Evaluation of Roasting and Grilling Effects on Chemical Composition, Volatile Profiles, and Toxicity of Stink Bugs (Tessaratoma papillosa): Implications for Utilization as Functional Food Ingredients
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Cells, and Cultures
2.2. Sample Preparation
2.3. Determination of Proximate Composition
2.4. HPLC Determination of Phenolic Acids and Flavonoids
2.5. Extraction and Determination of γ-Oryzanol and α-, δ-, and γ-Tocopherols
2.6. Determination of Amino Acid Content
2.7. Determination of Volatile Compounds
2.8. Determination of Molecular Weights of Protein Components
2.8.1. Protein Extraction Procedure
2.8.2. Evaluation of Protein Molecular Weight
2.9. Cell Proliferation Assay
2.10. Statistical Analysis
3. Results
3.1. General Characteristics of Stink Bugs
3.2. Phenolic Acid and Flavonoid Contents
3.3. Oryzanol and Tocopherols Contents
3.4. Proximate Composition
3.5. Amino Acid Content
3.6. Volatile Compounds Content
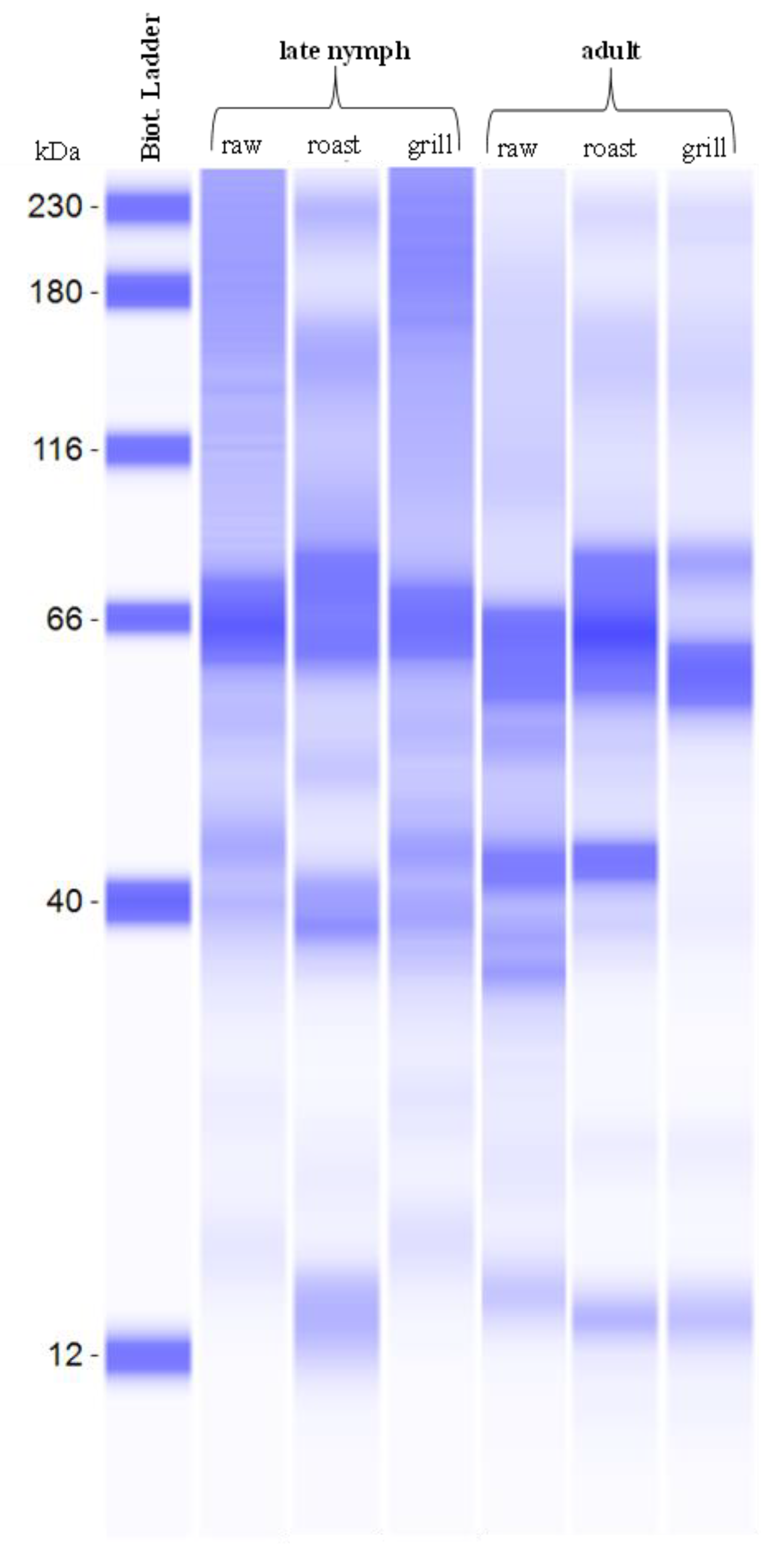
3.7. Protein Molecular Weight in T. papillosa with Different Cooking Methods
3.8. Anti-Proliferative Activity and Cytotoxicity of Stink Bug Extract
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kinyuru, J.N.; Mogendi, J.B.; Riwa, C.A.; Ndung’u, N.W. Edible insects—A novel source of essential nutrients for human diet: Learning from traditional knowledge. Anim. Front. 2015, 5, 14–19. [Google Scholar] [CrossRef]
- Wu, R.A.; Ding, Q.; Yin, L.; Chi, X.; Sun, N.; He, R.; Luo, L.; Ma, H.; Li, Z. Comparison of the nutritional value of mysore thorn borer (Anoplophora chinensis) and mealworm larva (Tenebrio molitor): Amino acid, fatty acid, and element profiles. Food Chem. 2020, 323, 126818. [Google Scholar] [CrossRef]
- Fogang Mba, A.R.; Kansci, G.; Viau, M.; Hafnaoui, N.; Meynier, A.; Demmano, G.; Genot, C. Lipid and amino acid profiles support the potential of Rhynchophorus phoenicis larvae for human nutrition. J. Food Compos. Anal. 2017, 60, 64–73. [Google Scholar] [CrossRef]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; Van Huis, A.; Van Boekel, M.A.J.S. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Köhler, R.; Kariuki, L.; Lambert, C.; Biesalski, H.K. Protein, amino acid and mineral composition of some edible insects from Thailand. J. Asia-Pac. Entomol. 2019, 22, 372–378. [Google Scholar] [CrossRef]
- Raksakantong, P.; Meeso, N.; Kubola, J.; Siriamornpun, S. Fatty acids and proximate composition of eight Thai edible terricolous insects. Food Res. Int. 2010, 43, 350–355. [Google Scholar] [CrossRef]
- Ordoez-Araque, R.; Egas-Montenegro, E. Edible insects: A food alternative for the sustainable development of the planet. Int. J. Gastron. Food Sci. 2021, 23, 100304. [Google Scholar] [CrossRef]
- Aiello, D.; Barbera, M.; Bongiorno, D.; Cammarata, M.; Censi, V.; Indelicato, S.; Mazzotti, F.; Napoli, A.; Piazzese, D.; Saiano, F. Edible insects an alternative nutritional source of bioactive compounds: A review. Molecules 2023, 28, 699. [Google Scholar] [CrossRef] [PubMed]
- da Silva Lucas, A.J.; de Oliveira, L.M.; da Rocha, M.; Prentice, C. Edible insects: An alternative of nutritional, functional and bioactive compounds. Food Chem. 2020, 311, 126022. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, D.; Gao, J.; Peng, Z. Determination of the volatile composition of Tessaratoma papillosa NYMPH by GC/MS. Chem. Nat. Compd. 2015, 51, 166–168. [Google Scholar] [CrossRef]
- Hlongwane, Z.T.; Siwela, M.; Slotow, R.; Munyai, T.C. Effect of geographical location, insect type and cooking method on the nutritional composition of insects consumed in South Africa. J. Insects Food Feed 2022, 8, 537–556. [Google Scholar] [CrossRef]
- Ssepuuya, G.; Nakimbugwe, D.; De Winne, A.; Smets, R.; Claes, J.; Van Der Borght, M. Effect of heat processing on the nutrient composition, colour, and volatile odour compounds of the long-horned grasshopper Ruspolia differens serville. Food Res. Int. 2020, 129, 108831. [Google Scholar] [CrossRef] [PubMed]
- Chumroenphat, T.; Somboonwatthanakul, I.; Saensouk, S.; Siriamornpun, S. Changes in curcuminoids and chemical components of turmeric (Curcuma longa L.) under freeze-drying and low-temperature drying methods. Food Chem. 2021, 339, 128121. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S.; Meeso, N. Phytochemicals, vitamin C and sugar content of Thai wild fruits. Food Chem. 2011, 126, 972–981. [Google Scholar] [CrossRef]
- Chen, M.; Bergman, C.J. A rapid procedure for analysing rice bran tocopherol, tocotrienol and γ-oryzanol contents. J. Food Compos. Anal. 2005, 18, 139–151. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, W.; Li, G. Study of the alarming volatile characteristics of Tessaratoma papillosa using SPME-GC-MS. J. Chromatogr. Sci. 2009, 47, 291–296. [Google Scholar] [CrossRef][Green Version]
- Siripong, P.; Rassamee, K.; Piyaviriyakul, S.; Yahuafai, J.; Kanokmedhakul, K. Anti-metastatic effects on B16F10 melanoma cells of extracts and two prenylated xanthones isolated from Maclura amboinensis Bl roots. Asian Pac. J. Cancer Prev. 2012, 13, 3519–3528. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koch, R.L.; Pezzini, D.T.; Michel, A.P.; Hunt, T.E. Identification, biology, impacts, and management of stink bugs (Hemiptera: Heteroptera: Pentatomidae) of soybean and corn in the midwestern United States. J. Integr. Pest Manag. 2017, 8, 11. [Google Scholar] [CrossRef]
- Sanders, D.; Nickel, H.; Grützner, T.; Platner, C. Habitat structure mediates top–down effects of spiders and ants on herbivores. Basic Appl. Ecol. 2008, 9, 152–160. [Google Scholar] [CrossRef]
- Hassan, A.B.; Al Maiman, S.A.; Alshammari, G.M.; Mohammed, M.A.; Alhuthayli, H.F.; Ahmed, I.A.M.; Alfawaz, M.A.; Yagoub, A.E.A.; Fickak, A.; Osman, M.A. Effects of boiling and roasting treatments on the content of total phenolics and flavonoids and the antioxidant activity of peanut (Arachis hypogaea L.) pod shells. Processes 2021, 9, 1542. [Google Scholar] [CrossRef]
- Kamalaja, T.; Prashanthi, M.; Rajeswari, K. Evaluation of antioxidant activity and bioactive compounds on domestic cooking method. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 4090–4097. [Google Scholar] [CrossRef]
- Chukwumah, Y.; Walker, L.; Vogler, B.; Verghese, M. Changes in the phytochemical composition and profile of raw, boiled, and roasted peanuts. J. Agric. Food Chem. 2007, 55, 9266–9273. [Google Scholar] [CrossRef]
- Mohamed Ahmed, I.A.; Al Juhaimi, F.Y.; Osman, M.A.; Al Maiman, S.A.; Hassan, A.B.; Alqah, H.A.S.; Babiker, E.E.; Ghafoor, K. Effect of oven roasting treatment on the antioxidant activity, phenolic compounds, fatty acids, minerals, and protein profile of Samh (Mesembryanthemum forsskalei Hochst) seeds. LWT 2020, 131, 109825. [Google Scholar] [CrossRef]
- Adisakwattana, S. Cinnamic acid and its derivatives: Mechanisms for prevention and management of diabetes and its complications. Nutrients 2017, 9, 163. [Google Scholar] [CrossRef]
- Jannat, B.; Oveisi, M.R.; Sadeghi, N.; Hajimahmoodi, M.; Behzad, M.; Nahavandi, B.; Tehrani, S.; Sadeghi, F.; Oveisi, M. Effect of roasting process on total phenolic compounds and γ-tocopherol contents of Iranian sesame seeds (Sesamum indicum). Iran. J. Pharm. Res. 2013, 12, 751–758. [Google Scholar] [PubMed]
- Silva, M.P.; Martinez, M.J.; Casini, C.; Grosso, N.R. Tocopherol content, peroxide value and sensory attributes in roasted peanuts during storage. Int. J. Food Sci. Technol. 2010, 45, 1499–1504. [Google Scholar] [CrossRef]
- Oluwaniyi, O.O.; Dosumu, O.O.; Awolola, G.V. Effect of cooking method on the proximate, amino acid and fatty acid compositions of Clarias gariepinus and Oreochromis niloticus. J. Turk. Chem. Soc. A 2016, 4, 115. [Google Scholar] [CrossRef][Green Version]
- Markmanuel, D.P.; Godwin, J. Effects of culinary methods on the proximate composition of an edible insect (Rhynchophorus Phoenicis) larvae obtained from Bayelsa State, Nigeria. Eur. J. Agric. Food Sci. 2020, 2. [Google Scholar] [CrossRef]
- Thomas, S.; Vásquez-Benítez, J.D.; Cuéllar-Cepeda, F.A.; Mosquera-Vásquez, T.; Narváez-Cuenca, C.E. Vitamin C, protein, and dietary fibre contents as affected by genotype, agro-climatic conditions, and cooking method on tubers of Solanum tuberosum Group Phureja. Food Chem. 2021, 349, 129207. [Google Scholar] [CrossRef]
- Cano-Estrada, A.; Castañeda-Ovando, A.; Ramírez-Godinez, J.; Contreras-López, E. Proximate and fatty acid composition in raw and cooked muscle tissue of farmed rainbow trout (Oncorhynchus mykiss) fed with commercial fishmeal. J. Food Process. Preserv. 2018, 42, e13674. [Google Scholar] [CrossRef]
- Adu, O.B.; Ogundeko, T.O.; Ogunrinola, O.O.; Saibu, G.M.; Elemo, B.O. The effect of thermal processing on protein quality and free amino acid profile of Terminalia catappa (Indian almond) seed. J. Food Sci. Technol. 2015, 52, 4637–4641. [Google Scholar] [CrossRef]
- Lopes, A.F.; Alfaia, C.M.M.; Partidário, A.M.C.P.C.; Lemos, J.P.C.; Prates, J.A.M. Influence of household cooking methods on amino acids and minerals of Barrosã-PDO veal. Meat Sci. 2015, 99, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, I.; Zubair, M.; Rizwan, K.; Rasool, N.; Jamil, M.; Khan, S.A.; Tareen, R.B.; Ahmad, V.U.; Mahmood, A.; Riaz, M.; et al. Chemical composition, antioxidant and antimicrobial potential of essential oils from different parts of Daphne mucronata Royle. Chem. Cent. J. 2018, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J.M. Effect of different cooking methods on lipid oxidation and formation of volatile compounds in foal meat. Meat Sci. 2014, 97, 223–230. [Google Scholar] [CrossRef]
- Van, H.; Hwang, I.; Jeong, D.; Touseef, A. Principle of meat aroma flavors and future prospect. In Latest Research into Quality Control; Akyar, I., Ed.; IntechOpen: London, UK, 2012; pp. 145–176. [Google Scholar]
- Yu, P. Protein secondary structures (α-helix and β-sheet) at a cellular level and protein fractions in relation to rumen degradation behaviours of protein: A new approach. Br. J. Nutr. 2005, 94, 655–665. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Mikołajczak, B. The effect of roasting on the protein profile and antiradical capacity of flaxseed meal. Foods 2020, 9, 1383. [Google Scholar] [CrossRef] [PubMed]
- Manditsera, F.A.; Luning, P.A.; Fogliano, V.; Lakemond, C.M.M. Effect of domestic cooking methods on protein digestibility and mineral bioaccessibility of wild harvested adult edible insects. Food Res. Int. 2019, 121, 404–411. [Google Scholar] [CrossRef]

| Samples | Size | Morphology | Local Style of Cooking | References |
|---|---|---|---|---|
| Late nymph | 1.5–10.4 mm long | Yellow-brown in color Moderate pungent smell Wingless | Grilling Roasting Frying Light curry with vegetables | [6,18,19] |
| Adult | 11.0–15.0 mm long | Brown marbled appearance Strong pungent smell Wing | Chili paste Dipping Grilling Roasting Frying | [6,18,19] |
| Parameter | Late Nymph | Adult | ||||
|---|---|---|---|---|---|---|
| Raw | Roasted | Grilled | Raw | Roasted | Grilled | |
| Phenolic acid (μg/g) | ||||||
| gallic acid | 158.8 ± 9.0 b | 381.4 ± 3.2 a | 155.7 ± 1.8 b | 98.0 ± 0.5 c | 109.8 ± 0.3 a | 107.6 ± 0.5 b |
| protocatechuic acid | 20.4 ± 0.5 b | 40.4 ± 4.4 a | 21.1 ± 0.5 b | 14.0 ± 0.1 b | 14.9 ± 0.7 a | 13.9 ± 0.3 b |
| p-hydroxybenzoic acid | 30.5 ± 0.6 c | 96.4 ± 4.9 a | 38.2 ± 0.4 b | 20.0 ± 0.0 b | 21.8 ± 0.1 a | 21.9 ± 0.1 a |
| chlorogenic acid | - | - | - | - | - | - |
| vanillic acid | 20.8 ± 0.2 c | 58.3 ± 0.5 a | 26.2 ± 0.2 b | - | - | - |
| caffeic acid | - | - | 1.5 ± 0.0 a | - | - | - |
| syringic acid | 266.4 ± 1.9 a | - | 171.1 ± 0.8 b | 121.8 ± 0.9 a | 60.3 ± 0.5 c | 71.5 ± 0.3 b |
| vanillin | 24.2 ± 1.2 c | 62.4 ± 0.4 a | 26.1 ± 0.3 b | 10.4 ± 0.1 a | 8.9 ± 0.2 b | 8.9 ± 0.1 b |
| p-coumaric acid | - | - | 1.0 ± 0.1 a | - | - | - |
| ferulic acid | 2.1 ± 0.2 b | - | 3.2 ± 0.1 a | 0.8 ± 0.0 a | 0.8 ± 0.0 a | 0.8 ± 0.0 a |
| sinapic acid | 0.7 ± 0.1 c | 7.1 ± 0.4 a | 1.2 ± 0.0 b | 0.6 ± 0.0 c | 0.7 ± 0.0 b | 0.8 ± 0.0 a |
| cinamic acid | 160.8 ± 8.9 c | 552.8 ± 10.6 a | 246.1 ± 4.5 b | 1438.6 ± 23.1 c | 1867.7 ± 23.3 a | 1733.0 ± 6.5 b |
| genistic acid | 18.1 ± 0.7 c | 39.1 ± 2.3 a | 29.1 ± 1.5 b | 147.8 ± 0.8 b | 153.9 ± 10.7 b | 180.2 ± 0.9 a |
| total | 702.8 ± 25.8 b | 1237.8 ± 25.3 a | 720.5 ± 10.2 b | 1852.0 ± 25.7 c | 2238.8 ± 35.9 a | 2138.6 ± 8.6 b |
| Flavonoid (μg/g) | ||||||
| rutin | 29.7 ± 0.8 b | 58.2 ± 0.2 a | 25.1 ± 0.2 c | 5.5 ± 0.2 b | 42.4 ± 0.7 a | 5.6 ± 0.1 b |
| quercetin | 106.9 ± 6.7 c | 397.5 ± 1.8 a | 207.3 ± 8.6 b | 83.9 ± 0.6 b | 117.9 ± 1.8 a | 69.4 ± 1.0 c |
| apigenin | 103.4 ± 5.8 b | 133.4 ± 9.5 a | 116.7 ± 7.0 b | 90.4 ± 2.5 b | 26.7 ± 1.1 c | 99.4 ± 5.5 a |
| kaempferol | 25.5 ± 0.4 c | 96.8 ± 3.5 a | 36.4 ± 0.6 b | - | - | - |
| myricetin | 169.1 ± 4.8 c | 750.0 ± 9.8 a | 567.7 ± 7.2 b | 80.8 ± 4.0 b | 123.9 ± 2.5 a | 122.6 ± 0.7 a |
| total | 434.6 ± 4.7 c | 1435.9 ± 4.9 a | 953.2 ± 4.7 a | 260.7 ± 1.5 c | 310.9 ± 2.0 a | 297.0 ± 1.5 b |
| Parameter | Late Nymph | Adult | ||||
|---|---|---|---|---|---|---|
| Raw | Roasted | Grilled | Raw | Roasted | Grilled | |
| Protein (g/100 g) | 55.6 ± 0.4 a | 55.5 ± 0.5 a | 57.5 ± 1.3 a | 31.0 ± 0.6 b | 33.3 ± 0.1 a | 31.9 ± 0.1 b |
| Fat (g/100 g) | 23.5 ± 0.6 a | 23.4 ± 0.1 a | 23.0 ± 1.2 a | 50.5 ± 0.2 a | 50.7 ± 0.0 a | 51.4 ± 0.5 a |
| Fiber (g/100 g) | 14.2 ± 0.0 a | 12.7 ± 0.6 b | 12.5 ± 0.0 b | 14.3 ± 0.5 a | 12.1 ± 0.0 b | 11.7 ± 0.3 b |
| Ash (g/100 g) | 3.3 ± 0.1 b | 4.9 ± 0.0 a | 2.8 ± 0.0 c | 1.4 ± 0.1 b | 1.1 ± 0.0 b | 1.8 ± 0.1 a |
| Carbohydrate (g/100 g) | 3.4 ± 0.1 b | 3.5 ± 0.0 b | 4.1 ± 0.2 a | 2.8 ± 0.2 b | 2.9 ± 0.1 ab | 3.2 ± 0.0 a |
| δ-tocopherol (μg/g) | 0.5 ± 0.0 c | 0.7 ± 0.0 b | 1.4 ± 0.1 a | 2.7 ± 0.1 a | 2.3 ± 0.2 b | 0.7 ± 0.0 c |
| γ-tocopherol (μg/g) | 5.7 ± 0.0 b | 7.6 ± 0.2 a | 5.2 ± 0.3 c | 4.4 ± 0.3 b | 18.5 ± 0.7 a | 2.9 ± 0.2 c |
| α-tocopherol (μg/g) | 1.7 ± 0.0 c | 6.1 ± 0.1 a | 2.9 ± 0.2 b | 3.1 ± 0.1 b | 2.8 ± 0.1 c | 4.7 ± 0.3 a |
| γ-oryzanol (mg/g) | - | - | - | - | - | - |
| Parameter | Late Nymph | Adult | ||||
|---|---|---|---|---|---|---|
| Raw | Roasted | Grilled | Raw | Roasted | Grilled | |
| Essential | ||||||
| arginine | 13.6 ± 0.1 b | 14.2 ± 0.2 a | 13.8 ± 0.1 b | 13.8 ± 0.0 b | 14.9 ± 0.3 a | 13.5 ± 0.2 b |
| histidine | 24.9 ± 0.8 a | 24.2 ± 0.5 a | 23.9 ± 0.4 a | 20.4 ± 0.3 a | 19.2 ± 0.3 b | 18.8 ± 0.1 b |
| isoleucine | 13.3 ± 0.1 b | 13.9 ± 0.2 a | 14.0 ± 0.2 a | 13.3 ± 0.3 b | 14.3 ± 0.2 a | 13.2 ± 0.2 b |
| leucine | 11.7 ± 0.1 b | 12.4 ± 0.1 a | 12.4 ± 0.2 a | 11.7 ± 0.0 b | 12.7 ± 0.2 a | 11.6 ± 0.1 b |
| lysine | 4.5 ± 0.1 a | 4.0 ± 0.1 b | 4.0 ± 0.2 b | 4.2 ± 0.1 b | 4.3 ± 0.1 a | 3.8 ± 0.0 c |
| methionine | 53.0 ± 1.1 b | 54.0 ± 0.6 ab | 55.2 ± 0.5 a | 74.2 ± 2.5 b | 80.5 ± 1.4 a | 72.0 ± 1.9 b |
| phenylalanine | 10.7 ± 0.2 c | 11.0 ± 0.1 b | 11.3 ± 0.1 a | 10.9 ± 0.2 b | 12.0 ± 0.1 a | 10.7 ± 0.1 c |
| threonine | 5.9 ± 0.1 a | 6.0 ± 0.1 a | 5.9 ± 0.1 a | 5.4 ± 0.1 b | 5.8 ± 0.1 a | 4.9 ± 0.0 c |
| tryptophan | - | - | - | - | - | - |
| valine | 18.5 ± 0.1 c | 19.4 ± 0.0 a | 19.1 ± 0.0 b | 16.4 ± 0.1 a | 16.7 ± 0.3 a | 16.0 ± 0.1 b |
| sum-essential | 155.9 ± 0.6 b | 158.9 ± 1.2 a | 159.4 ± 0.7 a | 170.3 ± 2.5 b | 180.2 ± 1.4 a | 164.4 ± 2.0 c |
| Non-Essential | ||||||
| alanine | 15.5 ± 0.2 a | 16.0 ± 0.1 a | 14.6 ± 0.7 b | 14.3 ± 0.1 a | 14.1 ± 0.1 a | 13.1 ± 0.1 b |
| asparagine | - | - | - | - | - | - |
| aspartic acid | 11.1 ± 0.1 a | 11.2 ± 0.2 a | 11.1 ± 0.2 a | 11.5 ± 0.1 b | 12.9 ± 0.2 a | 11.2 ± 0.2 c |
| cysteine | 0.1 ± 0.0 b | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 b |
| glutamine | 4.8 ± 0.1 a | 4.4 ± 0.1 b | 4.4 ± 0.0 b | 4.5 ± 0.1 b | 4.7 ± 0.1 a | 4.1 ± 0.1 c |
| glutamic acid | 13.0 ± 0.3 a | 12.7 ± 0.3 a | 12.9 ± 0.1 a | 12.9 ± 0.1 b | 14.4 ± 0.2 a | 12.3 ± 0.4 b |
| glycine | 4.0 ± 0.1 a | 3.8 ± 0.2 a | 3.7 ± 0.2 a | 4.2 ± 0.1 a | 4.1 ± 0.1 a | 3.7 ± 0.1 b |
| proline | 11.4 ± 0.8 a | 11.0 ± 0.4 ab | 10.1 ± 0.2 b | 11.2 ± 0.4 a | 11.3 ± 0.4 a | 10.6 ± 0.3 a |
| serine | 8.5 ± 0.2 ab | 8.7 ± 0.0 a | 8.3 ± 0.0 ab | 7.6 ± 0.1 a | 7.8 ± 0.1 a | 6.9 ± 0.1 b |
| tyrosine | 70.5 ± 0.8 b | 74.6 ± 0.1 a | 74.2 ± 0.6 a | 45.5 ± 0.3 a | 45.8 ± 0.6 a | 42.7 ± 0.1 b |
| sum-non-essential | 138.8 ± 0.7 b | 142.4 ± 1.1 a | 139.5 ± 0.8 b | 111.8 ± 0.5 b | 115.1 ± 1.0 a | 104.6 ± 0.3 c |
| total amino acids | 294.7 ± 0.8 b | 301.4 ± 1.7 a | 298.9 ± 1.4 a | 282.1 ± 2.3 b | 295.3 ± 2.2 a | 268.9 ± 1.9 c |
| EAAI | 0.48 | 0.49 | 0.49 | 0.42 | 0.43 | 0.40 |
| No | Volatile Compounds a | Ri b | Late Nymph | Adult | ||||
|---|---|---|---|---|---|---|---|---|
| Raw | Roasted | Grilled | Raw | Roasted | Grilled | |||
| 1 | Disulfide, dimethyl | 741 | 0.10 | 0.21 | 0.28 | 0.62 | 0.74 | 0.26 |
| 2 | Trisulfide, dimethyl | 969 | - | - | - | 0.25 | - | - |
| 3 | 2-Hexenoic acid | 1060 | - | - | - | 1.62 | 0.89 | 0.12 |
| 4 | Undecane | 1100 | - | - | - | 0.09 | 0.12 | 0.06 |
| 5 | Butane,3-methyl-1-(methylthio)- | 1137 | - | - | - | 0.07 | ||
| 6 | Dodecane | 1200 | 1.57 | 1.91 | 1.46 | 2.86 | 3.15 | 2.48 |
| 7 | 2-Octenoic acid | 1241 | 0.52 | - | - | - | - | - |
| 8 | 1-Tridecene | 1293 | 0.28 | 0.27 | 0.36 | 0.33 | 0.33 | |
| 9 | Octadecane, 5-methyl- | 1309 | 88.59 | 87.5 | 87.48 | 92.24 | 92.79 | 89.47 |
| 10 | Pentadecane | 1502 | 0.11 | 0.18 | 0.17 | 0.14 | 0.09 | 0.08 |
| 11 | cis-9-Hexadecenoic acid | 1957 | - | - | 0.49 | - | - | - |
| 12 | Palmitoleic acid | 1958 | - | 0.50 | - | 0.07 | - | 0.35 |
| 13 | cis-10-Heptadecenoic acid | 1960 | 0.20 | - | - | - | 0.05 | - |
| 14 | n-Hexadecanoic acid | 1976 | 2.19 | 3.24 | 3.24 | 0.70 | 0.68 | 3.36 |
| 15 | n-Heptanoicacid,methyl (tetramethylene)silyl ester | 1991 | - | - | - | - | 0.07 | - |
| 16 | 10(E),12(Z)-Conjugated linoleic acid | 2149 | 0.76 | 0.78 | 0.94 | - | - | - |
| 17 | Oleic Acid | 2153 | 3.96 | 3.77 | 4.73 | 1.04 | 0.90 | 3.33 |
| 18 | Octadecanoic acid | 2177 | 1.28 | 1.50 | 0.24 | 0.12 | ||
| 19 | Heneicosane | 2504 | 0.15 | 0.14 | 0.21 | 0.01 | - | 0.05 |
| 20 | Cyclohexane,1,1′-tetradecylidenebis- | 2567 | - | - | - | - | - | 0.08 |
| 21 | 9-Tricosanol, acetate | 2690 | 0.14 | 0.13 | 0.25 | - | - | - |
| 22 | Tetracontane | 2706 | 0.24 | - | - | - | ||
| 23 | Hexatriacontane | 2707 | 0.15 | 0.14 | - | - | - | 0.03 |
| IC50 (µg/mL) | Doxorubicin | Extraction | Late Nymph | Adult | ||||
|---|---|---|---|---|---|---|---|---|
| Raw | Roasted | Grilled | Raw | Roasted | Grilled | |||
| Hela | 0.06 ± 0.01 | cold extraction * | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| heat extraction ** | 747.5 ± 21.5 | 394.9 ± 30.0 | 715.8 ± 52.1 | 625.2 ± 26.8 | 585.2 ± 25.9 | 257.7 ± 21.9 | ||
| KB | 0.26 ± 0.01 | cold extraction | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| heat extraction | 592.2 ± 25.3 | 285.4 ± 5.2 | 298.0 ± 6.8 | 558.4 ± 28.9 | 395.0 ± 25.7 | 303.1 ± 16.0 | ||
| Vero | 1.91 ± 0.39 | cold extraction | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| heat extraction | 1282.1 ± 10.3 | 670.7 ± 35.3 | 737.4 ± 35.8 | 1279.0 ± 5.8 | 639.8 ± 14.2 | 647.2 ± 17.6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Chumroenphat, T.; Boonarsa, P.; Yahuafai, J.; Wrigley, C.; Siriamornpun, S. Evaluation of Roasting and Grilling Effects on Chemical Composition, Volatile Profiles, and Toxicity of Stink Bugs (Tessaratoma papillosa): Implications for Utilization as Functional Food Ingredients. Foods 2023, 12, 3053. https://doi.org/10.3390/foods12163053
Li H, Chumroenphat T, Boonarsa P, Yahuafai J, Wrigley C, Siriamornpun S. Evaluation of Roasting and Grilling Effects on Chemical Composition, Volatile Profiles, and Toxicity of Stink Bugs (Tessaratoma papillosa): Implications for Utilization as Functional Food Ingredients. Foods. 2023; 12(16):3053. https://doi.org/10.3390/foods12163053
Chicago/Turabian StyleLi, Hua, Theeraphan Chumroenphat, Parinya Boonarsa, Jantana Yahuafai, Colin Wrigley, and Sirithon Siriamornpun. 2023. "Evaluation of Roasting and Grilling Effects on Chemical Composition, Volatile Profiles, and Toxicity of Stink Bugs (Tessaratoma papillosa): Implications for Utilization as Functional Food Ingredients" Foods 12, no. 16: 3053. https://doi.org/10.3390/foods12163053
APA StyleLi, H., Chumroenphat, T., Boonarsa, P., Yahuafai, J., Wrigley, C., & Siriamornpun, S. (2023). Evaluation of Roasting and Grilling Effects on Chemical Composition, Volatile Profiles, and Toxicity of Stink Bugs (Tessaratoma papillosa): Implications for Utilization as Functional Food Ingredients. Foods, 12(16), 3053. https://doi.org/10.3390/foods12163053







