Preparation and Characterization of Ferulic Acid Wheat Gluten Nanofiber Films with Excellent Antimicrobial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Blending Electrospinning
2.3. Physicochemical Properties of Electrospinning Solution
2.4. Morphology
2.5. Compatibility of Components
2.6. Physical Form
2.7. Active Molecules Release
2.8. Antimicrobial Test
2.9. Statistical Analysis
3. Results and Discussion
3.1. Solution Properties
3.2. Morphology
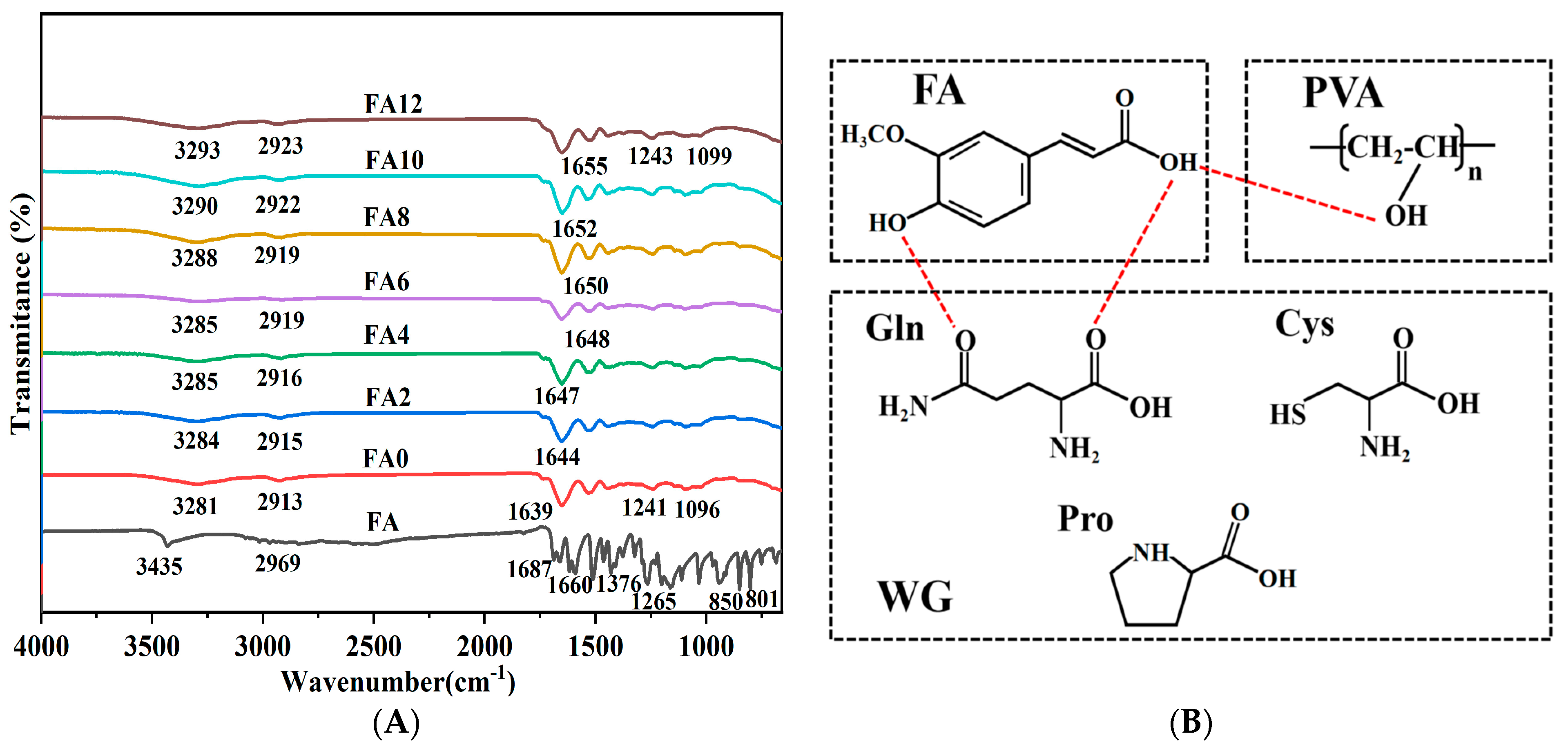
3.3. Component Interaction Analysis
3.4. Physical State Analysis
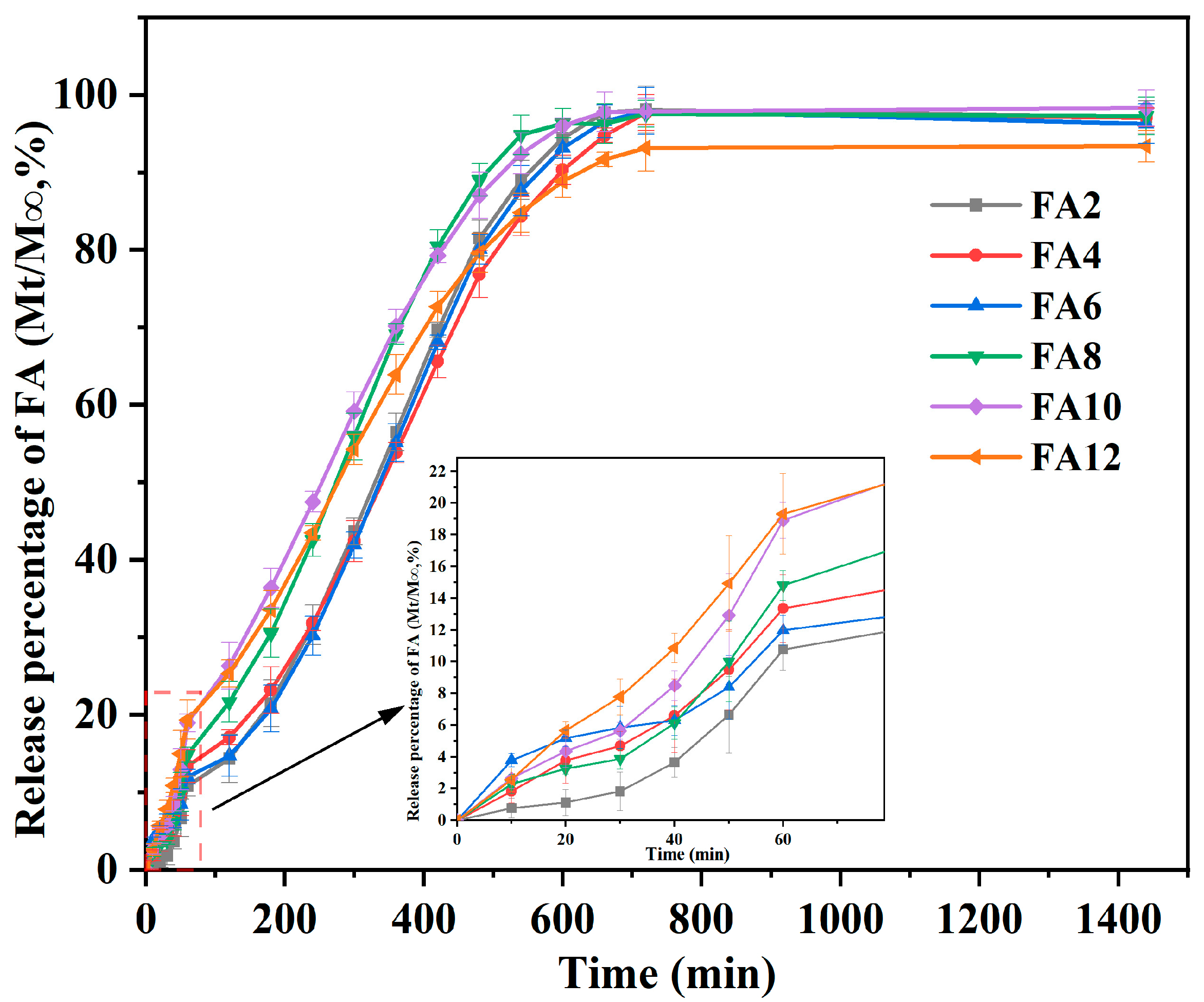
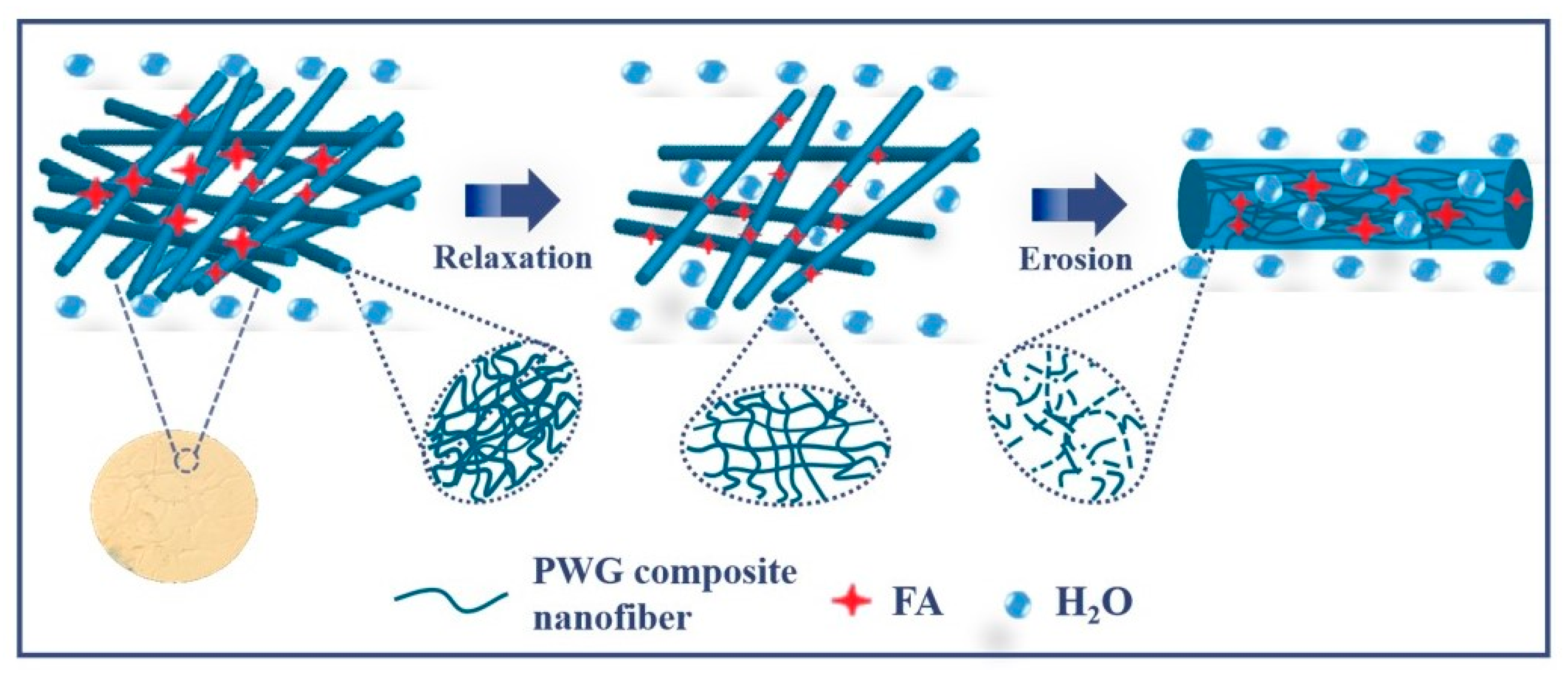
3.5. Release Performance
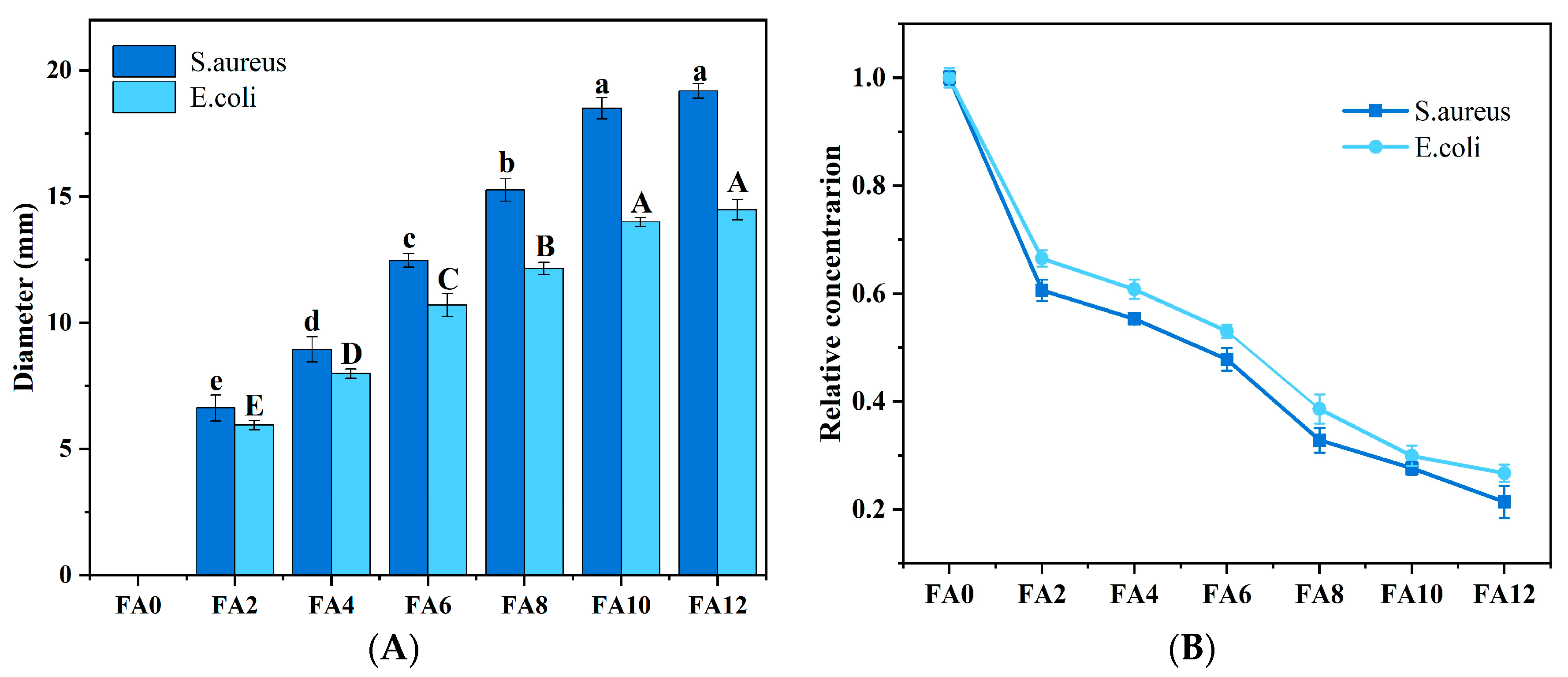
3.6. Antibacterial Property Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wen, P.; Wen, Y.; Zong, M.H.; Linhardt, R.J.; Wu, H. Encapsulation of Bioactive Compound in Electrospun Fibers and Its Potential Application. J. Agric. Food Chem. 2017, 65, 9161–9179. [Google Scholar] [CrossRef]
- Kumar, C.S.; Soloman, A.M.; Thangam, R.; Perumal, R.K.; Gopinath, A.; Madhan, B. Ferulic acid-loaded collagen hydrolysate and polycaprolactone nanofibres for tissue engineering applications. Iet Nanobiotechnol. 2020, 14, 202–209. [Google Scholar] [CrossRef]
- Li, T.; Xia, N.; Xu, L.N.; Zhang, H.; Zhang, H.J.; Chi, Y.J.; Zhang, Y.L.; Li, L.L.; Li, H.Y. Preparation, characterization and application of SPI-based blend film with antioxidant activity. Food Packag. Shelf Life 2021, 27, 100614. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, L.; Zhong, H.; Zou, Y.C.; Qin, Z.Y.; Li, Y.; Zhang, H. Impact of glycation on physical properties of composite gluten/zein nanofibrous films fabricated by blending electrospinning. Food Chem. 2022, 366, 130586. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, Y.; Zhang, C.; Que, F.; Weiss, J.; Zhang, H. Characterization and antioxidant activity of trilayer gelatin/dextran-propyl gallate/gelatin films: Electrospinning versus solvent casting. LWT-Food Sci. Technol. 2020, 128, 109536. [Google Scholar] [CrossRef]
- Li, W.H.; Zhang, C.; Chi, H.; Li, L.; Lan, T.Q.; Han, P.; Chen, H.Y.; Qin, Y.Y. Development of Antimicrobial Packaging Film Made from Poly(Lactic Acid) Incorporating Titanium Dioxide and Silver Nanoparticles. Molecules 2017, 22, 1170. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2015, 11, 1165–1188. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Cen, Z.; Yang, L.; Peng, W.; Hui, Z. Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 479–502. [Google Scholar] [CrossRef]
- Liu, F.G.; Li, X.Z.; Wang, L.; Yan, X.J.; Ma, D.X.; Liu, Z.G.; Liu, X.B. Sesamol incorporated cellulose acetate-zein composite nanofiber membrane: An efficient strategy to accelerate diabetic wound healing. Int. J. Biol. Macromol. 2020, 149, 627–638. [Google Scholar] [CrossRef]
- Riaz, A.; Lagnika, C.; Luo, H.; Dai, Z.Q.; Nie, M.M.; Hashim, M.M.; Liu, C.Q.; Song, J.F.; Li, D.J. Chitosan-based biodegradable active food packaging film containing Chinese chive (Allium tuberosum) root extract for food application. Int. J. Biol. Macromol. 2020, 150, 595–604. [Google Scholar] [CrossRef]
- Nassar, S.F.; Dombre, C.; Gastaldi, E.; Touchaleaume, F.; Chalier, P. Soy protein isolate nanocomposite film enriched with eugenol, an antimicrobial agent: Interactions and properties. J. Appl. Polym. Sci. 2018, 135, 45941. [Google Scholar] [CrossRef]
- Vn, A.; Mkm, B.; St, A. Electrospinning preparation and spectral characterizations of the inclusion complex of ferulic acid and γ-cyclodextrin with encapsulation into polyvinyl alcohol electrospun nanofibers. J. Mol. Struct. 2020, 1221, 128767. [Google Scholar]
- Zhang, Y.; Deng, L.; Zhong, H.; Pan, J.; Li, Y.; Zhang, H. Superior water stability and antimicrobial activity of electrospun gluten nanofibrous films incorporated with glycerol monolaurate. Food Hydrocoll. 2020, 109, 106–116. [Google Scholar] [CrossRef]
- Wang, H.; She, Y.; Chu, C.; Liu, H.; Jiang, S.; Sun, M.; Jiang, S. Preparation, antimicrobial and release behaviors of nisin-poly (vinyl alcohol)/wheat gluten/ZrO2 nanofibrous membranes. J. Mater. Sci. 2015, 50, 5068–5078. [Google Scholar] [CrossRef]
- Zhang, H.J.; Jin, C.M.; Lv, S.H.; Ren, F.Y.; Wang, J. Study on electrospinning of wheat gluten: A review. Food Res. Int. 2023, 169, 112851. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chen, H. Enhancement of nanofiber elasticity by using wheat glutenin as an addition. Polym. Sci. 2013, 55, 320–326. [Google Scholar] [CrossRef]
- Ugur, M.H.; Oktay, B.; Gungor, A.; Kayaman-Apohan, N. Highly thermally resistant, hydrophobic poly(vinyl alcohol)-silica hybrid nanofibers. J. Serbian Chem. Soc. 2018, 83, 885–897. [Google Scholar] [CrossRef]
- Aziz, S.; Hosseinzadeh, L.; Arkan, E.; Azandaryani, A.H. Preparation of electrospun nanofibers based on wheat gluten containing azathioprine for biomedical application. Int. J. Polym. Mater. 2019, 68, 639–646. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Moghadasian, M.H. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef]
- Huang, X.Y.; Jiang, W.L.; Zhou, J.F.; Yu, D.G.; Liu, H. The Applications of Ferulic-Acid-Loaded Fibrous Films for Fruit Preservation. Polymers 2022, 14, 4947. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Peppas, N. Chemical and Physical Structure of Polymers as Carriers for Controlled Release of Bioactive Agents: A Review. Polym. Rev. 1983, 23, 61–126. [Google Scholar] [CrossRef]
- Lobo, C. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Neo, Y.P.; Swift, S.; Ray, S.; Gizdavic-Nikolaidis, M.; Jin, J.Y.; Perera, C.O. Evaluation of gallic acid loaded zein sub-micron electrospun fibre mats as novel active packaging materials. Food Chem. 2013, 141, 3192–3200. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Yang, J.L.; Yu, K.; Tsuji, T.; Jha, R.; Zuo, Y.Y. Determining the surface dilational rheology of surfactant and protein films with a droplet waveform generator. J. Colloid Interface Sci. 2019, 537, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.; Golmakani, M.T.; Niakousari, M.; Hosseini, S.M.H.; Ghorani, B.; Lopez-Rubio, A. Active Food Packaging Coatings Based on Hybrid Electrospun Gliadin Nanofibers Containing Ferulic Acid/Hydroxypropyl-Beta-Cyclodextrin Inclusion Complexes. Nanomaterials 2018, 8, 919. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Development of ferulic acid/cyclodextrin inclusion complex nanofibers for fast -dissolving drug delivery system. Int. J. Pharm. 2020, 584, 119395. [Google Scholar] [CrossRef]
- Irani, M.; Sadeghi, G.M.M.; Haririan, I. The sustained delivery of temozolomide from electrospun PCL-Diol-b-PU/gold nanocompsite nanofibers to treat glioblastoma tumors. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 75, 165–174. [Google Scholar] [CrossRef]
- Deng, L.L.; Li, Y.; Feng, F.Q.; Wu, D.; Zhang, H. Encapsulation of allopurinol by glucose cross-linked gelatin/zein nanofibers: Characterization and release behavior. Food Hydrocoll. 2019, 94, 574–584. [Google Scholar] [CrossRef]
- Jia, Q.Q.; Lin, X.H.; Yang, Y.W.; Duan, B. Multifunctional edible chitin nanofibers/ferulic acid composite coating for fruit preservation. J. Polym. Sci. 2023, 24, 1–15. [Google Scholar] [CrossRef]
- Panwar, R.; Sharma, A.K.; Kaloti, M.; Dutt, D.; Pruthi, V. Characterization and anticancer potential of ferulic acid-loaded chitosan nanoparticles against ME-180 human cervical cancer cell lines. Appl. Nanosci. 2016, 6, 803–813. [Google Scholar] [CrossRef]
- Yang, H.; Feng, K.; Wen, P.; Zong, M.H.; Lou, W.Y.; Wu, H. Enhancing oxidative stability of encapsulated fish oil by incorporation of ferulic acid into electrospun zein mat. Lwt-Food Sci. Technol. 2017, 84, 82–90. [Google Scholar] [CrossRef]
- Narayanan, G.; Boy, R.; Gupta, B.S.; Tonelli, A.E. Analytical techniques for characterizing cyclodextrins and their inclusion complexes with large and small molecular weight guest molecules. Polym. Test. 2017, 62, 402–439. [Google Scholar] [CrossRef]
- Yu, D.G.; Li, J.J.; Williams, G.R.; Zhao, M. Electrospun amorphous solid dispersions of poorly water-soluble drugs: A review. J. Control. Release 2018, 292, 91–110. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.D.; Yang, Y.Y.; Zhao, B.W.; Liang, G.Q.; Liu, S.W.; Liu, X.L.; Yu, D.G. Fast Dissolving of Ferulic Acid via Electrospun Ternary Amorphous Composites Produced by a Coaxial Process. Pharmaceutics 2018, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, M.R.; Kim, M.S.; Kwon, O.H. Polyphenol-loaded polycaprolactone nanofibers for effective growth inhibition of human cancer cells. Mater. Chem. Phys. 2012, 133, 674–680. [Google Scholar] [CrossRef]
- Yakub, G.; Ignatova, M.; Manolova, N.; Rashkov, I.; Markova, N. Chitosan/ferulic acid-coated poly(ε-caprolactone) electrospun materials with antioxidant, antibacterial and antitumor properties. Int. J. Biol. Macromol. 2018, 107, 689–702. [Google Scholar] [CrossRef]
- Yu, D.G.; Yang, J.M.; Branford-White, C.; Lu, P.; Zhang, L.; Zhu, L.M. Third generation solid dispersions of ferulic acid in electrospun composite nanofibers. Int. J. Pharm. 2010, 400, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Tatham, A.S. Improving wheat to remove coeliac epitopes but retain functionality. J. Cereal Sci. 2016, 67, 12–21. [Google Scholar] [CrossRef]
- Kajdic, S.; Planinsek, O.; Gasperlin, M.; Kocbek, P. Electrospun nanofibers for customized drug-delivery systems. J. Drug Deliv. Sci. Technol. 2019, 51, 672–681. [Google Scholar] [CrossRef]
- Kalu, V.D.; Odeniyi, M.A.; Jaiyeoba, K.T. Matrix properties of a new plant gum in controlled drug delivery. Arch. Pharmacal Res. 2007, 30, 884–889. [Google Scholar] [CrossRef]
- Ferrari, P.C.; Oliveira, G.F.; Chibebe, F.C.S.; Evangelista, R.C. In vitro characterization of coevaporates containing chitosan for colonic drug delivery. Carbohydr. Polym. 2009, 78, 557–563. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Concheiro, A.; Alvarez-Lorenzo, C. Crosslinked Hyaluronan Electrospun Nanofibers for Ferulic Acid Ocular Delivery. Pharmaceutics 2020, 12, 274. [Google Scholar] [CrossRef]



| Sample (FA-PWG) | Conductivity (ms/cm) | Viscosity (Pa.s) | Surface Tension (mN/m) |
|---|---|---|---|
| FA0 | 1.03 ± 0.05 e | 2.01 ± 0.08 e | 29.81 ± 0.13 d |
| FA2 | 1.06 ± 0.04 de | 2.06 ± 0.02 e | 29.92 ± 0.48 cd |
| FA4 | 1.15 ± 0.02 bc | 2.15 ± 0.05 d | 30.96 ± 0.71 cd |
| FA6 | 1.18 ± 0.02 bc | 2.18 ± 0.02 cd | 31.26 ± 0.95 bcd |
| FA8 | 1.23 ± 0.04 ab | 2.26 ± 0.03 c | 31.57 ± 0.28 bc |
| FA10 | 1.28 ± 0.02 a | 2.36 ± 0.04 b | 32.48 ± 0.47 b |
| FA12 | 1.12 ± 0.07 cd | 2.45 ± 0.05 a | 34.19 ± 0.96 a |
| Formulation | Zero-Order | First-Order | Higuchi | Korsmeyer–Peppas | Release Rate (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| KZ | R2 | KF | R2 | KH | R2 | KK | n | R2 | ||
| FA2 | 0.0904 | 0.6931 | 0.0034 | 0.7578 | 4.0457 | 0.8533 | 0.0501 | 1.1809 | 0.9844 | 97.15 |
| FA4 | 0.0901 | 0.7499 | 0.0031 | 0.7962 | 3.7053 | 0.8884 | 0.3171 | 0.8696 | 0.9796 | 96.25 |
| FA6 | 0.0906 | 0.7314 | 0.0032 | 0.7721 | 3.7247 | 0.8661 | 0.3823 | 0.8393 | 0.9664 | 96.17 |
| FA8 | 0.0902 | 0.6986 | 0.0033 | 0.7623 | 3.8357 | 0.8853 | 0.2083 | 0.9697 | 0.9765 | 96.37 |
| FA10 | 0.1186 | 0.5302 | 0.0034 | 0.8167 | 3.7368 | 0.9007 | 0.3263 | 0.9018 | 0.9686 | 97.38 |
| FA12 | 0.0815 | 0.732 | 0.0025 | 0.8219 | 3.4398 | 0.9141 | 0.7767 | 0.7303 | 0.9737 | 93.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, C.; Zhang, H.; Ren, F.; Wang, J.; Yin, S. Preparation and Characterization of Ferulic Acid Wheat Gluten Nanofiber Films with Excellent Antimicrobial Properties. Foods 2023, 12, 2778. https://doi.org/10.3390/foods12142778
Jin C, Zhang H, Ren F, Wang J, Yin S. Preparation and Characterization of Ferulic Acid Wheat Gluten Nanofiber Films with Excellent Antimicrobial Properties. Foods. 2023; 12(14):2778. https://doi.org/10.3390/foods12142778
Chicago/Turabian StyleJin, Chengming, Huijuan Zhang, Feiyue Ren, Jing Wang, and Sheng Yin. 2023. "Preparation and Characterization of Ferulic Acid Wheat Gluten Nanofiber Films with Excellent Antimicrobial Properties" Foods 12, no. 14: 2778. https://doi.org/10.3390/foods12142778
APA StyleJin, C., Zhang, H., Ren, F., Wang, J., & Yin, S. (2023). Preparation and Characterization of Ferulic Acid Wheat Gluten Nanofiber Films with Excellent Antimicrobial Properties. Foods, 12(14), 2778. https://doi.org/10.3390/foods12142778








