Complex Modification Orders Alleviate the Gelling Weakening Behavior of High Microbial Transglutaminase (MTGase)-Catalyzed Fish Gelatin: Gelling and Structural Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Gelatin Gels
2.3. Gelling Properties
2.3.1. Gel Strength
2.3.2. Texture Profile Analysis (TPA)
2.4. Gelation Point and Melting Point Analysis
2.5. Transparency
2.6. Structure Properties
2.6.1. Fluorescence Intensity
2.6.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.6.3. Scanning Electron Microscope
3. Results and Discussion
3.1. Gel Strength and TPA
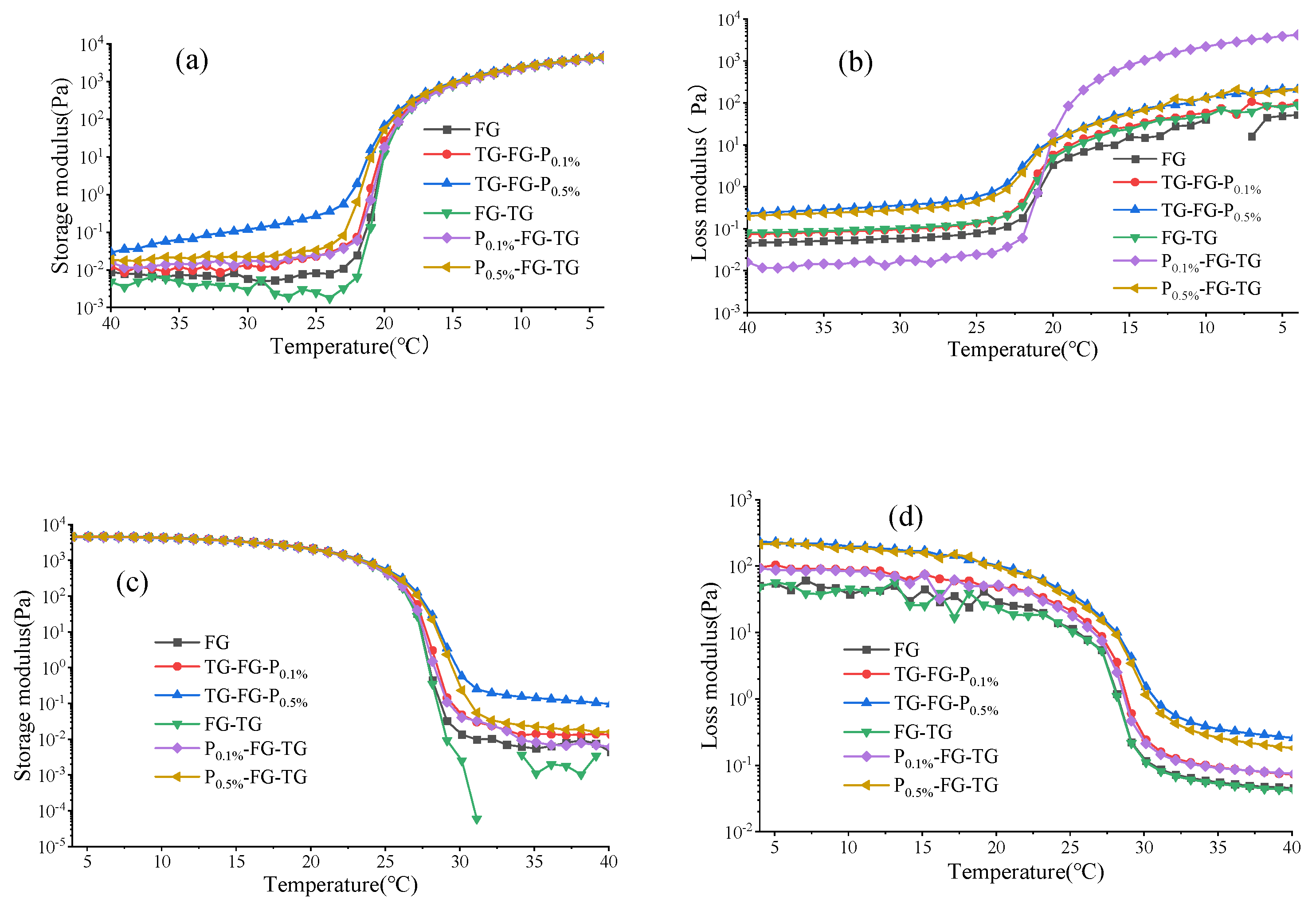
3.2. Gelation Point and Melting Point Analysis
3.3. Turbidity Analysis
3.4. Structure Properties
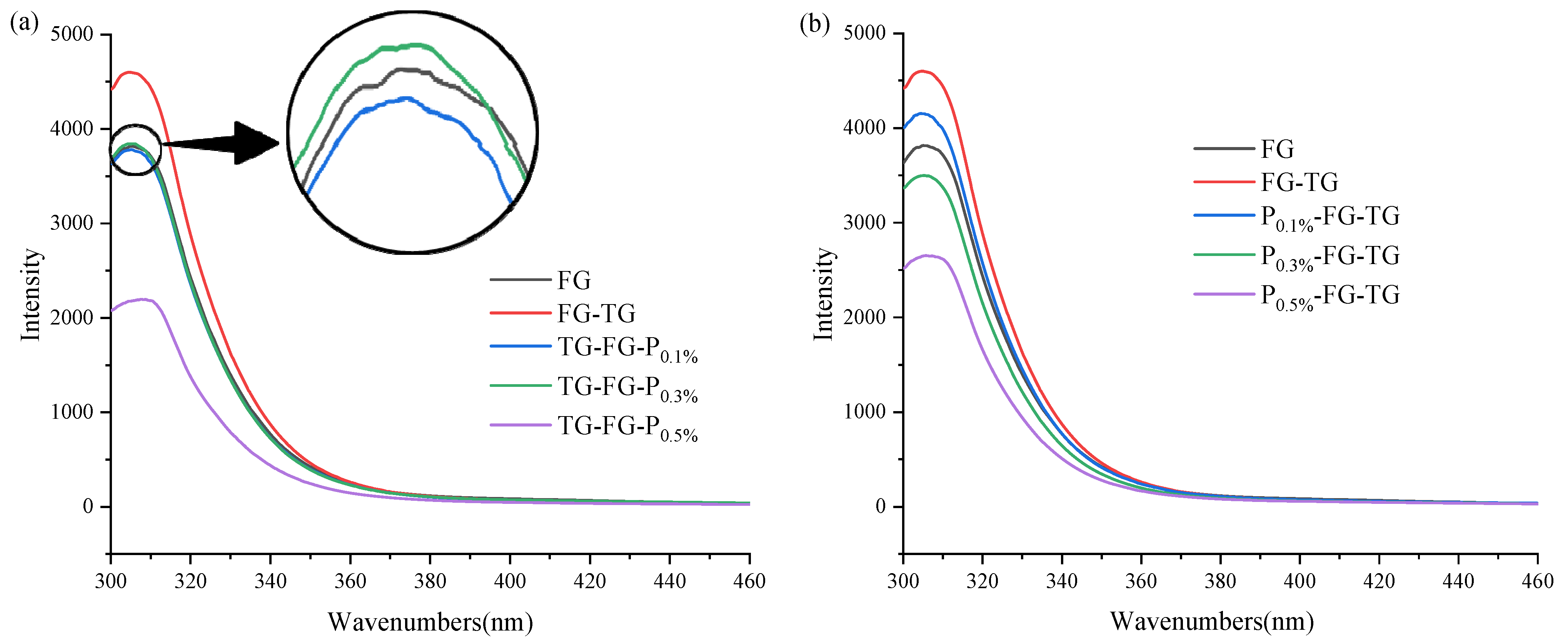
3.4.1. Fluorescence Analysis
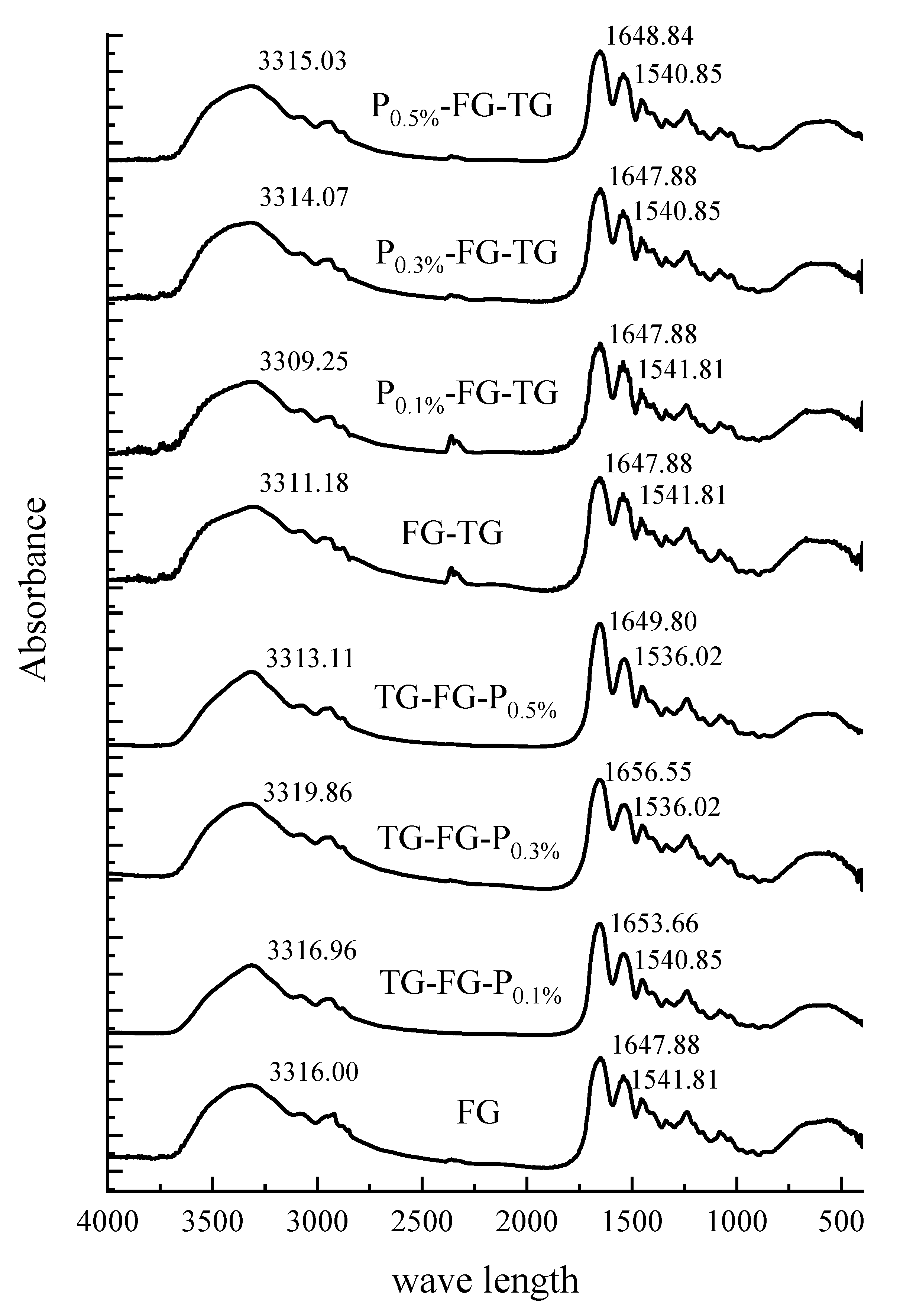
3.4.2. FTIR Analysis
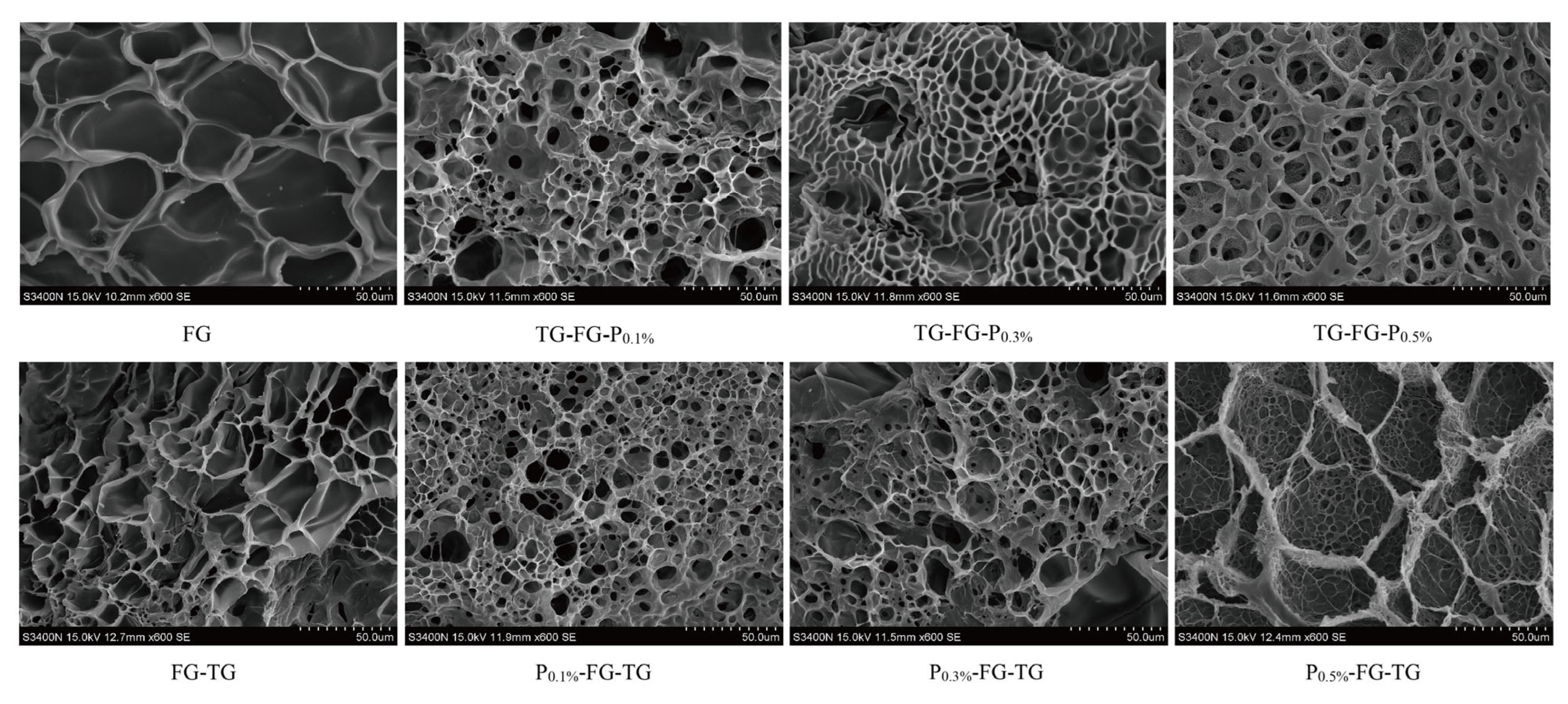
3.4.3. Electron Microscope Scanning
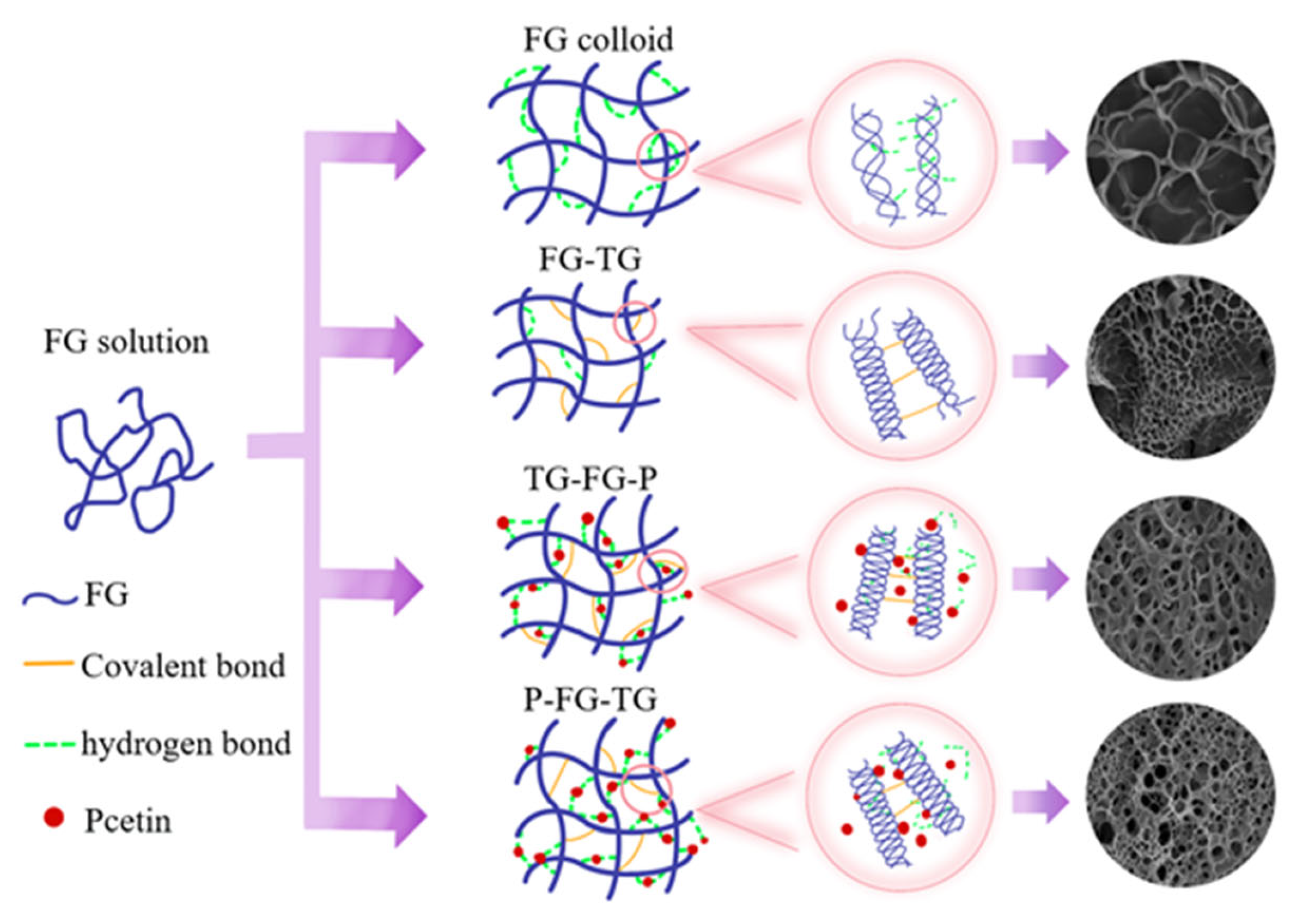
3.5. Graphical Representation of the Gelation Mode
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azam, A.; Hamid, B.; Simindokht, J.; Faezeh, A.; Farangis, T.; Abdorreza, M.N.; Nurul, N.S.; Nurul, H.; Ahmadreza, A. Application of poultry gelatin to enhance the physicochemical, mechanical, and rheological properties of fish gelatin as alternative mammalian gelatin films for food packaging. Foods 2023, 12, 670. [Google Scholar]
- Zhao, H.; Kang, X.; Zhou, X.; Tong, L.; Yu, W.; Zhang, J.; Yang, W.; Lou, Q.; Huang, T. Glycosylation fish gelatin with gum Arabic: Functional and structural properties. LWT 2021, 139, 110634. [Google Scholar] [CrossRef]
- Ahmadreza, A.; Faezeh, A.; Farangis, T.; Najibeh, R.; Mohammad, R.S.A.; Fazilah, A.; Abdorreza, M.N.; Nurul, H.; Jumardi, R. Characterization and cell viability of probiotic/prebiotics film based on duck feet gelatin: A novel poultry gelatin as a suitable matrix for probiotics. Foods 2021, 10, 1761. [Google Scholar]
- Huang, T.; Tu, Z.C.; Shangguan, X.; Sha, X.; Wang, H.; Zhang, L.; Bansal, N. Fish gelatin modifications: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 260–269. [Google Scholar] [CrossRef]
- Saber, M.M. Strategies for surface modification of gelatin-based nanoparticles. Colloids Surf. B 2019, 183, 110407. [Google Scholar]
- Lv, L.C.; Huang, Q.Y.; Ding, W.; Xiao, X.H.; Zhang, H.Y.; Xiong, L.X. Fish gelatin: The novel potential applications. J. Funct. Foods 2019, 63, 103581. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Wang, H.; Liu, W.; Zhang, L.; Zhang, Y.; Shangguan, X.C. Comparison of rheological behaviors and nanostructure of bighead carp scales gelatin modified by different modification methods. J. Food Sci. Technol. 2017, 54, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhao, H.; Fang, Y.; Lu, J.; Yang, W.; Qiao, Z.; Lou, Q.; Xu, D.; Zhang, J. Comparison of gelling properties and flow behaviors of microbial transglutaminase (MTGase) and pectin modified fish gelatin. J. Texture Stud. 2019, 50, 400–409. [Google Scholar] [CrossRef]
- Wang, C.C.; Su, K.Y.; Sun, W.Y.; Huang, T.; Lou, Q.M.; Zhan, S.N. Comparative investigations of various modification methods on the gelling, rheological properties and mechanism of fish gelatin. Food Chem. 2023, 426, 136632. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Wang, H.; Shangguan, X.; Zhang, L.; Zhang, N.H.; Bansal, N. Pectin and enzyme complex modified fish scales gelatin: Rheological behavior, gel properties and nanostructure. Carbohyd. Polym. 2017, 156, 294–302. [Google Scholar] [CrossRef]
- Anvari, M.; Chung, D. Dynamic rheological and structural characterization of fish gelatin—Gum arabic coacervate gels cross-linked by tannic acid. Food Hydrocoll. 2016, 60, 516–524. [Google Scholar] [CrossRef]
- Wu, B.C.; McClements, D.J. Functional hydrogel microspheres: Parameters affecting electrostatic assembly of biopolymer particles fabricated from gelatin and pectin. Food Res. Int. 2015, 72, 231–240. [Google Scholar] [CrossRef]
- Sha, X.M.; Hu, Z.Z.; Ye, Y.H.; Xu, H.; Tu, Z.C. Effect of extraction temperature on the gelling properties and identification of porcine gelatin. Food Hydrocoll. 2019, 92, 163–172. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Shangguan, X.C.; Wang, H.; Sha, X.M.; Bansal, N. Rheological behavior, emulsifying properties and structural characterization of phosphorylated fish gelatin. Food Chem. 2018, 246, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.P.; Fang, Q.; Ma, N.; Yang, W.G.; Zhang, L.Y.; Huang, T. Gelation behaviour of fish skin gelatin in the presence of methanol-water and ethanol-water solvent system. Int. J. Food Sci. Technol. 2022, 57, 1598–1608. [Google Scholar] [CrossRef]
- Fang, Q.; Ma, N.; Ding, K.Y.; Zhan, S.N.; Lou, Q.M.; Huang, T. Interaction between negatively charged fish gelatin and cyclodextrin in aqueous solution: Characteristics and formation mechanism. Gels 2021, 7, 260. [Google Scholar] [CrossRef]
- Sarbon, N.M.; Badii, F.; Howell, N.K. The effect of chicken skin gelatin and whey protein interactions on rheological and thermal properties. Food Hydrocoll. 2015, 45, 83–92. [Google Scholar] [CrossRef]
- Sow, L.C.; Yang, H.S. Effects of salt and sugar addition on the physicochemical properties and nanostructure of fish gelatin. Food Hydrocoll. 2015, 45, 72–82. [Google Scholar] [CrossRef]
- Varela, M.S.; Palacio, M.A.; Navarro, A.S.; Yamul, D.K. Structural and functional properties and digital image texture analysis of gelatin, pectin, and carrageenan gels with honey addition. J. Texture Stud. 2023, 1–13. [Google Scholar] [CrossRef]
- Kumar, D.P.; Chandra, M.V.; Elavarasan, K.; Shamasundar, B.A. Structural properties of gelatin extracted from croaker fish (Johnius sp.) skin waste. Int. J. Food Prop. 2018, 20, S2612–S2625. [Google Scholar] [CrossRef]
- Da Silva, M.A.; Bode, F.; Grillo, I.; Dreiss, C.A. Exploring the kinetics of gelation and final architecture of enzymatically cross-linked chitosan/gelatin gels. Biomacromolecules 2015, 16, 1401–1409. [Google Scholar] [CrossRef]
- Sezer, P.; Okur, I.; Oztop, M.H.; Alpas, H. Improving the physical properties of fish gelatin by high hydrostatic pressure (HHP) and ultrasonication (US). Int. J. Food Sci. Technol. 2020, 55, 1468–1476. [Google Scholar] [CrossRef]
- Hu, Z.Z.; Sha, X.M.; Huang, T.; Zhang, L.; Wang, G.Y.; Tu, Z. CMicrobial transglutaminase (MTGase) modified fish gelatin-γ-polyglutamic acid (γ-PGA): Rheological behavior, gelling properties, and structure. Food Chem. 2021, 348, 129093. [Google Scholar] [CrossRef]
- Cheng, C.; Tu, Z.; Wang, H. pH-induced complex coacervation of fish gelatin and carboxylated chitosan: Phase behavior and structural properties. Food Res. Int. 2023, 167, 112652. [Google Scholar] [CrossRef]
- Sow, L.C.; Toh NZ, Y.; Wong, C.W.; Yang, H. Combination of sodium alginate with tilapia fish gelatin for improved texture properties and nanostructure modification. Food Hydrocoll. 2019, 94, 459–467. [Google Scholar] [CrossRef]
- Guo, Y.N.; Liu, C.H.; Ma, Y.T.; Shen, L.L.; Gong, Q.; Hu, Z.D.; Wang, Z.J.; Liu, X.; Guo, Z.W.; Zhou, L.Y. Study on the structure, function, and interface characteristics of soybean protein isolate by industrial phosphorylation. Foods 2023, 12, 1108. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ding, K.; Su, K.; Sun, W.; Zhan, S.; Lou, Q.; Huang, T. Ultrasonic-assisted glycosylation with glucose on the functional and structural properties of fish gelatin. Gels 2023, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Tu, Z.C.; Sha, X.M.; Hu, Y.M.; Chen, N.; Wang, H. Fabrication and performance evaluation of pectin–fish gelatin–resveratrol preservative films. Food Chem. 2021, 361, 129832. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Geng, H.; Guo, W.; Sun, W.; Zhan, S.; Lou, Q.; Huang, T. Ultrasonic-assisted glycosylation with kappa-carrageenan on the functional and structural properties of fish gelatin. J. Sci. Food Agric. 2023, 103, 5322–5331. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Kang, X.; Fang, Q.; Yang, W.; Cen, S.; Lou, Q.; Huang, T. Rheological properties and interactions of fish gelatin-kappa-carrageenan polyelectrolyte hydrogels: The effects of salt. J. Texture Stud. 2022, 53, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Cen, S.; Zhang, L.; Liu, L.; Lou, Q.; Wang, C.; Huang, T. Phosphorylation modification on functional and structural properties of fish gelatin: The effects of phosphate contents. Food Chem. 2023, 380, 132209. [Google Scholar] [CrossRef] [PubMed]
| Content | TG-FG-P | P-FG-TG | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | FG | TG-FG-P0.1% | TG-FG-P0.3% | TG-FG-P0.5% | FG-TG | P0.1%-FG-TG | P0.3%-FG-TG | P0.5%-FG-TG |
| Gel strength(g) | 701.78 ± 11.81 bc | 744.02 ± 5.45 a | 730.18 ± 11.43 ab | 687.67 ± 20.98 c | 685.84 ± 11.69 c | 728.35 ± 10.93 ab | 737.67 ± 12.05 ab | 751.99 ± 10.97 a |
| Hardness(N) | 13.51 ± 0.28 cd | 14.36 ± 0.27 ab | 13.98 ± 0.24 bc | 12.18 ± 0.45 de | 13.29 ± 0.28 cd | 14.46 ± 0.12 ab | 14.83 ± 0.28 a | 14.91 ± 0.33 a |
| Springiness | 0.95 ± 0.03 a | 0.96 ± 0.02 a | 0.95 ± 0.02 a | 0.97 ± 0.01 a | 0.97 ± 0.01 a | 0.95 ± 0.01 a | 0.97 ± 0.00 a | 0.96 ± 0.06 a |
| Cohesiveness | 0.92 ± 0.02 a | 0.93 ± 0.01 a | 0.92 ± 0.01 a | 0.92 ± 0.01 a | 0.93 ± 0.01 a | 0.92 ± 0.02 a | 0.93 ± 0.01 a | 0.91 ± 0.01 a |
| Gumminess(N) | 12.65 ± 0.58 b | 13.36 ± 0.23 ab | 12.92 ± 0.31 ab | 11.25 ± 0.29 c | 12.31 ± 0.17 b | 13.37 ± 0.24 ab | 13.75 ± 0.20 a | 13.84 ± 0.30 ab |
| Chewiness(N) | 12.27 ± 0.27 ab | 12.81 ± 0.47 ab | 12.34 ± 0.54 ab | 10.96 ± 0.24 c | 11.99 ± 0.91 b | 12.75 ± 0.30 ab | 13.34 ± 0.19 ab | 13.65 ± 0.33 a |
| Resilience | 0.78 ± 0.01 b | 0.79 ± 0.01 ab | 0.79 ± 0.01 ab | 0.79 ± 0.04 ab | 0.79 ± 0.01 ab | 0.79 ± 0.03 ab | 0.80 ± 0.00 ab | 0.79 ± 0.00 ab |
| Transparency | 2.90 ± 0.00 a | 1.43 ± 0.00 b | 1.09 ± 0.00 c | 1.04 ± 0.00 c | 2.96 ± 0.00 a | 1.44 ± 0.00 b | 1.10 ± 0.00 c | 1.01 ± 0.00 c |
| Content | Mp (°C) | Gp (°C) |
|---|---|---|
| FG | 27.41 ± 0.00 c | 20.56 ± 0.00 c |
| TG-FG-P0.1% | 27.99 ± 0.01 b | 20.89 ± 0.01 b |
| TG-FG-P0.5% | 28.85 ± 0.00 a | 21.61 ± 0.00 a |
| FG-TG | 27.79 ± 0.02 bc | 20.65 ± 0.01 c |
| P0.1%-FG-TG | 27.84 ± 0.00 bc | 20.71 ± 0.00 bc |
| P0.5%-FG-TG | 28.85 ± 0.01 a | 21.35 ± 0.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, K.; Sun, W.; Li, Z.; Huang, T.; Lou, Q.; Zhan, S. Complex Modification Orders Alleviate the Gelling Weakening Behavior of High Microbial Transglutaminase (MTGase)-Catalyzed Fish Gelatin: Gelling and Structural Analysis. Foods 2023, 12, 3027. https://doi.org/10.3390/foods12163027
Su K, Sun W, Li Z, Huang T, Lou Q, Zhan S. Complex Modification Orders Alleviate the Gelling Weakening Behavior of High Microbial Transglutaminase (MTGase)-Catalyzed Fish Gelatin: Gelling and Structural Analysis. Foods. 2023; 12(16):3027. https://doi.org/10.3390/foods12163027
Chicago/Turabian StyleSu, Kaiyuan, Wanyi Sun, Zhang Li, Tao Huang, Qiaoming Lou, and Shengnan Zhan. 2023. "Complex Modification Orders Alleviate the Gelling Weakening Behavior of High Microbial Transglutaminase (MTGase)-Catalyzed Fish Gelatin: Gelling and Structural Analysis" Foods 12, no. 16: 3027. https://doi.org/10.3390/foods12163027
APA StyleSu, K., Sun, W., Li, Z., Huang, T., Lou, Q., & Zhan, S. (2023). Complex Modification Orders Alleviate the Gelling Weakening Behavior of High Microbial Transglutaminase (MTGase)-Catalyzed Fish Gelatin: Gelling and Structural Analysis. Foods, 12(16), 3027. https://doi.org/10.3390/foods12163027







