Ultrasound- and Microwave-Assisted Extraction of Pectin from Apple Pomace and Its Effect on the Quality of Fruit Bars
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Productions of Apple Pomace (AP)
2.3. Characterizations of Apple Pomace Powder
2.4. Methods of Extractions and Purifications of Pectin from Apple Pomace
2.5. Pectin Characterization
2.5.1. The Preparation of Pectin Solutions for Titrimetric and Spectroscopic Analysis
2.5.2. The Determination of Equivalent Weight (EW)
2.5.3. The Determination of the Methoxyl Groups (MeO)
2.5.4. The Determination of the Content of the Anhydrogalacturonic Acid (AUA)
2.5.5. The Determination of the Degree of Esterification (DE)
2.6. The Production of Fruit Bars
2.6.1. Sensory Analysis of Fruit Bars
2.6.2. The Physicochemical Analysis of the Fruit Bars
2.6.3. The Microbiological Analysis of the Fruit Bars
2.7. Preparation of Apple Pomace and Fruit Bar Extracts for Spectrophotometric Analysis
2.8. Total Polyphenols and Flavonoids by Folin–Ciocâlteu
2.9. Total Tannins by Folin–Ciocâlteu
2.10. Total Carotenoids
2.11. Determination of DPPH Free Radical-Scavenging Activity
2.12. Mathematical Modeling
2.13. The Statistical Analysis of the Results
3. Results
3.1. Characterization of Apple Pomace Powder
3.2. Characterization of Pectin from Apple Pomace
3.2.1. Pectin Yield (PY)
3.2.2. Equivalent Weight (EW)
3.2.3. Methoxyl Content (MeO)
3.2.4. Anhydrogalacturonic Acid (AUA) Content
3.2.5. Degree of Esterification (DE)
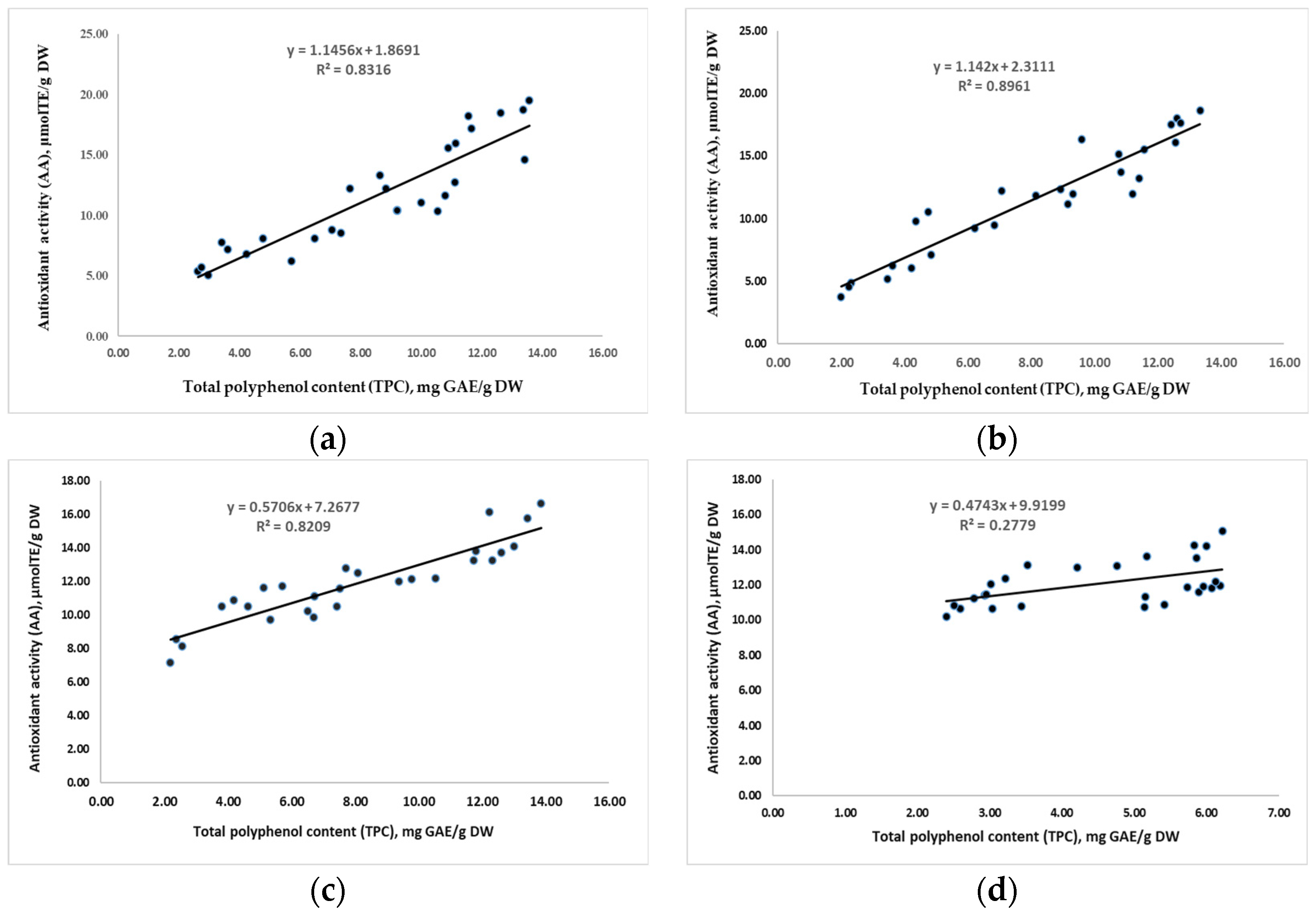
3.2.6. The Antioxidant Activity (AA) of Pectin
3.3. Mathematical Modeling
3.4. The Characteristics of Fruit Bars
3.4.1. Sensory Assessment
3.4.2. Evolution of Physicochemical Parameters and Microbiological Stability of Fruit Bars during Storage
3.4.3. The Biologically Active Compounds and Antioxidant Activity (AA) in Fruit Bars
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Study “Internal and External Analysis of the Fruit Production Sector in the Republic of Moldova”. Available online: http://madrm.gov.md/sites/default/files/Documente%20atasate%20Advance%20Pagines/Studiu%20Sector%20Pomicol%20RM%20-%20%20APM_FARM%20final.pdf (accessed on 2 March 2023). (In Romanian)
- Gołębiewska, E.; Kalinowska, M.; Yildiz, G. Sustainable Use of Apple Pomace (AP) in Different Industrial Sectors. Materials 2022, 15, 1788. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Vergara-Valencia, N.; Granados-Perez, E.; Agama-Acevedo, E.; Tovar, J.; Ruales, J.; Bello-Péreza, L.A. Fibre concentrate from mango fruit: Characteri-zation, associated antioxidant capacity and application as a bakery productingredient. LWT Food Sci. Technol. 2007, 40, 722–729. [Google Scholar] [CrossRef]
- Wicker, L.; Kim, Y.; Kim, M.J.; Thirkield, B.; Lin, Z.; Jung, J. Pectin as a bioactive polysaccharide extracting tailored function from less. Food Hydrocoll. 2014, 42, 251–259. [Google Scholar] [CrossRef]
- Belkheiri, A.; Forouhar, A.; Ursu, A.V.; Dubessay, P.; Pierre, G.; Delattre, C.; Djelveh, G.h.; Abdelkafi, S.; Hamdam, N.; Michaud, P. Extraction, Characterization, and Applications of Pectins from Plant By-Products. Appl. Sci. 2021, 11, 6596. [Google Scholar] [CrossRef]
- Arrutia, F.; Adam, M.; Calvo-Carrascal, M.Á.; Mao, Y.; Binner, E. Development of a continuous-flow system for microwaveassisted extraction of pectin-derived oligosaccharides from food waste. Chem. Eng. J. 2020, 395, 125056. [Google Scholar] [CrossRef]
- Ponmurugan, K.; Al-Dhabi, N.A.; Maran, J.P.; Karthikeyan, K.; Moothy, I.G.; Sivarajasekar, N.; Manoj, J.J.B. Ultrasound assisted pectic polysaccharide extraction and its caracterization from waste heads of Helianthus annus. Carbohydr. Polym. 2017, 173, 707–713. [Google Scholar] [CrossRef]
- Zhang, L.; Xinqian, Y.; Ding, T.; Sun, X.; Xu, Y.; Liu, D. Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrason. Sonochem. 2013, 20, 222–231. [Google Scholar] [CrossRef]
- Vorobiev, E.; Lebovka, N. Pulse Eletric Field-Assisted Extraction. In Enhancing Extraction Processes in the Food Industry; CRC Press: Boca Raton, FL, USA, 2016; pp. 25–84. [Google Scholar]
- Wang, X.; Chen, Q.; L¨u, X. Pectin extracted from apple pomace and citrus peel by subcritical water. Food Hydrocoll. 2014, 38, 129–137. [Google Scholar] [CrossRef]
- Calvete-Torre, I.; Muñoz-Almagro, N.; Pacheco, M.T.; Ant´on, M.J.; Dapena, E.; Ruiz, L.; Margolles, A.; Villamiel, M.; Moreno, F.J. Apple pomaces derived from mono-varietal Asturian ciders production are potential source of pectins with appealing functional properties. Carbohyd. Polym. 2021, 264, 117980. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B.; Guo, Y. Ultrasound-microwave assisted extraction of pectin from fig (Ficus carica L.) skin: Optimization, characterization and bioactivity. Carb. Polym. 2019, 222, 114992. [Google Scholar] [CrossRef]
- Abou-Elseoud, W.S.; Hassan, E.A.; Hassan, M.L. Extraction of pectin from sugar beet pulp by enzymatic and ultrasound-assisted treatments. Carbohydr. Polym. Technol. Appl. 2021, 2, 100042. [Google Scholar] [CrossRef]
- Liew, S.Q.; Ngoh, G.C.; Yusoff, R.; Teoh, W.H. Sequential ultrasound-microwave assisted acid extraction (UMAE) of pectin from pomelo peels. Int. J. Biol. Macromol. 2016, 93, 426–435. [Google Scholar] [CrossRef]
- Wang, S.; Chen, F.; Wu, J.; Wang, Z.; Liao, X.; Hu, X. Optimization of pectin extraction assisted by microwave from apple pomace using response surface methodology. J. Food Eng. 2007, 78, 693–700. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Microwave heating extraction of pectin from lime peel: Characterization and properties compared with the conventional heating method. Food Chem. 2019, 278, 364–372. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Montilla, A.; Moreno, F.J.; Villamiel, M. Modification of citrus and apple pectin by power ultrasound: Effects of acid and enzymatic treatment. Ultrason. Sonochem. 2017, 38, 807–819. [Google Scholar] [CrossRef]
- Wikiera, A.; Kozioł, A.; Mika, M.; Stodolak, B. Structure and bioactivity of apple pectin isolated with arabinanase and mannanase. Food Chem. 2022, 388, 133020. [Google Scholar] [CrossRef]
- Smirnov, V.V.; Golovchenko, V.V.; Vityazev, F.V.; Patova, O.A.; Selivanov, N.Y.; Selivanova, O.G.; Popov, S.V. The Antioxidant Properties of Pectin Fractions Isolated from Fruits Using a Simulated Gastric Fluid. J. Chem. 2017, 2017, 5898594. [Google Scholar] [CrossRef]
- Karbuz, P.; Tugrul, N. Microwave and ultrasound assisted extraction of pectin from various fruits. J. Food Sci. Technol. 2021, 58, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Qadir, S.; Abidi, S.; Azhar, I.; Alam Mahmood, Z. Antioxidant activity and cytotoxicity of pectin extracted from orange peels. Pak. J. Pharm. 2019, 36, 15–24. [Google Scholar]
- Ro, J.; Kim, Y.; Kim, H.; Jang, S.B.; Lee, H.J.; Chakma, S.; Jeong, J.H.; Lee, J. Anti-Oxidative Activity of Pectin and Its Stabilizing Effect on Retinyl Palmtiate. Coreian Physiol. Pharmacol. 2013, 17, 197–201. [Google Scholar]
- Chen, R.; Jin, C.; Tong, Z.; Lu, J.; Tan, L.; Tian, L.; Chang, Q. Optimization extraction, characterization and antioxidant activities of pectic polysaccharide from tangerine peels. Carbohydr. Polym. 2016, 136, 187–197. [Google Scholar] [CrossRef]
- Ogutu, F.O.; Mu, T.H. Ultrasonic degradation of sweet potato pectin and its antioxidant activity. Ultrason. Sonochem. 2017, 38, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lü, X. Characterization of pectic polysaccharides extracted from apple pomace by hot-compressed water. Carbohydr. Polym. 2014, 102, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, P.; Zhang, H. Pectin in cancer therapy: A review. Trends Food Sci. Technol. 2015, 44, 258–271. [Google Scholar] [CrossRef]
- Zhang, T.; Shuai, M.; Ma, P.; Huang, J.; Sun, C.h.; Yao, X.; Chen, Z.; Min, X.; Yan, S. Purification, chemical analysis and antioxidative activity of polysaccharides from pH-modified citrus pectin after dialyzation. LWT 2020, 128, 109513. [Google Scholar] [CrossRef]
- Leclere, L.; Van Cutsem, P.; Michiels, C. Anti-cancer activities of pH-or heat-modified pectin. Front. Pharm. 2013, 4, 128. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.h.; Nie, S.h.; Yu, Q.; Xie, M. Reviews on echanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxidative Med. Cell. Longev. 2016, 64, 5692852. [Google Scholar]
- Celus, M.; Kyomugasho, C.; van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Influence of pectin structural properties on interactions with divalent cations and its associated functionalities. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1576–1594. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Mohamed, A.K. Novel derived pectin hydrogel from mandarin peel based metal-organic frameworks composite for enhanced Cr(VI) and Pb(II) ions removal. Int. J. Biol. Macromol. 2020, 164, 920–931. [Google Scholar] [CrossRef]
- Celus, M.; Salvia-Trujillo, L.; Kyomugasho, C.; Maes, I.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Structurally modified pectin for targeted lipid antioxidant capacity in linseed/sunflower oil-in-water emulsions. Food Chem. 2018, 241, 86–96. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.J.; Gill, C.I.R.; Brien, G.; Rao, J.R.; McRoberts, W.C.; Hughes, P.; McEntee, R.; Rowland, I.R. Anti-cancer properties of phenolics from apple waste on colon carcinogenesis in vitro. Food Chem. Toxicol. 2007, 45, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chauhan, G.S. Extraction and characterization of pectin from apple pomace and its evaluation as lipase (steapsin) inhibitor. Carbohydr. Polym. 2010, 82, 454–459. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K.C.; Li, Y.; Fu, L. In vitro study of possible role of dietaryfibre in lowering postprandial serum glucose. J. Agric. Food Chem. 2001, 49, 1026–1029. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Abari, A.; Amini Rourani, H.; Ghasemi, S.M.; Kim, H.; Kim, Y.G. Investigation of antioxidant and anticancer activities of unsaturated oligo-galacturonic acids produced by pectinase of Streptomyces hydrogenans YAM1. Sci. Rep. 2021, 11, 8491. [Google Scholar] [CrossRef] [PubMed]
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological Activity and Pharmacological Application of Pectic Polysaccharides: A Review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef]
- Popov, S.V.; Ovodov, Y.S. Polypotency of the immunomodulatory effect of pectins. Biochemistry 2013, 78, 823–835. [Google Scholar] [CrossRef]
- Lootens, D.; Capel, F.; Durand, D.; Nicolai, T.; Boulenguer, P.; Langendorff, V. Influence of pH, Ca concentration, temperature and amidation on the gelation of low methoxyl pectin. Food Hydrocoll. 2003, 17, 237–244. [Google Scholar] [CrossRef]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef]
- Lee, Y.; Yoon, Y.R.K.; Chang, H. Effect of pectic oligosaccharide on probiotic survival and physicochemical properties of hydrogel beads for synbiotic encapsulation of Lactobacillus bulgaricus. Food Biosci. 2023, 51, 102260. [Google Scholar] [CrossRef]
- Rehman, A.; Ahmad Rana, T.; Aadil Maria, M.; Spotti Amr, J.; Bakry Imran, M.; Khan, M.; Zhao, L.; Riaz, T.; Tong, Q. Pectin polymers as wall materials for the nano-encapsulation of bioactive compounds. Trends Food Sci. Technol. 2019, 90, 35–46. [Google Scholar] [CrossRef]
- Rohasmizah, H.; Azizah, M. Pectin-based edible coatings and nanoemulsion for the preservation of fruits and fruits: A review. Appl. Food Res. 2022, 2, 100221. [Google Scholar] [CrossRef]
- Mendes, J.F.; Norcino, L.B.; Manrich, A.; Pinheiro, A.C.M.; Oliveira, J.E.; Mattoso, L.H.C. Characterization of pectin films integrated with cocoa butter by continuous casting: Physical, thermal and barrier properties. J. Polym. Environ. 2020, 28, 2905–2917. [Google Scholar] [CrossRef]
- Eça, K.S.; Machado, M.T.C.; Hubinger, M.D.; Menegalli, F.C. Development of active films from pectin and fruit extracts: Light protection, antioxidant capacity, and compounds stability. J. Food Sci. 2015, 80, 2389–2396. [Google Scholar] [CrossRef]
- Muñoz-Labrador, A.; Moreno, R.; Villamiel, M.; Montilla, A. Preparation of citrus pectin gels by power ultrasound and its application as an edible coating in strawberries. J. Sci. Food Agric. 2018, 98, 4866–4875. [Google Scholar] [CrossRef] [PubMed]
- ISO 750:1998; Fruit and Fruit Products—Determination of Titratable Acidity. International Organization for Standardization: Geneva, Switzerland, 1998.
- Nollet, L.M.L. Handbook of Food Analysis, 2nd ed.; Dekker, M., Ed.; CRC Press: Boca Raton, FL, USA, 2004; p. 912. [Google Scholar]
- ISO 659:2009; Oilseeds—Determination of Oil Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 20483:2013; Cereals and Pulses—Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 2013.
- AOAC 985.29; Total Dietary Fibre in Foods. Enzymatic-Gravimetric Method. Official Methods of Analysis. 14th ed. Association of Official Analytical Chemists: Washington, DC, USA, 1985.
- AOAC 991.42; Insoluble Dietary Fibre in Foods and Food Products. Enzymatic-Gravimetric Method. Official Methods of Analysis. 14th ed. Association of Official Analytical Chemists: Washington, DC, USA, 1985.
- Ranganna, S. Handbook of Analysis and Quality Control of Fruit and Fruit Products; Tata McGraw Hill Publishing Co., Ltd.: New Delhi, India, 1986; p. 1112. [Google Scholar]
- Suhaila, M.; Zahariah, H. Extraction and characterisation of pectin from various tropical agrowastes. ASEAN Food J. 1995, 2, 43–50. [Google Scholar]
- ISO 8586:2012; Sensory Analysis General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. Technical Committee, ISO/TC 34/SC 12 Sensory Analysis. International Organization for Standardization: Geneva, Switzerland, 2012.
- ISO 1842:1991; Fruit and Vegetable Products—Determination of pH. Technical Committee ISO/TC 34/SC 3 Fruits and Vegetables and Their Derived Products. International Organization for Standardization: Geneva, Switzerland, 1991.
- ISO 1026:1982; Fruit and Vegetable Products—Determination of Dry Matter Content by Drying under Reduced Pressure and of Water Content by Azeotropic Distillation. Technical Committee ISO/TC 34/SC 9 Microbiology. International Organization for Standardization: Geneva, Switzerland, 1982.
- ISO 21807:2004; Microbiology of Food and Animal Feeding Stuffs—Determination of Water Activity. Technical Committee, ISO/TC 34/SC 9 Microbiology. International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 21149:2017; Microbiology—Enumeration and Detection of Aerobic Mesophilic Bacteria. Technical Committee ISO/TC 217, Cosmetics. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 21527-2:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Mould. Technical Committee ISO/TC 34, Food Products, Subcommittee SC 9, Microbiology. International Organization for Standardization: Geneva, Switzerland, 2008.
- Popescu, L.; Ceșco, T.; Gurev, A.; Ghendov-Mosanu, A.; Sturza, R.; Tarna, R. Impact of Apple Pomace Powder on the Bioactivity, and the Sensory and Textural Characteristics of Yogurt. Foods 2022, 11, 3565. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Dakka, N.; Talbaoui, A.; Moussaoui, N.E.; Abrini, J.; Bakri, Y. Phenolic contents and antiradical capacity of fruit oil from Pistacia lentiscus (L.). J. Mater. Environ. Sci. 2018, 9, 1518–1524. [Google Scholar]
- Singleton, V.; Rossi, J. Colorimetry of total phenolic compounds with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Blackwell Scientific Publication: Oxford, UK, 1994; p. 248. [Google Scholar]
- Cristea, E.; Ghendov-Mosanu, A.; Patras, A.; Socaciu, C.; Pintea, A.; Tudor, C.; Sturza, R. The Influence of Temperature, Storage Conditions, pH, and Ionic Strength on the Antioxidant Activity and Color Parameters of Rowan Berry Extracts. Molecules 2021, 26, 3786. [Google Scholar] [CrossRef]
- Britton, G.; Liaaen, S.; Pfander, H.P. Carotenoids, Vol. 1A: Isolation and Analysis; Birkhauser Verlag: Basel, Switzerland, 1995; pp. 104–107. [Google Scholar]
- Paulpriya, K.; Packia Lincy, M.; Tresina Soris, P.; Veerabahu Ramasamy, M. In vitro antioxidant activity, total phenolic and total flavonoid contents of aerial part extracts of Daphniphyllum neilgherrense (wt.) Rosenth. Ethnopharm. J. Bio Innov. 2015, 4, 257–268. [Google Scholar]
- Paninski, L. Estimation of entropy and mutual information. Neural Comput. 2003, 15, 1191–1253. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, X.; Miao, Z.; Hassan, H.; Song, Y.; Fan, M. Screening for antioxidant and antibacterial activities of phenolics from Golden Delicious apple pomace. Chem. Cent. J. 2010, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Krasnova, I.; Segliņa, D. Content of phenolic compounds and antioxidant activity in fresh apple, pomace and pomace water Extract—Effect of cultivar. Proc. Latv. Acad. Sci. 2019, 73, 513–518. [Google Scholar] [CrossRef]
- Ćetković, G.; Čanadanović-Brunet, J.; Djilas, S.; Savatović, S.; Mandić, A.; Tumbas, V. Assessment of Polyphenolic Content and in Vitro Antiradical Characteristics of Apple Pomace. Food Chem. 2008, 109, 340–347. [Google Scholar] [CrossRef]
- Radenkovs, V.; Kviesis, J.; Juhnevica-Radenkova, K.; Valdovska, A.; Püssa, T.; Klavins, M.; Drudze, I. Valorization of Wild Apple (Malus spp.) By-Products as a Source of Essential Fatty Acids, Tocopherols and Phytosterols with Antimicrobial Activity. Plants 2018, 7, 90. [Google Scholar] [CrossRef]
- Gorjanović, S.; Micić, D.; Pastor, F.; Tosti, T.; Kalušević, A.; Ristić, S.; Zlatanović, S. Evaluation of Apple Pomace Flour Obtained Industrially by Dehydration as a Source of Biomolecules with Antioxidant, Antidiabetic and Antiobesity Effects. Antioxidants 2020, 9, 413. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Ultrasound-Assisted Extraction of Pectin from Malus domestica ‘Falticeni’ Apple Pomace. Processes 2019, 7, 488. [Google Scholar] [CrossRef]
- Bagherian, H.; Zokaee Ashtiani, F.; Fouladitajar, A.; Mohtashamy, M. Comparisons between conventional, microwave- and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem. Eng. Process. Process Intensif. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Kazemi, M.; Najari, Z. Optimization and characterization of pectin extracted from sour orange peel by ultrasound assisted method. Int. J. Biol. Macromol. 2018, 125, 621–629. [Google Scholar] [CrossRef]
- Dranca, F.; Vargas, M.; Oroıan, M. Physicochemical properties of pectin from Malus domestica ‘Fălticeni’ apple pomace as affected by non-conventional extraction techniques. Food Hydrocoll. 2020, 100, 105383. [Google Scholar] [CrossRef]
- Rascón-Chu, A.; Martínez-López, A.L.; Carvajal-Millán, E.; León-Renova, N.E.P.; Márquez-Escalante, J.A.; Romo, A. Pectin from low quality ‘golden delicious’ apples: Composition and gelling capability. Food Chem. 2009, 116, 101–103. [Google Scholar] [CrossRef]
- Paniagua, C.; Pose, S.; Morris, V.J.; Kirby, A.R.; Quesada, M.A.; Mercado, J.A. Fruit softening and pectin disassembly: An overview of nanostructural pectin modifications assessed by atomic force microscopy. Ann. Bot. 2014, 114, 1375–1383. [Google Scholar] [CrossRef]
- Yang, H.; Lai, S.; An, H.; Li, Y. Atomic force microscopy study of the ultrastructural changes of chelate-soluble pectin in peaches under controlled atmosphere storage. Postharvest Biol. Technol. 2006, 39, 75–83. [Google Scholar] [CrossRef]
- Kute, A.; Mohapatra, D.; Babu, B.; Sawant, B. Optimization of microwave assisted extraction of pectin from orange peel using response surface methodology. J. Food Res. Technol. 2015, 3, 62–70. [Google Scholar]
- Azad, A.; Ali, M.; Akter, M.S.; Rahman, M.J.; Ahmed, M. Isolation and characterization of pectin extracted from lemon pomace during ripening. J. Food Nutr. Sci. 2014, 2, 30–35. [Google Scholar] [CrossRef]
- Food Chemical Codex; IV monographs; according to the specifications on purity characteristics of the Joint FAO/WHO Expert 302 Committee on Food Additives and the European Commission; National Academy Press: Washington, DC, USA, 1996; p. 283.
- Sengar, A.S.; Rawson, A.; Muthiah, M.; Kalakandan, S.K. Comparison of different ultrasound assisted extraction techniques for pectin from tomato processing waste. Ultrason. Sonochem. 2020, 61, 104812. [Google Scholar] [CrossRef] [PubMed]
- Guandalini, B.B.V.; Rodrigues, N.P.; Marczak, L.D.F. Sequential extraction of phenolics and pectin from mango peel assisted by ultrasound. Food Res. Int. 2018, 119, 455–461. [Google Scholar] [CrossRef]
- Canteri-Schemin, M.H.; Fertonani, H.C.R.; Waszczynskyj, N.; Wosiacki, G. Extraction of pectin from apple pomace. Braz. Arch. Biol. Technol. 2005, 48, 259–266. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Yarmand, M.S. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr. Polym. 2016, 140, 59–65. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Antioxidant and Radical Scavenging Activities of Polyphenols from Apple Pomace. Food Chem. 2000, 68, 81–85. [Google Scholar] [CrossRef]
- Ghendov-Mosanu, A.; Cristea, E.; Patras, A.; Sturza, R.; Niculaua, M. Rose Hips, a valuable source of antioxidants to improve gingerbread characteristics. Molecules 2020, 25, 5659. [Google Scholar] [CrossRef] [PubMed]
- Ghendov-Mosanu, A.; Cojocari, D.; Balan, G.; Patras, A.; Lung, I.; Soran, M.-L.; Opriş, O.; Cristea, E.; Sturza, R. Chemometric Optimization of Biologically Active Compounds Extraction from Grape Marc: Composition and Antimicrobial Activity. Molecules 2022, 27, 1610. [Google Scholar] [CrossRef]
- Youssef, M.A.; EL Kady, A.A. Evaluation the Activity of Edible Coating for Maintenance the Shelf life of Raisins and Prunes. Curr. Sci. Int. 2016, 5, 103–110. [Google Scholar]
- Jayeola, V.; Farber, J. Induction of the Viable but Non-Culturable State in Salmonella Contaminating Dried Fruit. Appl. Environ. Microbiol. 2021, 88, e0173321. [Google Scholar] [CrossRef]
- Arendse, W.; Jideani, V. Storage Stability and Consumer Acceptability of Dried Apple: Impact of Citric Acid, Potassium Sorbate and Moringa oleifera Leaf Extract Powder. Foods 2022, 11, 984. [Google Scholar] [CrossRef]
- Nunes, J.; Silva, P.; Gaspar, P.; Pires, L.; Andrade, L. The cherry drying as a complementary conservation process to conservation in cold. In Proceedings of the CYTEF 2016−VIII Congresso Ibérico, VI Congresso Ibero-Americano de Ciências e Técnicas do Frio, Coimbra, Portugal, 3–6 May 2016. [Google Scholar]
- Quitral, V.; Sepúlveda, M.; Schwartz, M. Antioxidant Capacity and Total Polyphenol Content in Different Apple Varieties Cultivated in Chile. Rev. Iberoam. Tecnol. Postcosecha 2013, 14, 31–39. [Google Scholar]
- Średnicka-Tober, D.; Ponder, A.; Hallmann, E.; Głowacka, A.; Rozpara, E. The Profile and Content of Polyphenols and Carotenoids in Local and Commercial Sweet Cherry Fruits (Prunus avium L.) and Their Antioxidant Activity In Vitro. Antioxidants 2019, 8, 534. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Nowicka, P.; Laskowski, P.; Oszmiański, J. Evaluation of Sour Cherry (Prunus cerasus L.) Fruits for Their Polyphenol Content, Antioxidant Properties, and Nutritional Components. J. Agric. Food Chem. 2014, 62, 12332–12345. [Google Scholar] [CrossRef]
- Prvulovic, D.; Malenčić, Đ.; Popović, M.; Ljubojević, M.; Ognjanov, V. Antioxidant properties of sweet cherries (Prunus avium L.)—Role of phenolic compounds. World Acad. Sci. Eng. Technol. 2011, 59, 1149–1152. [Google Scholar]
- Zhang, H.; Pu, J.; Tang, Y.; Wang, M.; Tian, K.; Wang, Y.; Luo, X.; Deng, Q. Changes in Phenolic Compounds and Antioxidant Activity during Development of ‘Qiangcuili’ and ‘Cuihongli’ Fruit. Foods 2022, 11, 3198. [Google Scholar] [CrossRef]
- Miletić, N.; Mitrović, O.; Popović, B.; Nedović, V.; Zlatković, B.; Kandić, M. Polyphenolics of Plums and Prunes. J. Food Qual. 2013, 36, 229–237. [Google Scholar] [CrossRef]
- Ishiwata, K.; Yamaguchl, T.; Takamura, H.; Matova, T. DPPH Radical-Scavenging Activity and Polyphenol Content in Dried Fruits. Food Sci. Technol. Res. 2004, 10, 152–156. [Google Scholar] [CrossRef]
- Orhan, D.D.; Özlük, Ö.; Coşkun, S.H. Antioxidant capacities, ascorbic acid and total phenol contents of the plants sold as rose hip in Turkey. FABAD J. Pharm. Sci. 2012, 37, 161–167. [Google Scholar]
- Koczka, N.; Stefanovits-Bányai, É.; Ombódi, A. Total Polyphenol Content and Antioxidant Capacity of Rosehips of Some Rosa Species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Szatan, D.; Zielonka-Brzezicka, J.; Florkowska, K.; Muzykiewicz, A.; Klimowicz, A. Antioxidant Activity of Selected Parts of Prunus domestica L. Harvested at Two Ripening Stages. Pomeranian J. Life Sci. 2020, 66, 65–69. [Google Scholar] [CrossRef]
| Parameters | Mean Value ± SD |
|---|---|
| Moisture content, % | 12.0 ± 0.13 |
| Titratable acidity, % expressed in malic acid | 0.19 ± 0.01 |
| Soluble solids content, o Brix | 15.54 ± 0.03 |
| Fat content, % | 2.74 ± 0.21 |
| Protein content, % | 4.67 ± 0.11 |
| Total dietary fiber content, % | 61.22 ± 1.71 |
| Insoluble dietary fiber content, % | 13.45 ± 0.84 |
| Ash content, % | 2.07± 0.14 |
| Compounds | Mean Value ± SD |
|---|---|
| Total polyphenol content, mg GAE/g DW | 6.03 ± 0.21 |
| Total flavonoid content, mg QE/g DW | 2.13 ± 0.13 |
| Total tannins, mg TAE/g DW | 0.61± 0.03 |
| Total carotenoid content, mg/g DW | 0.04 ± 0.01 |
| Antioxidant activity (DPPH), µmol TE/g DW | 22.82 ± 0.19 |
| Parameters | Hydromodule | Duration of Application, Min | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ultrasounds | Microwaves | ||||||||||||
| 15 | 30 | 5 | 10 | ||||||||||
| pH 1.5 | pH 2.0 | pH 2.5 | pH 1.5 | pH 2.0 | pH 2.5 | pH 1.5 | pH 2.0 | pH 2.5 | pH 1.5 | pH 2.0 | pH 2.5 | ||
| Pectin yield (PY), % | 1:10 | 2.74 ± 0.15 b | 1.50 ± 0.09 a,b | 0.98 ± 0.05 a | 7.04 ± 0.37 e | 2.03 ± 0.10 b | 1.68 ± 0.07 b | 10.71 ± 0.31 h | 4.49 ± 0.19 c,d | 1.18 ± 0.02 a | 16.94 ± 0.23 j | 10.17 ± 0.20 g | 4.50 ± 0.05 c,d |
| 1:15 | 3.87 ± 0.32 c | 1.91 ± 0.08 b | 1.18 ± 0.06 a | 8.73 ± 0.48 f,g | 3.70 ± 0.29 c | 2.23 ± 0.15 b | 11.71 ± 0.29 h | 4.61 ± 0.14 c,d | 1.81 ± 0.05 b | 19.56 ± 0.06 k | 11.49 ± 0.16 h | 6.78 ± 0.08 e | |
| 1:20 | 4.20 ± 0.29 c | 2.06 ± 0.12 b | 1.39 ± 0.11 a | 9.91 ± 0.41 g | 4.30 ± 0.26 c,d | 2.88 ± 0.19 b,c | 13.86 ± 0.33 i | 5.26 ± 0.20 d | 2.28 ± 0.08 b | 19.88 ± 0.29 k | 13.54 ± 0.04 i | 6.37 ± 0.11 e | |
| Equivalent weight (EW), g/mol | 1:10 | 768.1 ± 15.3 d | 1128.0 ± 27.8 f | 1799.2 ± 21.6 h,i | 401.7 ± 7.9 a | 613.1 ± 9.8 c | 907.7 ± 12.9 d,e | 803.7 ± 10.5 d | 1180.0 ± 18.2 f | 1879.3 ± 12.8 i | 526.3 ± 2.4 b | 739.6 ± 4.8 c | 1092.7 ± 11.3 f |
| 1:15 | 652.1 ± 10.5 c | 1117.2 ± 29.6 f | 1927.1 ± 12.9 i | 401.9 ± 9.1 a | 559.6 ± 5.3 b | 900.0 ± 15.2 d,e | 773.7 ± 9.7 d | 1360.0 ± 10.0 g | 1947.9 ± 2.1 i | 490.6 ± 5.6 b | 663.0 ± 5.1 c | 1068.1 ± 10.4 f | |
| 1:20 | 652.5 ± 18.6 c | 1057.3 ± 11.5 e,f | 1659.0 ± 9.0 h | 378.3 ± 6.4 a | 546.8 ± 10.1 b | 849.1 ± 11.3 d | 594.8 ± 8.6 b,c | 1028.8 ± 12.4 e | 2261.7 ± 9.3 j | 421.6 ± 3.1 a | 596.4 ± 3.5 b | 979.9 ± 7.5 e | |
| Methoxyl content (MeO), % | 1:10 | 5.47 ± 0.02 d | 6.40 ± 0.04 j | 6.67 ± 0.04 k,l | 5.14 ± 0.02 b,c | 6.13 ± 0.06 h | 6.63 ± 0.08 k,l | 5.02 ± 0.01 b | 6.17 ± 0.05 h,i | 6.24 ± 0.06 h,i | 5.01 ± 0.4 a,b | 5.87 ± 0.02 f | 6.20 ± 0.03 h,i |
| 1:15 | 5.50 ± 0.03 d | 6.43 ± 0.06 j | 6.71 ± 0.07 k,l | 5.27 ± 0.02 c | 6.25 ± 0.04 i | 6.29 ± 0.05 i | 5.13 ± 0.03 b,c | 6.00 ± 0.04 g | 6.23 ± 0.05 h,i | 4.91 ± 0.03 a | 6.08 ± 0.04 g,h | 6.24 ± 0.03 i | |
| 1:20 | 5.55 ± 0.03 d | 6.38 ± 0.05 i,j | 6.81 ± 0.05 l,m | 5.05 ± 0.02 b | 6.30 ± 0.04 i | 6.63 ± 0.07 k,l | 5.04 ± 0.02 b | 6.07 ± 0.05 g,h | 6.39 ± 0.07 i,j | 4.88 ± 0.02 a | 5.75 ± 0.03 e,f | 6.25 ± 0.04 i | |
| Anhydrogalacturonic acid content (AUA), % | 1:10 | 56.85 ± 0.34 e | 53.96 ± 0.41 d | 50.31 ± 0.28 c | 76.52 ± 0.58 m | 66.16 ± 0.37 i | 61.42 ± 0.31 g | 53.86 ± 0.26 d | 49.62 ± 0.26 b | 47.31 ± 0.18 a | 65.66 ± 0.28 i | 61.74 ± 0.46 g | 58.92 ± 0.30 f |
| 1:15 | 59.45 ± 0.53 f | 54.47 ± 0.50 d | 49.16 ± 0.25 b | 77.18 ± 0.49 m,n | 68.25 ± 0.40 j | 62.37 ± 0.28 g,h | 55.25 ± 0.29 d,e | 48.94 ± 0.30 b | 48.17 ± 0.21 b | 71.19 ± 0.45 k | 64.59 ± 0.37 h | 57.55 ± 0.27 e,f | |
| 1:20 | 61.92 ± 0.51 g | 54.84 ± 0.47 d,e | 53.73 ± 0.35 d | 78.71 ± 0.67 n | 68.95 ± 0.43 j | 62.31 ± 0.31 g,h | 61.67 ± 0.32 g | 52.12 ± 0.25 c | 47.47 ± 0.20 a,b | 73.02 ± 0.47 l | 65.68 ± 0.30 i | 61.04 ± 0.31 g | |
| Degree of esterification (DE), % | 1:10 | 59.87 ± 0.35 h | 64.71 ± 0.41 i | 72.91 ± 0.51 k | 38.16 ± 0.22 b | 51.43 ± 0.38 e | 56.18 ± 0.40 g | 49.86 ± 0.27 d,e | 62.55 ± 0.48 h,i | 71.37 ± 0.59 j,k | 43.76 ± 0.32 c | 53.11 ± 0.48 e,f | 55.55 ± 0.43 f,g |
| 1:15 | 50.73 ± 0.29 e | 63.35 ± 0.38 i | 73.78 ± 0.47 k | 38.77 ± 0.24 b | 48.61 ± 0.29 d | 56.69 ± 0.43 g | 51.83 ± 0.35 e | 61.36 ± 0.32 h | 68.88 ± 0.41 j | 44.74 ± 0.38 c | 50.44 ± 0.37 e | 56.02 ± 0.52 f,g | |
| 1:20 | 48.54 ± 0.31 d | 63.29 ± 0.45 i | 73.50 ± 0.41 k | 36.47 ± 0.29 a | 48.30 ± 0.31 d | 54.67 ± 0.37 f | 46.40 ± 0.31 c,d | 59.16 ± 0.45 g,h | 71.02 ± 0.55 j,k | 38.69 ± 0.31 b | 47.74 ± 0.41 d | 52.45 ± 0.48 e,f | |
| Total polyphenol content (TPC), mg GAE/g DW | 1:10 | 3.93 ± 0.04 c | 6.77 ± 0.07 f | 8.14 ± 0.06 g | 3.92 ± 0.05 c | 9.14 ± 0.11 h | 12.98 ± 0.18 k | 5.42 ± 0.08 d | 7.90 ± 0.09 g | 12.82 ± 0.21 k | 2.50 ± 0.02 a | 3.24 ± 0.07 b | 5.81 ± 0.04 e |
| 1:15 | 4.10 ± 0.05 c | 12.26 ± 0.05 k | 12.07 ± 0.07 j | 4.56 ± 0.07 c | 6.54 ± 0.08 e,f | 12.59 ± 0.15 k | 2.28 ± 0.05 a | 7.12 ± 0.11 f | 10.56 ± 0.23 k | 3.27 ± 0.06 b | 6.02 ± 0.05 e | 5.98 ± 0.06 e | |
| 1:20 | 2.68 ± 0.08 a | 10.40 ± 0.08 i | 11.02 ± 0.10 i | 2.16 ± 0.04 a | 11.14 ± 0.09 i | 10.60 ± 0.11 i | 4.00 ± 0.03 c | 5.93 ± 0.07 e | 13.05 ± 0.07 k | 2.86 ± 0.04 b | 4.97 ± 0.06 d | 5.63 ± 0.04 d,e | |
| DPPH antioxidant activity (AA), μmol TE/g DW | 1:10 | 6.96 ± 0.17 c | 8.43 ± 0.24 d | 12.76 ± 0.22 h | 6.15 ± 0.09 b | 12.14 ± 0.19 g | 18.32 ± 0.35 l | 11.66 ± 0.26 g | 12.65 ± 0.20 g,h | 13.91 ± 0.27 i | 10.43 ± 0.28 f | 10.71 ± 0.31 f | 11.41 ± 0.35 f,g |
| 1:15 | 7.89 ± 0.20 d | 13.67 ± 0.31 h,i | 18.86 ± 0.41 m,l | 10.15 ± 0.14 f | 9.36 ± 0.07 e | 17.55 ± 0.31 k,l | 7.85 ± 0.16 d | 11.34 ± 0.24 f,g | 12.62 ± 0.21 g,h | 12.58 ± 0.19 g,h | 14.68 ± 0.37 i,j | 11.70 ± 0.30 g | |
| 1:20 | 5.53 ± 0.13 b | 11.35 ± 0.26 f,g | 15.76 ± 0.29 j | 4.32 ± 0.05 a | 13.44 ± 0.25 h | 15.94 ± 0.27 j | 10.70 ± 0.21 f | 9.95 ± 0.09 e,f | 16.39 ± 0.35 j,k | 11.33 ± 0.24 f,g | 13.36 ± 0.28 h | 11.47 ± 0.39 f,g | |
| DPPH antioxidant activity, % | 1:10 | 13.37 ± 0.37 b | 14.82 ± 0.15 c | 22.18 ± 1.25 g,h | 11.17 ± 0.21 a | 21.88 ± 0.40 g,h | 30.75 ± 0.76 l,m | 21.68 ± 1.06 g,h | 23.17 ± 1.06 g,h | 24.44 ± 0.41 i | 22.28 ± 0.11 h | 25.57 ± 0.72 j | 18.21 ± 0.91 e,f |
| 1:15 | 14.61 ± 0.35 b,c | 16.78 ± 0.61 d | 29.0 ± 0.41 k | 18.32 ± 0.86 e,f | 22.04 ± 0.29 g,h | 29.96 ± 0.24 l | 20.28 ± 1.21 f,g | 22.25 ± 0.57 g,h | 21.63 ± 1.02 f,g | 28.07 ± 0.07 k | 31.07 ± 0.40 l,m | 19.85 ± 0.43 f | |
| 1:20 | 14.98 ± 0.47 c | 15.67 ± 1.17 c,d | 22.15 ± 0.40 g,h | 14.86 ± 0.27 c | 23.68 ± 0.55 h,i | 29.85 ± 1.62 l,m | 20.11 ± 0.76 f,g | 23.21 ± 0.61 h,i | 22.78 ± 0.64 h | 22.68 ± 0.35 h | 28.78 ± 0.51 k | 19.57 ± 0.61 e,f | |
| Pectin Properties | Influence of pH on Pectin Properties (Bits) | |||
|---|---|---|---|---|
| Ultrasound-Assisted Extraction | Microwave-Assisted Extraction | |||
| 15 min | 30 min | 5 min | 10 min | |
| Pectin yield | 0.885 | 0.873 | 0.998 | 0.998 |
| Equivalent weight (ME) | 0.998 | 0.998 | 0.996 | 0.982 |
| Methoxyl content (MeO) | 0.958 | 0.836 | 0.755 | 0.755 |
| Anhydrogalacturonic acid content (AUA) | 0.836 | 0.985 | 0.821 | 0.645 |
| Degree of esterification (DE) | 0.995 | 0.996 | 0.996 | 0.591 |
| Total polyphenol content (TPC) | 0.491 | 0.812 | 0.916 | 0.522 |
| Antioxidant activity (AA) | 0.684 | 0.915 | 0.325 | 0.101 |
| Sensory Characteristics | Description |
|---|---|
| Appearance, shape, and surface | Glossy surface, slightly sticky. Rectangular shape, susceptible to deformation |
| Consistency | Semi-hard |
| Color | Uniform color. A pronounced dark shade interspersed with pieces of dried yellow apples |
| Taste and smell | Sweet, typical for cherries and dried prunes, with a taste of dried apples and rosehips. No foreign tastes or smells have been identified |
| Parameters | Storage Period, Days | ||||
|---|---|---|---|---|---|
| 1st | 90th | 180th | 270th | 360th | |
| Moisture content, % | 30.0 ± 0.1 e | 28.5 ± 0.1 d | 26.4 ± 0.0 c | 25.1 ± 0.1 b | 23.6 ± 0.1 a |
| pH | 3.61 ± 0.03 a | 3.61 ± 0.02 a | 3.64 ± 0.0 a | 3.75 ± 0.02 b | 3.95 ± 0.01 c |
| Titratable acidity, % expressed in citric acid | 1.12 ± 0.02 c | 1.08 ± 0.01 b | 1.05 ± 0.01 b | 0.84 ± 0.02 a | 0.83 ± 0.02 a |
| Water activity (aw), c.u. | 0.571 ± 0.002 d | 0.565 ± 0.003 d | 0.543 ± 0.001 c | 0.510 ± 0.002 b | 0.496 ± 0.001 a |
| Total viable count (TVC), CFU/g | 0 ± 0 a | 2.0 ± 0.1 b | 2.0 ± 0.1 b | 2.0 ± 0.1 b | 2.0 ± 0.1 b |
| Total polyphenol content, mg GAE/g DW | 7.68 ± 0.12 c | 7.63 ± 0.13 c | 7.57 ± 0.11 c | 6.24 ± 0.13 b | 5.59 ± 0.07 a |
| Total flavonoid content, mg EQ/g DW | 2.75 ± 0.05 d | 2.71 ± 0.09 d | 2.48 ± 0.02 c | 2.13 ± 0.04 b | 1.85 ± 0.05 a |
| Inhibition DPPH, % | 84.09 ± 1.33 d,e | 82.62 ± 1.35 d,e | 77.91 ± 0.48 c,d | 72.29 ± 0.39 b | 67.80 ± 0.56 a |
| Antioxidant activity DPPH, µmol TE/g DW | 24.85 ± 0.14 d | 24.80 ± 0.09 d | 23.52 ± 0.05 c | 22.31 ± 0.07 b | 20.14 ± 0.0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurev, A.; Cesko, T.; Dragancea, V.; Ghendov-Mosanu, A.; Pintea, A.; Sturza, R. Ultrasound- and Microwave-Assisted Extraction of Pectin from Apple Pomace and Its Effect on the Quality of Fruit Bars. Foods 2023, 12, 2773. https://doi.org/10.3390/foods12142773
Gurev A, Cesko T, Dragancea V, Ghendov-Mosanu A, Pintea A, Sturza R. Ultrasound- and Microwave-Assisted Extraction of Pectin from Apple Pomace and Its Effect on the Quality of Fruit Bars. Foods. 2023; 12(14):2773. https://doi.org/10.3390/foods12142773
Chicago/Turabian StyleGurev, Angela, Tatiana Cesko, Veronica Dragancea, Aliona Ghendov-Mosanu, Adela Pintea, and Rodica Sturza. 2023. "Ultrasound- and Microwave-Assisted Extraction of Pectin from Apple Pomace and Its Effect on the Quality of Fruit Bars" Foods 12, no. 14: 2773. https://doi.org/10.3390/foods12142773
APA StyleGurev, A., Cesko, T., Dragancea, V., Ghendov-Mosanu, A., Pintea, A., & Sturza, R. (2023). Ultrasound- and Microwave-Assisted Extraction of Pectin from Apple Pomace and Its Effect on the Quality of Fruit Bars. Foods, 12(14), 2773. https://doi.org/10.3390/foods12142773









