agriculture Wastes into Activated Carbons: Energy Balance and Arsenic Removal from Water. Environ. Technol. 2017, 38, 353–360. [Google Scholar] [CrossRef][Green Version]
- Beltrán-Ramírez, F.; Orona-Tamayo, D.; Cornejo-Corona, I.; Luz Nicacio González-Cervantes, J.; de Jesús Esparza-Claudio, J.; Quintana-Rodríguez, E. Agro-Industrial Waste Revalorization: The Growing Biorefinery. Biomass Bioenergy Recent Trends Futur. Chall. 2019. [Google Scholar] [CrossRef]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic Agriculture Wastes as Biomass Feedstocks for Second-Generation Bioethanol Production: Concepts and Recent Developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef]
- Caporaso, N.; Formisano, D.; Genovese, A. Use of Phenolic Compounds from Olive Mill Wastewater as Valuable Ingredients for Functional Foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2829–2841. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, N.; Jafari, S.M.; Wani, T.A. Bioactive Profile, Dehydration, Extraction and Application of the Bioactive Components of Olive Leaves. Trends Food Sci. Technol. 2015, 42, 150–172. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Lage, M.P.; Simal-gándara, J. Valorization of By-Products from Olive Oil Industry and Added-Value Applications for Innovative Functional Foods. Food Res. Int. 2020, 109683. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Green Extraction of Grape Skin Phenolics by Using Deep Eutectic Solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Poveda, J.M.; Loarce, L.; Alarcón, M.; Díaz-Maroto, M.C.; Alañón, M.E. Revalorization of Winery By-Products as Source of Natural Preservatives Obtained by Means of Green Extraction Techniques. Ind. Crops Prod. 2018, 112, 617–625. [Google Scholar] [CrossRef]
- Negro, V.; Mancini, G.; Ruggeri, B.; Fino, D. Citrus Waste as Feedstock for Bio-Based Products Recovery: Review on Limonene Case Study and Energy Valorization. Bioresour. Technol. 2016, 214, 806–815. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ciriminna, R.; Carnaroglio, D.; Tamburino, A.; Cravotto, G.; Grillo, G.; Ilharco, L.M.; Pagliaro, M. Eco-Friendly Extraction of Pectin and Essential Oils from Orange and Lemon Peels. ACS Sustain. Chem. Eng. 2016, 4, 2243–2251. [Google Scholar] [CrossRef]
- Liew, S.S.; Ho, W.Y.; Yeap, S.K.; Sharifudin, S.A. Bin Phytochemical Composition and in Vitro Antioxidant Activities of Citrus Sinensis Peel Extracts. PeerJ 2018, 6, e5331. [Google Scholar] [CrossRef] [PubMed]
- Sir Elkhatim, K.A.; Elagib, R.A.A.; Hassan, A.B. Content of Phenolic Compounds and Vitamin C and Antioxidant Activity in Wasted Parts of Sudanese Citrus Fruits. Food Sci. Nutr. 2018, 6, 1214–1219. [Google Scholar] [CrossRef]
- Montero-Calderon, A.; Cortes, C.; Zulueta, A.; Frigola, A.; Esteve, M.J. Green Solvents and Ultrasound-Assisted Extraction of Bioactive Orange (Citrus Sinensis) Peel Compounds. Sci. Rep. 2019, 9, 16120. [Google Scholar] [CrossRef]
- Szabo, K.; Cătoi, A.F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing By-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef]
- Szabo, K.; Diaconeasa, Z.; Cătoi, A.F.; Vodnar, D.C. Screening of Ten Tomato Varieties Processing Waste for Bioactive Components and Their Related Antioxidant and Antimicrobial Activities. Antioxidants 2019, 8, 292. [Google Scholar] [CrossRef]
- Tumbas Šaponjac, V.; Gironés-Vilaplana, A.; Djilas, S.; Mena, P.; Ćetković, G.; Moreno, D.A.; Čanadanović-Brunet, J.; Vulić, J.; Stajčić, S.; Vinčić, M. Chemical Composition and Potential Bioactivity of Strawberry Pomace. RSC Adv. 2015, 5, 5397–5405. [Google Scholar] [CrossRef]
- Afrin, S.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Reboredo-Rodriguez, P.; Mezzetti, B.; Varela-López, A.; Giampieri, F.; Battino, M. Promising Health Benefits of the Strawberry: A Focus on Clinical Studies. J. Agric. Food Chem. 2016, 64, 4435–4449. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Peñas, E. Health Benefits of Oat: Current Evidence and Molecular Mechanisms. Curr. Opin. Food Sci. 2017, 14, 26–31. [Google Scholar] [CrossRef]
- Lee, D.S. Antioxidative Packaging System. In Innovations in Food Packaging, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; pp. 111–131. ISBN 9780123946010. [Google Scholar]
- Boufi, S. Biocomposites from Olive-Stone Flour: A Step Forward in the Valorization of the Solid Waste from the Olive-Oil Industry; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081009666. [Google Scholar]
- Embuscado, M.E. Spices and Herbs: Natural Sources of Antioxidants—A Mini Review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- González-Gómez, X.; Cambeiro-Pérez, N.; Martínez-Carballo, E.; Simal-Gándara, J. Screening of Organic Pollutants in Pet Hair Samples and the Significance of Environmental Factors. Sci. Total Environ. 2018, 625, 311–319. [Google Scholar] [CrossRef]
- Sanguos, C.L.; Suárez, O.L.; Martínez-Carballo, E.; Couce, M.L. Postnatal Exposure to Organic Pollutants in Maternal Milk in North-Western Spain. Environ. Pollut. 2023, 318, 120903. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Use of EFSA Pesticide Residue Intake Model (EFSA PRIMo Revision 3). EFSA J. 2018, 16, e05147. [Google Scholar] [CrossRef]
- World Health Organization. Preventing Disease through Healthy Environments: Exposure to Highly Hazardous Pesticides: A Major Public Health Concern; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- EFSA. Statement on the Available Outcomes of the Human Health Assessment in the Context of the Pesticides Peer Review of the Active Substance Chlorpyrifos. EFSA J. 2019, 17, e05809. [Google Scholar] [CrossRef]
- EFSA. Updated Statement on the Available Outcomes of the Human Health Assessment in the Context of the Pesticides Peer Review of the Active Substance Chlorpyrifos-Methyl. EFSA J. 2019, 17, e05908. [Google Scholar] [CrossRef]
- Bellisai, G.; Bernasconi, G.; Brancato, A.; Carrasco Cabrera, L.; Ferreira, L.; Giner, G.; Greco, L.; Jarrah, S.; Kazocina, A.; Leuschner, R.; et al. Reasoned Opinion on the Toxicological Properties and Maximum Residue Levels (MRLs) for the Benzimidazole Substances Carbendazim and Thiophanate-Methyl. EFSA J. 2021, 19, e06773. [Google Scholar] [CrossRef] [PubMed]
- Greene, T.; Salley, J.; Polcher, A.; Gentry, R.; Rücker, T. Literature Review on Pyrethroid Common Metabolites. EFSA Support. Publ. 2021, 18, 7064E. [Google Scholar] [CrossRef]
- Hernandez-Jerez, A.F.; Adriaanse, P.; Aldrich, A.; Berny, P.; Coja, T.; Duquesne, S.; Gimsing, A.L.; Marinovich, M.; Millet, M.; Pelkonen, O.; et al. Scientific Opinion of the Scientific Panel on Plant Protection Products and Their Residues (PPR Panel) on the Genotoxic Potential of Triazine Amine (Metabolite Common to Several Sulfonylurea Active Substances). EFSA J. 2020, 18, e06053. [Google Scholar] [CrossRef]
- EC. Commission Decision of 13 June 2007 Concerning the Non-Inclusion of Carbofuran in Annex I to Council Directive 91/414/EEC and the Withdrawal of Authorisations for Plant Protection Products Containing That Substance. Off J. Eur. Union 2007, L156/30, 1–2. [Google Scholar]
- Kuchheuser, P.; Birringer, M. Pesticide Residues in Food in the European Union: Analysis of Notifications in the European Rapid Alert System for Food and Feed from 2002 to 2020. Food Control 2022, 133, 108575. [Google Scholar] [CrossRef]
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of Phenolics from Citrus Peels: I—Solvent Extraction Method. Sep. Purif. Technol. 2006, 48, 182–188. [Google Scholar] [CrossRef]
- Zema, D.A.; Calabrò, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of Citrus Processing Waste: A Review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef]
- Carr, A.C.; Lykkesfeldt, J. Discrepancies in Global Vitamin C Recommendations: A Review of RDA Criteria and Underlying Health Perspectives. Crit. Rev. Food Sci. Nutr. 2021, 61, 742–755. [Google Scholar] [CrossRef]
- EFSA. The 2017 European Union Report on Pesticide Residues. EFSA J. 2019, 17, e05743. [Google Scholar]
- EFSA; Carrasco Cabrera, L.; Medina Pastor, P. The 2020 European Union Report on Pesticide Residues in Food. EFSA J. 2022, 20, e07215. [Google Scholar] [CrossRef]
- EFSA; Anastassiadou, M.; Bellisai, G.; Bernasconi, G.; Brancato, A.; Carrasco Cabrera, L.; Ferreira, L.; Greco, L.; Jarrah, S.; Kazocina, A.; et al. Modification of the Existing Maximum Residue Levels and Setting of Import Tolerances for Thiabendazole in Various Crops. EFSA J. 2021, 19, e06586. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2020/856 of 9 June 2020 Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Cyantraniliprole, Cyazofamid, Cyprodinil, Fenpyroximate, Flud; European Commission: Brussels, Belgium, 2020.
- Kruve, A.; Lamos, A.; Kirillova, J.; Herodes, K. Pesticide Residues in Commercially Available Oranges and Evaluation of Potential Washing Methods. Proc. Est. Acad. Sci. Chem. 2007, 56, 134–141. [Google Scholar]
- Fares, N.V.; Hassan, Y.A.A.; Hussein, L.A.; Ayad, M.F. Determination of Fungicides’ Residues and Their Degradation Kinetics in Orange Tree Fruits Using Liquid Chromatography—Tandem Mass Spectrometry Coupled with QuEChERS Method. Microchem. J. 2021, 168, 106376. [Google Scholar] [CrossRef]
- Cabras, P.; Schirra, M.; Pirisi, F.M.; Garau, V.L.; Angioni, A. Factors Affecting Imazalil and Thiabendazole Uptake and Persistence in Citrus Fruits Following Dip Treatments. J. Agric. Food Chem. 1999, 47, 3352–3354. [Google Scholar] [CrossRef] [PubMed]
- EC. Regulation No 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC. Off. J. Eur. Union 2005, 70, 1–16. [Google Scholar]
- Commission Implementing Regulation (EU). 2020/585 of 27 April 2020 Concerning a Coordinated Multiannual Control Programme of the Union for 2021, 2022 and 2023 to Ensure Compliance with Maximum Residue Levels of Pesticides and to Assess the Consumer Exposure to Pesticide Residues in and on Food of Plant and Animal Origin. OJL 2020, 135, 1–12. [Google Scholar]
- Standard Method EN 15662; Food of Plant Origin. Multimethod for the Determination of Pesticide Residues Using GC- and LC-Based Analysis Following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive e SPE—Modular QuEChERS-Method. European Committee for Standardization (CEN): Brussels, Belgium, 2018.
- Lehotay, S. AOAC Official Method 2007.01 Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate. J. AOAC Int. 2007, 90, 485–520. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Kolberg, D.I.; Benkenstein, A.; Eichhorn, E.; Zechmann, S.; Mack, D.; Wildgrube, C.; Sigalov, I.; Dörk, D.; Barth, A. Quick Method for the Analysis of Numerous Highly Polar Pesticides in Foods of Plant Origin via LC-MS/MS Involving Simultaneous Extraction with Methanol (QuPPe-Method). In EU Reference Laboratory for Pesticides Requiring Single Residue Methods (EURL-SRM); CVUA: Stuttgart, Germany, 2015. [Google Scholar]
- Kresse, M.; Drinda, H.; Romanotto, A.; Speer, K. Simultaneous Determination of Pesticides, Mycotoxins, and Metabolites as Well as Other Contaminants in Cereals by LC-LC-MS/MS. J. Chromatogr. B 2019, 1117, 86–102. [Google Scholar] [CrossRef]
- EURL-FV EURL-FV (2019-M33); Validation of MRM Pesticides from the Working Document SANCO/12745/2013 Using Three Multiresidue Methods (QuEChERS, Ethyl Acetate and Dutch Mini-Luke). European Commission: Brussels, Belgium, 2019; pp. 1–37.
- López, A.; Yusà, V.; Muñoz, A.; Vera, T.; Borràs, E.; Ródenas, M.; Coscollà, C. Risk Assessment of Airborne Pesticides in a Mediterranean Region of Spain. Sci. Total Environ. 2017, 574, 724–734. [Google Scholar] [CrossRef]
- Kurek, M.; Barchańska, H.; Turek, M. Degradation Processes of Pesticides Used in Potato Cultivations. Rev. Env. Contam. Toxicol. 2017, 242, 105–151. [Google Scholar]
- Douglas, L.; MacKinnon, G.; Cook, G.; Duncan, H.; Briddon, A.; Seamark, S. Determination of Chlorpropham (CIPC) Residues, in the Concrete Flooring of Potato Stores, Using Quantitative (HPLC UV/VIS) and Qualitative (GCMS) Methods. Chemosphere 2018, 195, 119–124. [Google Scholar] [CrossRef]
- EFSA. Reasoned Opinion on the Setting of Temporary Maximum Residue Levels for Chlorpropham in Potatoes. EFSA J. 2020, 18, 6061. [Google Scholar]
- López Pérez, G.C.; Arias-Estévez, M.; López-Periago, E.; Soto-González, B.; Cancho-Grande, B.; Simal-Gándara, J. Dynamics of Pesticides in Potato Crops. J. Agric. Food Chem. 2006, 54, 1797–1803. [Google Scholar] [CrossRef]
- Quijano, L.; Yusà, V.; Font, G.; Pardo, O. Chronic Cumulative Risk Assessment of the Exposure to Organophosphorus, Carbamate and Pyrethroid and Pyrethrin Pesticides through Fruit and Vegetables Consumption in the Region of Valencia (Spain). Food Chem. Toxicol. 2016, 89, 39–46. [Google Scholar] [CrossRef]
- EFSA; Carrasco Cabrera, L.; Di Piazza, G.; Dujardin, B.; Medina Pastor, P. The 2021 European Union Report on Pesticide Residues in Food. EFSA J. 2023, 21, e07939. [Google Scholar] [CrossRef]
- Cortés, J.M.; Ana Vázquez, A.; Santa-María, G.; Blanch, G.P.; Villén, J. Pesticide Residue Analysis by RPLC–GC in Lycopene and Other Carotenoids Obtained from Tomatoes by Supercritical Fluid Extraction. Food Chem. 2009, 113, 280–284. [Google Scholar] [CrossRef]
- Pose-Juan, E.; Sánchez-Martín, M.J.; Andrades, M.S.; Rodríguez-Cruz, M.S.; Herrero-Hernández, E. Pesticide Residues in Vineyard Soils from Spain: Spatialand Temporal Distributions. Sci. Total Environ. 2015, 514, 351–358. [Google Scholar] [CrossRef]
- Murcia-Morales, M.; Cutillas, V.; Fernández-Alba, A.R. Supercritical Fluid Chromatography and Gas Chromatography Coupled to Tandem Mass Spectrometry for the Analysis of Pyrethroids in Vegetable Matrices: A Comparative Study. J. Agric. Food Chem. 2019, 67, 12626–12632. [Google Scholar] [CrossRef]
- Panthee, D.; Chen, F. Genomics of Fungal Disease Resistance in Tomato. Curr. Genom. 2009, 11, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kumar Singh, V.K.; Kishore Singh, A.K.; Kumar, A. Disease Management of Tomato through PGPB: Current Trends and Future Perspective. 3 Biotech 2017, 7, 255. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2020/1085 of 23 July 2020 Amending Annexes II and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Chlorpyrifos and Chlorpyrifos-Methyl in or on Certain Product; European Commission: Brussels, Belgium, 2020.
- European Commission. Commission Regulation (EU) 2021/155 of 9 February 2021 Amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Carbon Tetrachloride, Chlorothalonil, Chlorpropham, Di; European Commission: Brussels, Belgium, 2021.
- Fillâtre, Y.; Gray, F.-X.; Roy, C. Pesticides in Essential Oils: Occurrence and Concentration in Organic and Conventional Orange Essential Oils from Eleven Geographical Origins. Anal. Chim. Acta 2017, 992, 55–66. [Google Scholar] [CrossRef]
- EFSA; Carrasco Cabrera, L.; Medina Pastor, P. The 2019 European Union Report on Pesticide Residues in Food. EFSA J. 2021, 19, e06491. [Google Scholar] [CrossRef]
- González-Rodríguez, R.M.; Cancho-Grande, B.; Simal-Gándara, J. Multiresidue Determination of 11 New Fungicides in Grapes and Wines by Liquid–Liquid Extraction/Clean-up and Programmable Temperature Vaporization Injection with Analyte Protectants/Gas Chromatography/Ion Trap Mass Spectrometry. J. Chromatogr. A 2009, 1216, 6033–6042. [Google Scholar] [CrossRef] [PubMed]
- Payá, P.; Mulero, J.; Oliva, J.; Cámara, M.A.; Barba, A. Influence of the Matrix in Bioavailability of Flufenoxuron, Lufenuron, Pyriproxyfen and Fenoxycarb Residues in Grapes and Wine. Food Chem. Toxicol. 2013, 60, 419–423. [Google Scholar] [CrossRef]
- Martínez, F.; Flores, F.; Vázquez-Ortiz, E.; López-Medina, J. Persistence of Trichoderma Asperellum Population in Strawberry Soilless Culture Growing Systems. Acta Hortic. 2009, 842, 1003–1006. [Google Scholar] [CrossRef]
- López-Aranda, J.M.; Miranda, L.; Soria, C.; Domínguez, P.; Pérez-Jiménez, R.M.; Martín-Sanchez, P.M.; Talavera, M.; Romero, F.; De los Santos, B.; Medina, J.J. Strawberry Production in Spain: Alternatives to MB, 2008 Results. In Proceedings of the 2008 Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions, Orlando, FL, USA, 11–14 November 2008. [Google Scholar]
- Lozowicka, B.; Jankowska, M.; Hrynko, I.; Kaczynski, P. Removal of 16 Pesticide Residues from Strawberries by Washing with Tap and Ozone Water, Ultrasonic Cleaning and Boiling. Environ. Monit. Assess. 2016, 188, 51. [Google Scholar] [CrossRef]
- EFSA. Peer Review of the Pesticide Risk Assessment for Bees for the Active Substance Clothianidin Considering the Uses as Seed Treatments and Granules. EFSA J. 2018, 16, 5177. [Google Scholar]
- EFSA; Medina-Pastor, P.; Triacchini, G. The 2018 European Union Report on Pesticide Residues in Food. EFSA J. 2020, 18, e06057. [Google Scholar]
- Dáder, B.; Colomer, I.; Adán, A.; Medina, P.; Viñuela, E. Compatibility of Early Natural Enemy Introductions in Commercial Pepper and Tomato Greenhouses with Repeated Pesticide Applications. Insect Sci. 2020, 27, 1111–1124. [Google Scholar] [CrossRef]
- Giménez–Moolhuyzen, M.; van der Blom, J.; Lorenzo–Mínguez, P.; Cabello, T.; Crisol–Martínez, E. Photosynthesis Inhibiting Efects of Pesticides on Sweet Pepper Leaves. Insects 2020, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- EC Regulation No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union 2009, L 309, 1–50.
- EFSA. The 2012 European Union Report on Pesticide Residues in Food. EFSA J. 2014, 12, 3942. [Google Scholar]
- AESAN. Pesticide Residue Control Results. In National Summary Report 2018; Spanish Agency for Food Safety and Nutrition: Madrid, Spain, 2018. [Google Scholar]
- Cozma, P.; Apostol, L.C.; Hlihor, R.M.; Simion, I.M.; Gavrilescu, M. Overview of Human Health Hazards Posed by Pesticides in Plant Products. In Proceedings of the 2017 E-Health and Bioengineering Conference, EHB 2017, Sinaia, Romania, 22–24 June 2017; pp. 293–296. [Google Scholar]
- Gagnon-Chauvin, A.; Bastien, K.; Saint-Aour, D. Environmental Toxic Agents: The Impact of Heavy Metals and Organochlorides on Brain Development. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 423–442. [Google Scholar]
- Jokanović, M. Neurotoxic Effects of Organophosphorus Pesticides and Possible Association with Neurodegenerative Diseases in Man: A Review. Toxicology 2018, 410, 125–131. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Chunhua, W.; Xiaojuan, Q.; Shuai, J.; Zhou, T.; Xiao, H.; Li, W.; Lu, D.; Feng, C.; et al. Early-Life Carbamate Exposure and Intelligence Quotient of Seven-Year-Old Children. Environ. Int. 2020, 145, 106105. [Google Scholar] [CrossRef]
- Zhu, Q.; Yang, Y.; Zhong, Y.; Lao, Z.; O’Neill, P.; Hong, D.; Zhang, K.; Zhao, S. Synthesis, Insecticidal Activity, Resistance, Photodegradation and Toxicity of Pyrethroids (A Review). Chemosphere 2020, 254, 126779. [Google Scholar] [CrossRef]
- Richardson, J.R.; Fitsanakis, V.; Westerink, R.H.S.; Kanthasamy, A.G. Neurotoxicity of Pesticides. Acta Neuropathol. 2019, 138, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Berny, P. Pesticides and the Intoxication of Wild Animals. J. Vet. Pharmacol. Ther. 2007, 30, 93–100. [Google Scholar] [CrossRef]
- Bertero, A.; Chiari, M.; Vitale, N.; Zanoni, M.; Faggionato, E.; Biancardi, A.; Caloni, F. Types of Pesticides Involved in Domestic and Wild Animal Poisoning in Italy. Sci. Total Environ. 2020, 707, 136129. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of Pesticides Use in Agriculture: Their Benefits and Hazards. Interdiscip. Toxicol. 2009, 2, 1. [Google Scholar] [CrossRef]
- Harada, T.; Takeda, M.; Kojima, S.; Tomiyama, N. Toxicity and Carcinogenicity of Dichlorodiphenyltrichloroethane (DDT). Toxicol. Res. 2016, 32, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Crépet, A.; Luong, T.M.; Baines, J.; Boon, P.E.; Ennis, J.; Kennedy, M.; Massarelli, I.; Miller, D.; Nako, S.; Reuss, R.; et al. An International Probabilistic Risk Assessment of Acute Dietary Exposure to Pesticide Residues in Relation to Codex Maximum Residue Limits for Pesticides in Food. Food Control 2021, 121, 107563. [Google Scholar] [CrossRef]
- Panel, E.; Products, D. Guidance on the Scientific Requirements for Health Claims Related to the Immune System, the Gastrointestinal Tract and Defence against Pathogenic Microorganisms. EFSA Panel Diet. Prod. Nutr. Allerg. NDA 2016, 14, 4369. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Dietary Reference Values for Nutrients Summary Report. EFSA Support. Publ. 2017, 14, e15121. [Google Scholar] [CrossRef]
- Besil, N.; Rezende, S.; Alonzo, N.; Cesio, M.V.; Rivas, F.; Heinzen, H. Analytical Methods for the Routinely Evaluation of Pesticide Residues in Lemon Fruits and by Products. SN Appl. Sci. 2019, 1, 618. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Mendiola, J.A.; Rodríguez-Delgado, M.Á.; Ibáñez, E.; Cifuentes, A. Safety Assessment of Citrus and Olive By-Products Using a Sustainable Methodology Based on Natural Deep Eutectic Solvents. J. Chromatogr. A 2022, 1669, 462922. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Aparicio García, N.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Velázquez Escobar, F.; Blanch, G.P.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of Coffee By-Products as Novel Food Ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef]
- Sójka, M.; Miszczak, A.; Sikorski, P.; Zagibajło, K.; Karlińska, E.; Kosmala, M. Pesticide Residue Levels in Strawberry Processing By-Products That Are Rich in Ellagitannins and an Assessment of Their Dietary Risk to Consumers. NFS J. 2015, 1, 31–37. [Google Scholar] [CrossRef]
- Jean, S.; Benoît, N.M.; Bosco, T.J.; Srivastava, A.K.; Srivastava, L.P. Contamination of Cowpea and By-Products by Organophosphorous Pesticide Residues in Ngaoundere Markets: Dietary Risk Estimation and Degradation Study. Aust. J. Fr. Stud. 2013, 7, 92–102. [Google Scholar]
- Chaudry, M.M.; Nelson, A.I.; Perkins, E.G. Distribution of Chlorinated Pesticides in Soybeans, Soybean Oil, and Its by-Products during Processing. J. Am. Oil Chem. Soc. 1978, 55, 851–853. [Google Scholar] [CrossRef]
- Pereira, A.G.; Fraga-Corral, M.; Garcia-Oliveira, P.; Otero, P.; Soria-Lopez, A.; Cassani, L.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Single-Cell Proteins Obtained by Circular Economy Intended as a Feed Ingredient in Aquaculture. Foods 2022, 11, 2831. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, S.; Garcia-Garcia, G.; Duong, L.; Swainson, M.; Martindale, W. Codesign of Food System and Circular Economy Approaches for the Development of Livestock Feeds from Insect Larvae. Foods 2021, 10, 1701. [Google Scholar] [CrossRef] [PubMed]
- de Moraes Barros, H.R.; de Castro Ferreira, T.A.P.; Genovese, M.I. Antioxidant Capacity and Mineral Content of Pulp and Peel from Commercial Cultivars of Citrus from Brazil. Food Chem. 2012, 134, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, A.; Biradar, R.K. Comparative Evaluation of Soxhlet and Ultrasonics on the Structural Morphology and Extraction of Bioactive Compounds of Lemon (Citrus limon L.) Peel. J. Food Chem. Nanotechnol. 2019, 5, 56–64. [Google Scholar] [CrossRef]
- Ani, P.N.; Abel, H.C. Nutrient, Phytochemical, and Antinutrient Composition of Citrus Maxima Fruit Juice and Peel Extract. Food Sci. Nutr. 2018, 6, 653–658. [Google Scholar] [CrossRef]
- Hernández-Carranza, P.; Ávila-Sosa, R.; Guerrero-Beltrán, J.A.; Navarro-Cruz, A.R.; Corona-Jiménez, E.; Ochoa-Velasco, C.E. Optimization of Antioxidant Compounds Extraction from Fruit By-Products: Apple Pomace, Orange and Banana Peel. J. Food Process. Preserv. 2016, 40, 103–115. [Google Scholar] [CrossRef]
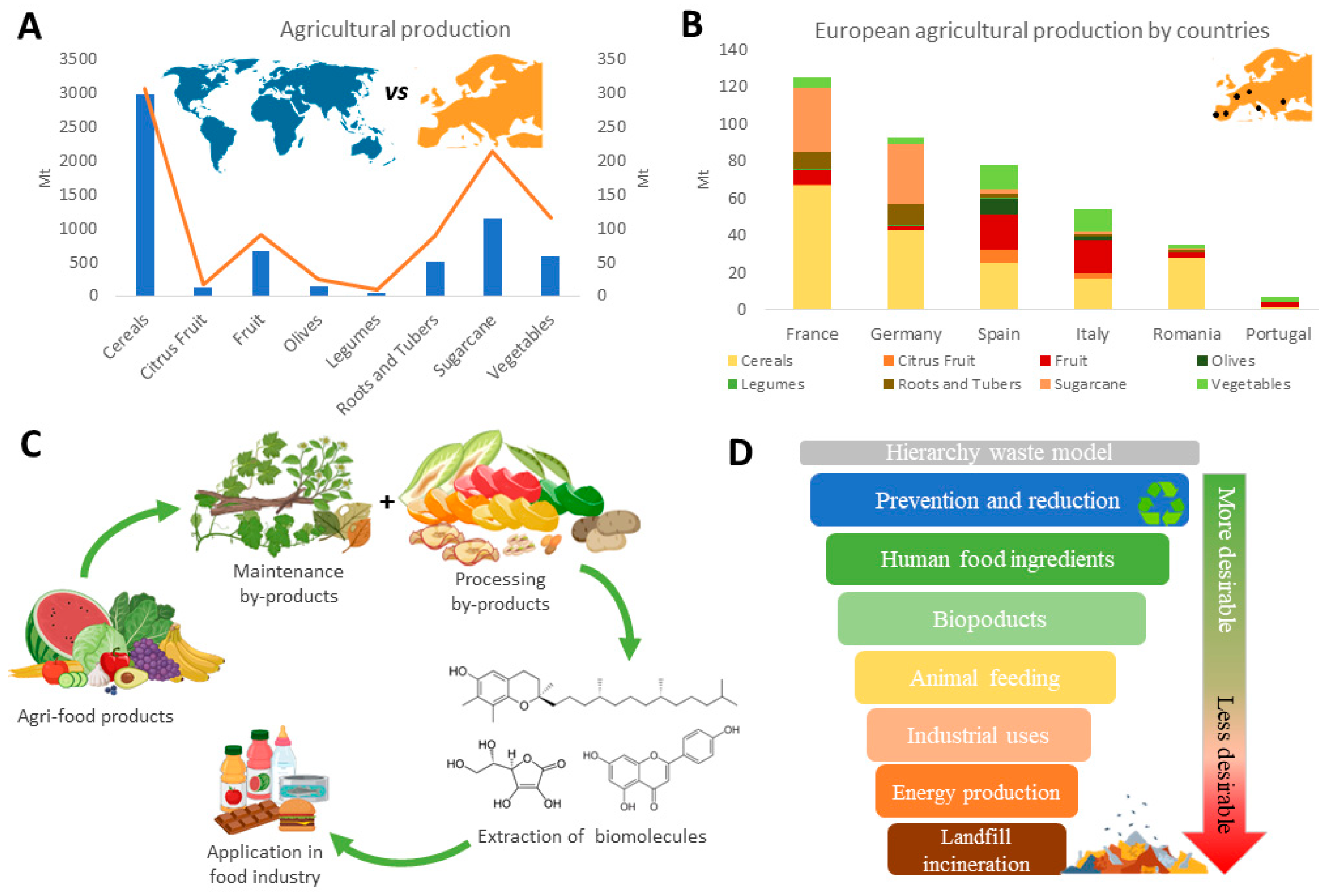

| Crops | Production | Residues | By-Products | Application | Refs. | ||
|---|---|---|---|---|---|---|---|
| World | Spain | World | Spain | ||||
| Orange | 70 mT | 3.9 mT | 30 mT | 1–1.8 mT | Pulp, skin, seed | Bio-refinery, bio-compounds, bio-composites, essential oils, bioethanol | [31,32] |
| Grapes | 279 mhL | 44.4 mhL | 18 mhL | 2–3 mhL | Pomace, lees, sludge, scrape | Bio-refinery, bio-compounds | [33] |
| Strawberry | 0.45 mT | 0.36 mT | 0.05 mT | 0.04 mT | Pulp, skin, seeds | Bio-compounds, fiber, colorants, bioethanol | [34] |
| Red berries | 18 mT | 0.45 mT | 1.8 mT | 0.05 mT | |||
| Peppers | 34,000 mT | 1082 mT | - | - | Seeds, stalks | Biofuel, bio-compounds, fertilizer | [35] |
| Olives and olive oil | 10 mT | 6.3 mT | 1.3 mT | 0.24 mT | Maintenance wastes, pulp, leaves, watermill | Biomass, fertilizer, bio-compounds, plastics | [36,37] |
| Cereals | 1370 mT | 23 mT | ±50% | ±50% | Stalk, peel, pulp, skin, seeds. | Livestock, paper, construction, fuels, fiber, bio-compounds, colorants | [38,39,40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otero, P.; Echave, J.; Chamorro, F.; Soria-Lopez, A.; Cassani, L.; Simal-Gandara, J.; Prieto, M.A.; Fraga-Corral, M. Challenges in the Application of Circular Economy Models to Agricultural By-Products: Pesticides in Spain as a Case Study. Foods 2023, 12, 3054. https://doi.org/10.3390/foods12163054
Otero P, Echave J, Chamorro F, Soria-Lopez A, Cassani L, Simal-Gandara J, Prieto MA, Fraga-Corral M. Challenges in the Application of Circular Economy Models to Agricultural By-Products: Pesticides in Spain as a Case Study. Foods. 2023; 12(16):3054. https://doi.org/10.3390/foods12163054
Chicago/Turabian StyleOtero, Paz, Javier Echave, Franklin Chamorro, Anton Soria-Lopez, Lucia Cassani, Jesus Simal-Gandara, Miguel A. Prieto, and Maria Fraga-Corral. 2023. "Challenges in the Application of Circular Economy Models to Agricultural By-Products: Pesticides in Spain as a Case Study" Foods 12, no. 16: 3054. https://doi.org/10.3390/foods12163054
APA StyleOtero, P., Echave, J., Chamorro, F., Soria-Lopez, A., Cassani, L., Simal-Gandara, J., Prieto, M. A., & Fraga-Corral, M. (2023). Challenges in the Application of Circular Economy Models to Agricultural By-Products: Pesticides in Spain as a Case Study. Foods, 12(16), 3054. https://doi.org/10.3390/foods12163054











