Microwave Hydrodiffusion and Gravity Extraction of Vitamin C and Antioxidant Compounds from Rosehips (Rosa canina L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Sample Extraction
2.2.1. Microwave Hydrodiffusion and Gravity (MHG) Extraction
2.2.2. Conventional Solvent Extraction
2.3. Design of Experiment (DoE)
2.3.1. Central Composite Design (CCD)
- Extraction yield % (Yld), calculated as the weight of lyophilized extract per 100 g of fresh-weight fruit;
- Total phenolic content (TPC), assessed as described in Section 2.4.1;
- Total flavonoid content (TFC), assessed as reported in Section 2.4.2;
- Total anthocyanin content (TAC), assessed as described in Section 2.4.3;
- Radical scavenging activity (DPPH), assessed as reported in Section 2.4.4;
- Vitamin C content (Vit. C), determined as described in Section 2.4.5.
2.4. Dry Extract Analysis
2.4.1. Total Phenolic Content (TPC)
2.4.2. Total Flavonoid Content (TFC)
2.4.3. Total Anthocyanin Content (TAC)
2.4.4. Radical Scavenging Activity
2.4.5. Vitamin C Content
3. Results and Discussion
3.1. DoE Analysis of MHG Process
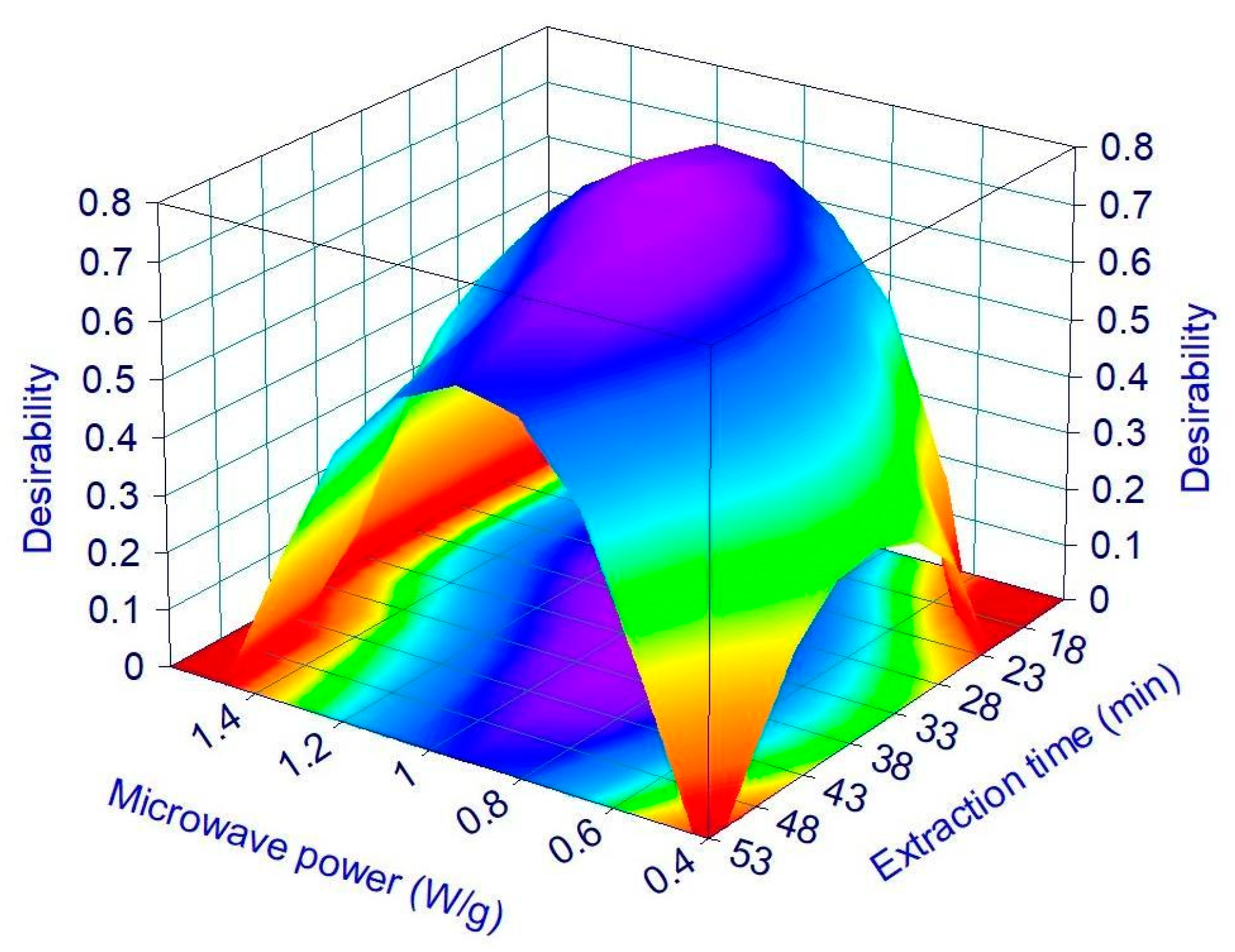
3.2. MHG Optimization
3.3. MHG vs. Conventional Extractions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arslan, E.S.; Akyol, A.; Örücü, Ö.K.; Sarıkaya, A.G. Distribution of rose hip (Rosa canina L.) under current and future climate conditions. Reg. Environ. Chang. 2020, 20, 107. [Google Scholar] [CrossRef]
- Enescu, C. Which shrub species should be used for the establishment of field shelterbelts in Romania? Sci. Pap. Ser. A Agron. 2018, 61, 464–469. [Google Scholar]
- Karakaya, T.; Yücel, E. Potential distribution modelling and mapping of dog rose (Rosa canina L.) in the nur mountains of gazİantep district, turkey. Appl. Ecol. Environ. Res. 2021, 19, 2741–2760. [Google Scholar] [CrossRef]
- Soare, R.; Bonea, D.; Iancu, P.; Niculescu, M. Biochemical and Technological Properties of Rosa canina L. Fruits from Spontaneous Flora of Oltenia, Romania. Bull. Univ. Agric. Sci. Vet. Med. Cluj.-Napoca Hortic. 2015, 72, 182–186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ghazghazi, H.; da Graça Miguel, M.; Hasnaoui, B.; Sebei, H.; Ksontini, M.; Figueiredo, A.C.; Pedro, L.G.; Barroso, J.G. Phenols, essential oils and carotenoids of Rosa canina from Tunisia and their antioxidant activities. Afr. J. Biotechnol. 2010, 9, 2709–2716. [Google Scholar]
- Medveckienė, B.; Kulaitienė, J.; Levickienė, D.; Hallmann, E. The Effect of Ripening Stages on the Accumulation of Carotenoids, Polyphenols and Vitamin C in Rosehip Species/Cultivars. Appl. Sci. 2021, 11, 6761. [Google Scholar] [CrossRef]
- Szentmihályi, K.; Vinkler, P.; Lakatos, B.; Illés, V.; Then, M. Rose hip (Rosa canina L.) oil obtained from waste hip seeds by different extraction methods. Bioresour. Technol. 2002, 82, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Petkova, N.; Ivanova, L.; Filova, G.; Ivanov, I.; Denev, P. Antioxidants and carbohydrate content in infusions and microwave extracts from eight medicinal plants. J. Appl. Pharm. Sci. 2017, 7, 55–61. [Google Scholar] [CrossRef]
- Koraqi, H.; Qazimi, B.; Çesko, C.; Petkoska, A.T. Environmentally Friendly Extraction of Bioactive Compounds from Rosa canina L. fruits Using Deep Eutectic Solvent (DES) as Green Extraction Media. Acta Chim. Slov. 2022, 69, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, M.; Moslavac, T.; Bilić, M.; Aladić, K.; Bakula, F.; Jokić, S. Supercritical CO2 extraction of oil from rose hips (Rosa canina L.) and cornelian cherry (Cornus mas L.) seeds. Croat. J. Food Sci. Technol. 2017, 10, 197–205. [Google Scholar] [CrossRef]
- Taneva, S.; Konakchiev, A.; Totzeva, I.; Kamenova-Nacheva, M.; Nikolova, Y.; Momchilova, S.; Dimitrov, V. Super-critical carbon dioxide extraction as an effective green technology for production of high quality rose hip oil. Bulg. Chem. Commun. 2017, 49, 126–131. [Google Scholar]
- Lakka, A.; Bozinou, E.; Stavropoulos, G.; Samanidis, I.; Athanasiadis, V.; Dourtoglou, V.G.; Makris, D.P.; Lalas, S.I. Enhancement of Polyphenols Recovery from Rosa canina, Calendula officinalis and Castanea sativa Using Pulsed Electric Field. Beverages 2021, 7, 63. [Google Scholar] [CrossRef]
- Khazayi, M.; Afshari, H.; Hashemi-Moghaddam, H. Evaluation of Extraction Method and Chemical Modifier on Chemical Composition of the Essential Oils from the Roots of Rosa canina L. J. Essent. Oil Bear. Plants 2019, 22, 131–140. [Google Scholar] [CrossRef]
- Singh Chouhan, K.B.; Tandey, R.; Sen, K.K.; Mehta, R.; Mandal, V. Critical analysis of microwave hydrodiffusion and gravity as a green tool for extraction of essential oils: Time to replace traditional distillation. Trends Food Sci. Technol. 2019, 92, 12–21. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Mazzara, E.; Abouelenein, D.; Angeloni, S.; Nunez, S.; Sagratini, G.; López, V.; Cespi, M.; Vittori, S.; Caprioli, G.; et al. Optimization of Solvent-Free Microwave-Assisted Hydrodiffusion and Gravity Extraction of Morus nigra L. Fruits Maximizing Polyphenols, Sugar Content, and Biological Activities Using Central Composite Design. Pharmaceuticals 2022, 15, 99. [Google Scholar] [CrossRef]
- Turk, M.; Perino, S.; Cendres, A.; Petitcolas, E.; Soubrat, T.; Chemat, F. Alternative process for strawberry juice processing: Microwave hydrodiffusion and gravity. LWT 2017, 84, 626–633. [Google Scholar] [CrossRef]
- Zill-e-Huma; Vian, M.A.; Fabiano-Tixier, A.S.; Elmaataoui, M.; Dangles, O.; Chemat, F. A remarkable influence of microwave extraction: Enhancement of antioxidant activity of extracted onion varieties. Food Chem. 2011, 127, 1472–1480. [Google Scholar] [CrossRef]
- Mazzara, E.; Carletti, R.; Petrelli, R.; Mustafa, A.M.; Caprioli, G.; Fiorini, D.; Scortichini, S.; Dall’Acqua, S.; Sut, S.; Nuñez, S.; et al. Green extraction of hemp (Cannabis sativa L.) using microwave method for recovery of three valuable fractions (essential oil, phenolic compounds and cannabinoids): A central composite design optimization study. J. Sci. Food Agric. 2022, 102, 6220–6235. [Google Scholar] [CrossRef] [PubMed]
- Mazzara, E.; Scortichini, S.; Fiorini, D.; Maggi, F.; Petrelli, R.; Cappellacci, L.; Morgese, G.; Morshedloo, M.R.; Palmieri, G.F.; Cespi, M. A design of experiment (Doe) approach to model the yield and chemical composition of ajowan (Trachyspermum ammi L.) essential oil obtained by microwave-assisted extraction. Pharmaceuticals 2021, 14, 816. [Google Scholar] [CrossRef]
- Fiorini, D.; Scortichini, S.; Bonacucina, G.; Greco, N.G.; Mazzara, E.; Petrelli, R.; Torresi, J.; Maggi, F.; Cespi, M. Cannabidiol-enriched hemp essential oil obtained by an optimized microwave-assisted extraction using a central composite design. Ind. Crops Prod. 2020, 154, 112688. [Google Scholar] [CrossRef]
- Munyaka, A.W.; Oey, I.; Van Loey, A.; Hendrickx, M. Application of thermal inactivation of enzymes during vitamin C analysis to study the influence of acidification, crushing and blanching on vitamin C stability in Broccoli (Brassica oleracea L. var. italica). Food Chem. 2010, 120, 591–598. [Google Scholar] [CrossRef]
- Angelov, G.; Boyadzhieva, S.S.; Georgieva, S.S. Rosehip extraction: Process optimization and antioxidant capacity of extracts. Cent. Eur. J. Chem. 2014, 12, 502–508. [Google Scholar] [CrossRef]
- Chatterjee, S.; Simonoff, J.S. Model Building. In Handbook of Regression Analysis; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Mustafa, A.M.; Maggi, F.; Öztürk, N.; Öztürk, Y.; Sagratini, G.; Torregiani, E.; Vittori, S.; Caprioli, G. Chemical and biological analysis of the by-product obtained by processing Gentiana lutea L. and other herbs during production of bitter liqueurs. Ind. Crops Prod. 2016, 80, 131–140. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Abouelenein, D.; Acquaticci, L.; Alessandroni, L.; Abd-Allah, R.H.; Borsetta, G.; Sagratini, G.; Maggi, F.; Vittori, S.; Caprioli, G. Effect of Roasting, Boiling, and Frying Processing on 29 Polyphenolics and Antioxidant Activity in Seeds and Shells of Sweet Chestnut (Castanea sativa Mill.). Plants 2021, 10, 2192. [Google Scholar] [CrossRef] [PubMed]
- Laurita, R.; Gozzi, G.; Tappi, S.; Capelli, F.; Bisag, A.; Laghi, G.; Gherardi, M.; Cellini, B.; Abouelenein, D.; Vittori, S.; et al. Effect of plasma activated water (PAW) on rocket leaves decontamination and nutritional value. Innov. Food Sci. Emerg. Technol. 2021, 73, 102805. [Google Scholar] [CrossRef]
- Avalos-Llano, K.R.; Martín-Belloso, O.; Soliva-Fortuny, R. Effect of pulsed light treatments on quality and antioxidant properties of fresh-cut strawberries. Food Chem. 2018, 264, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, G.; Fiorini, D.; Maggi, F.; Marangoni, M.; Papa, F.; Vittori, S.; Sagratini, G. Ascorbic acid content, fatty acid composition and nutritional value of the neglected vegetable Alexanders (Smyrnium olusatrum L., Apiaceae). J. Food Compos. Anal. 2014, 35, 30–36. [Google Scholar] [CrossRef]
- Oancea, S. A Review of the Current Knowledge of Thermal Stability of Anthocyanins and Approaches to Their Stabilization to Heat. Antioxidants 2021, 10, 1337. [Google Scholar] [CrossRef]
- Tumbas, V.T.; Čanadanović-Brunet, J.M.; Četojević-Simin, D.D.; Ćetković, G.S.; Ðilas, S.M.; Gille, L. Effect of rosehip (Rosa canina L.) phytochemicals on stable free radicals and human cancer cells. J. Sci. Food Agric. 2012, 92, 1273–1281. [Google Scholar] [CrossRef]
- Oprica, L.; Bucsa, C.; Zamfirache, M.M. Ascorbic acid content of rose hip fruit depending on altitude. Iran. J. Public Health 2015, 44, 138. [Google Scholar]
- Yoruk, I.H.; Turker, M.; Kazankaya, A.; Erez, M.E.; Batta, P.; Celik, F. Fatty acid, sugar and vitamin contents in rose hip species. Asian J. Chem. 2008, 20, 1357–1364. [Google Scholar]
- Georgieva, S.; Angelov, G.; Boyadzhieva, S. Concentration of vitamin C and antioxidant activity of rosehip extracts. J. Chem. Technol. Metall. 2014, 49, 451–454. [Google Scholar]
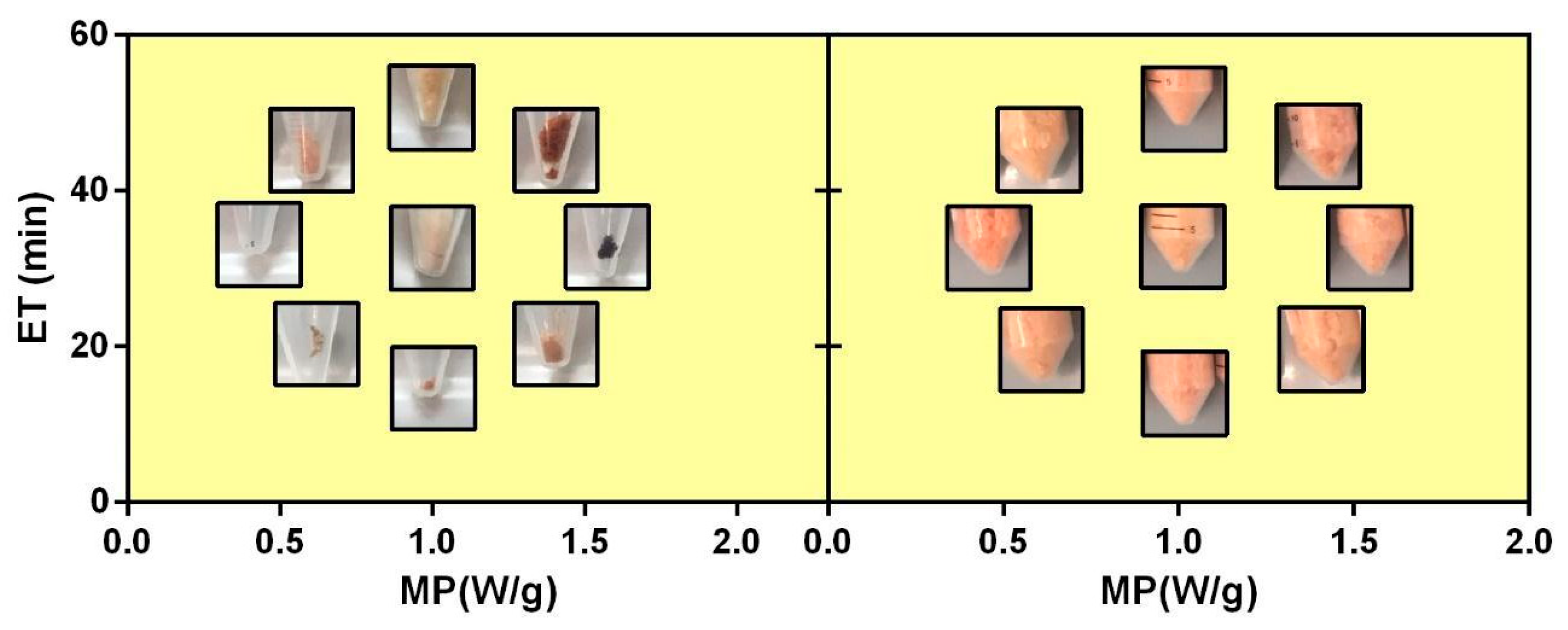




| Run | Point Type a | Coded Variables b | Uncoded Variables | ||||
|---|---|---|---|---|---|---|---|
| MP (W/g) | ET | MO | MP (W/g) | ET (min) | MO (y or n) | ||
| 1 | F | −1 | −1 | 1 | 0.6 | 20 | n |
| 2 | F | +1 | −1 | 1 | 1.4 | 20 | n |
| 3 | F | −1 | +1 | 1 | 0.6 | 45 | n |
| 4 | F | +1 | +1 | 1 | 1.4 | 45 | n |
| 5 | A | −1.41 | 0 | 1 | 0.4 | 33 | n |
| 6 | A | +1.41 | 0 | 1 | 1.6 | 33 | n |
| 7 | A | 0 | −1.41 | 1 | 1 | 15 | n |
| 8 | A | 0 | +1.41 | 1 | 1 | 50 | n |
| 9 | C | 0 | 0 | 1 | 1 | 33 | n |
| 10 | C | 0 | 0 | 1 | 1 | 33 | n |
| 11 | F | −1 | −1 | 2 | 0.6 | 20 | y |
| 12 | F | +1 | −1 | 2 | 1.4 | 20 | y |
| 13 | F | −1 | +1 | 2 | 0.6 | 45 | y |
| 14 | F | +1 | +1 | 2 | 1.4 | 45 | y |
| 15 | A | −1.41 | 0 | 2 | 0.4 | 33 | y |
| 16 | A | +1.41 | 0 | 2 | 1.6 | 33 | y |
| 17 | A | 0 | −1.41 | 2 | 1 | 15 | y |
| 18 | A | 0 | +1.41 | 2 | 1 | 50 | y |
| 19 | C | 0 | 0 | 2 | 1 | 33 | y |
| 20 | C | 0 | 0 | 2 | 1 | 33 | y |
| Response | Best Model a | R2 | R2adj | R2pred | Mallow’s Cp | p-Value Regr b | p-Value LOF b |
|---|---|---|---|---|---|---|---|
| Yield (gDE/100gWF) | y = −8.59 + 11.92 MP + 0.318 ET − 4.70 MP2 − 0.004 ET2 | 0.88 | 0.79 | 0.47 | 4.51 | * | ns |
| TPC (mgGAE/gDE) | None of the obtained models can describe the relationships between the MHG parameters and TPC | ||||||
| TFC (mgRE/gDE) | None of the obtained models can describe the relationships between the MHG parameters and TFC | ||||||
| TAC (mgCGE/gDE) | y = −1.045 + 2.458 MP − 0.017 ET − 0.021 W − 1.297 MP2 − 0.000384 ET2 | 0.88 | 0.78 | 0.50 | 5.68 | * | ns |
| DPPH (mgTE/gDE) | y = 162 + 128.9 MP + 2.85 ET + 0.1245 ET2 − 6.47 MP * ET | 0.80 | 0.72 | 0.67 | 4.78 | * | ns |
| Vit. C (mg/gDE) | None of the obtained models can describe the relationships between the MHG parameters and Vit. C content | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzara, E.; Caprioli, G.; Simonelli, G.; Mustafa, A.M.; Maggi, F.; Cespi, M. Microwave Hydrodiffusion and Gravity Extraction of Vitamin C and Antioxidant Compounds from Rosehips (Rosa canina L.). Foods 2023, 12, 3051. https://doi.org/10.3390/foods12163051
Mazzara E, Caprioli G, Simonelli G, Mustafa AM, Maggi F, Cespi M. Microwave Hydrodiffusion and Gravity Extraction of Vitamin C and Antioxidant Compounds from Rosehips (Rosa canina L.). Foods. 2023; 12(16):3051. https://doi.org/10.3390/foods12163051
Chicago/Turabian StyleMazzara, Eugenia, Giovanni Caprioli, Gianmarco Simonelli, Ahmed M. Mustafa, Filippo Maggi, and Marco Cespi. 2023. "Microwave Hydrodiffusion and Gravity Extraction of Vitamin C and Antioxidant Compounds from Rosehips (Rosa canina L.)" Foods 12, no. 16: 3051. https://doi.org/10.3390/foods12163051
APA StyleMazzara, E., Caprioli, G., Simonelli, G., Mustafa, A. M., Maggi, F., & Cespi, M. (2023). Microwave Hydrodiffusion and Gravity Extraction of Vitamin C and Antioxidant Compounds from Rosehips (Rosa canina L.). Foods, 12(16), 3051. https://doi.org/10.3390/foods12163051














