The Hypocholesterolemic Potential of the Edible Algae Fucus vesiculosus: Proteomic and Quantitative PCR Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Algae Extract Preparation and Identification of Compounds
2.3. Cell Culturing
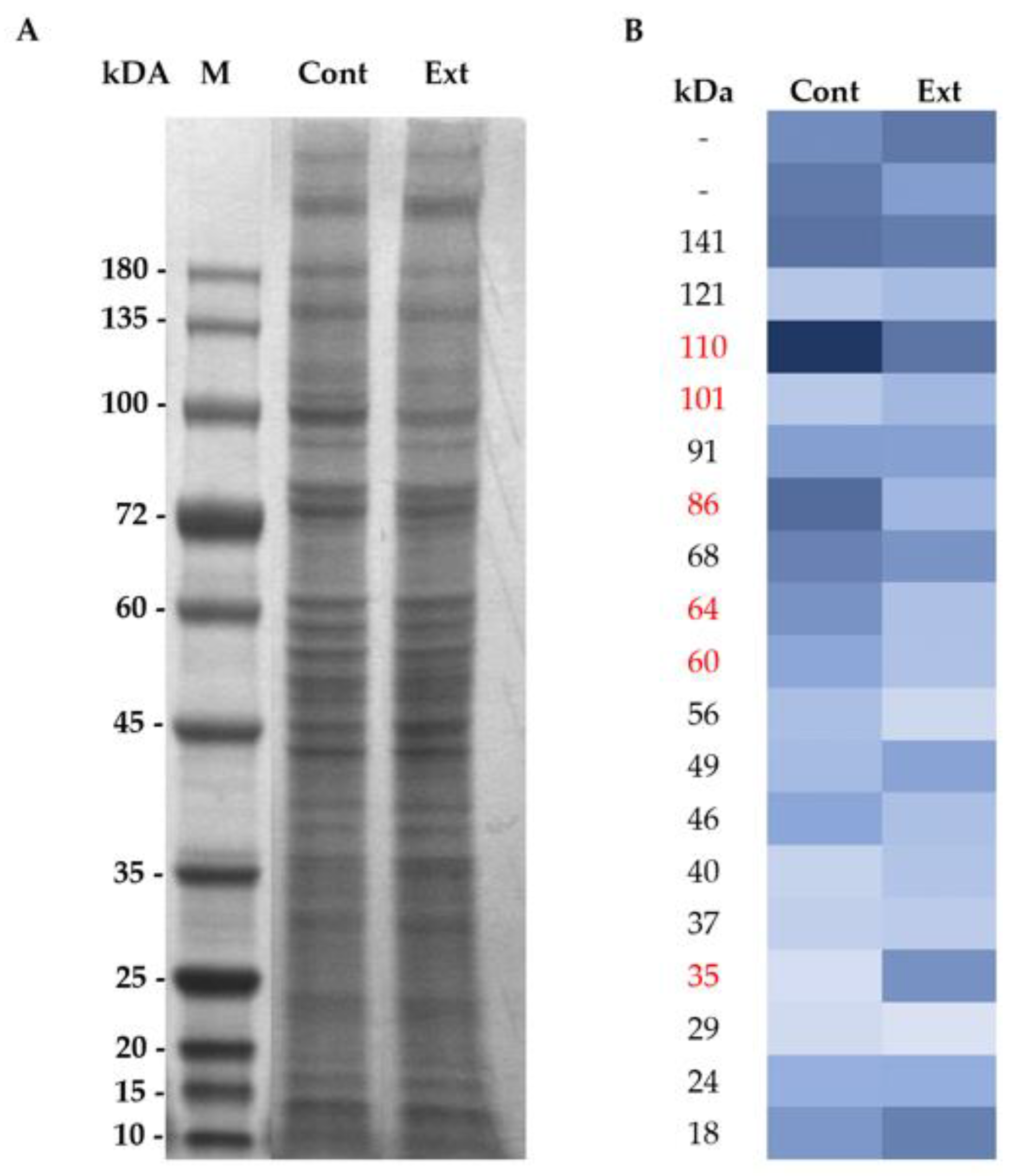
2.4. Membrane Protein Extraction and SDS-PAGE Electrophoresis
2.5. In-Gel Protein Digestion, Nano-LC−ESI−MS/MS and Data Analysis
2.6. Western Blot Analysis
2.7. Real Time Quantitative PCR
2.8. Statistical Analysis
3. Results and Discussion
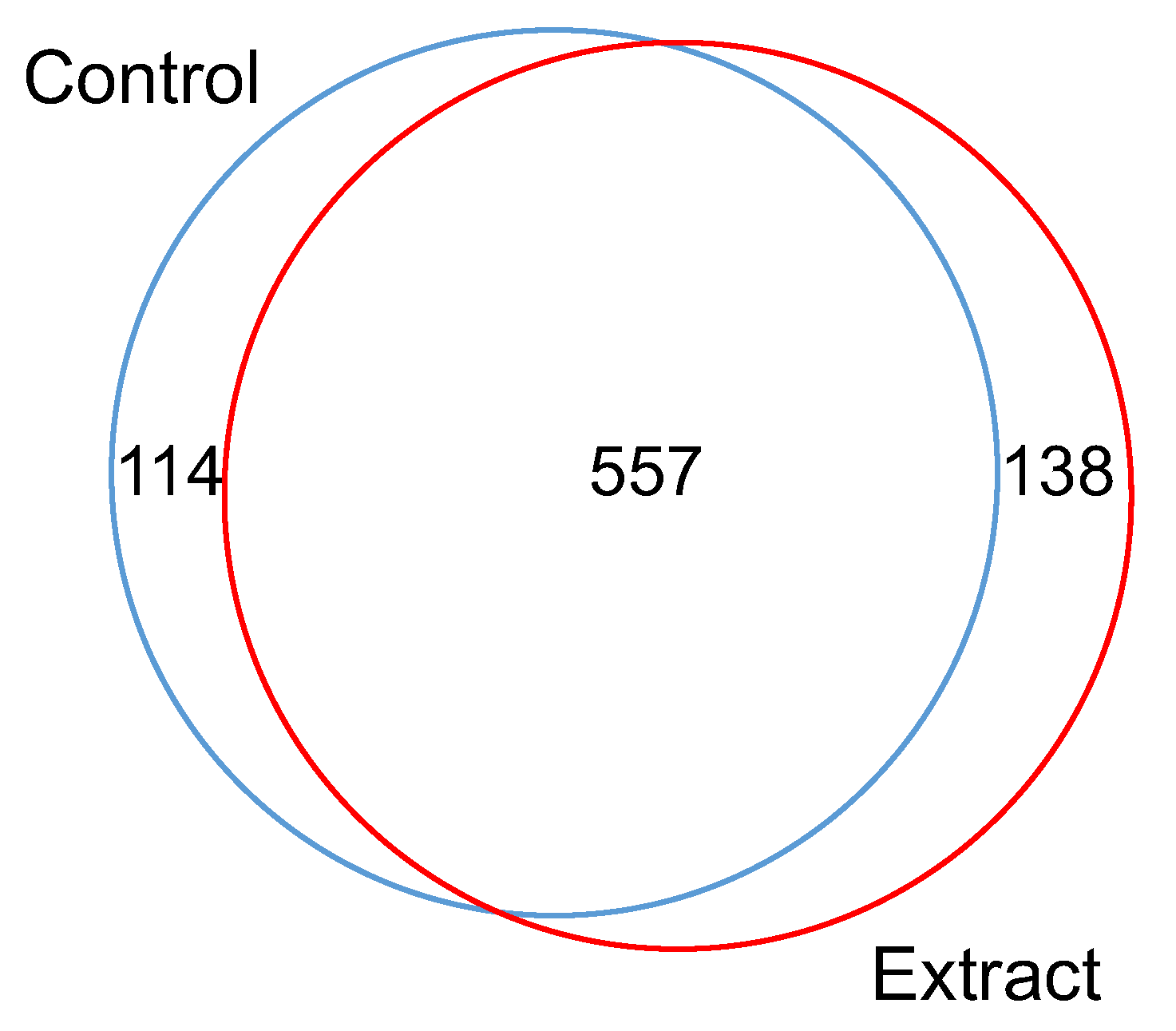
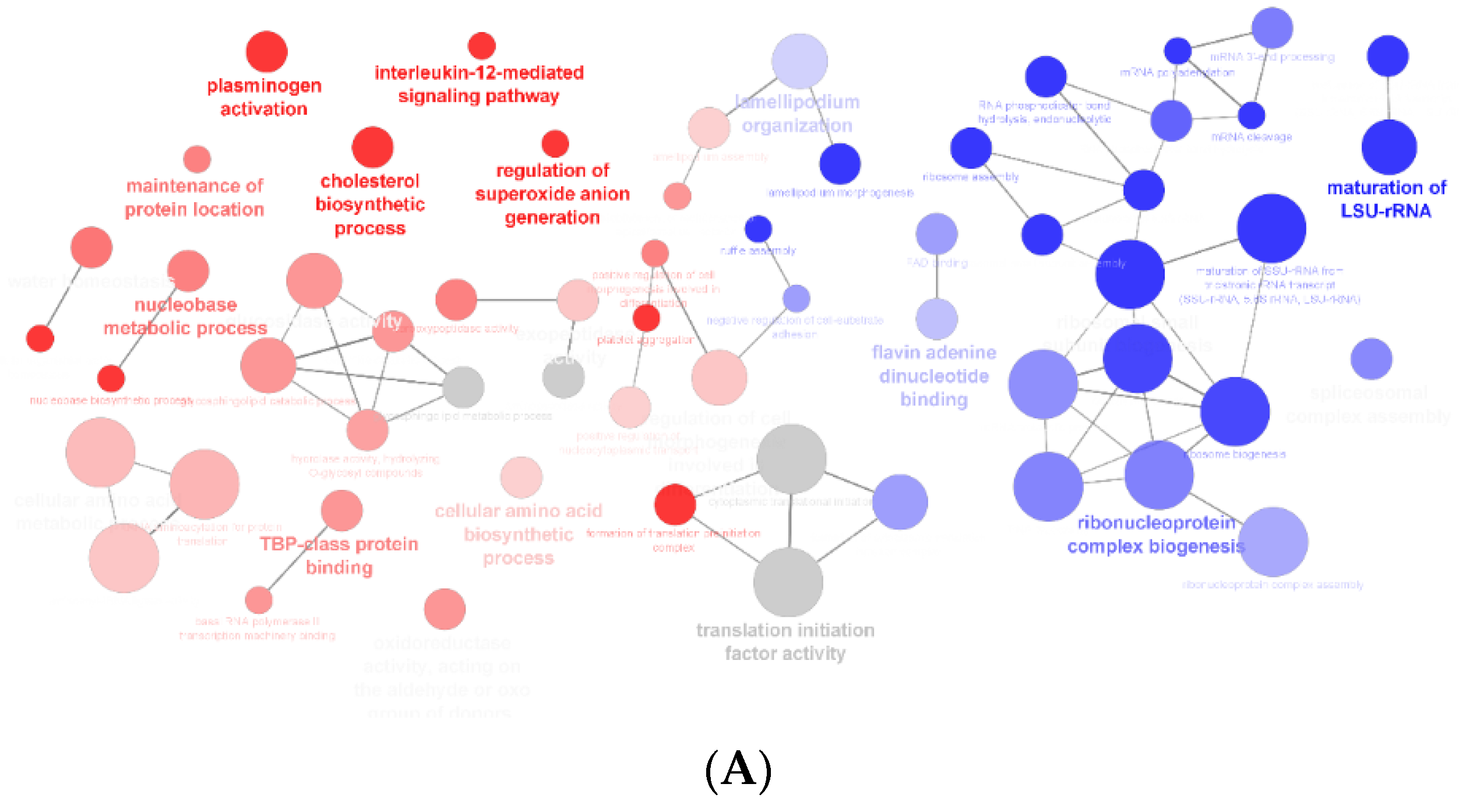
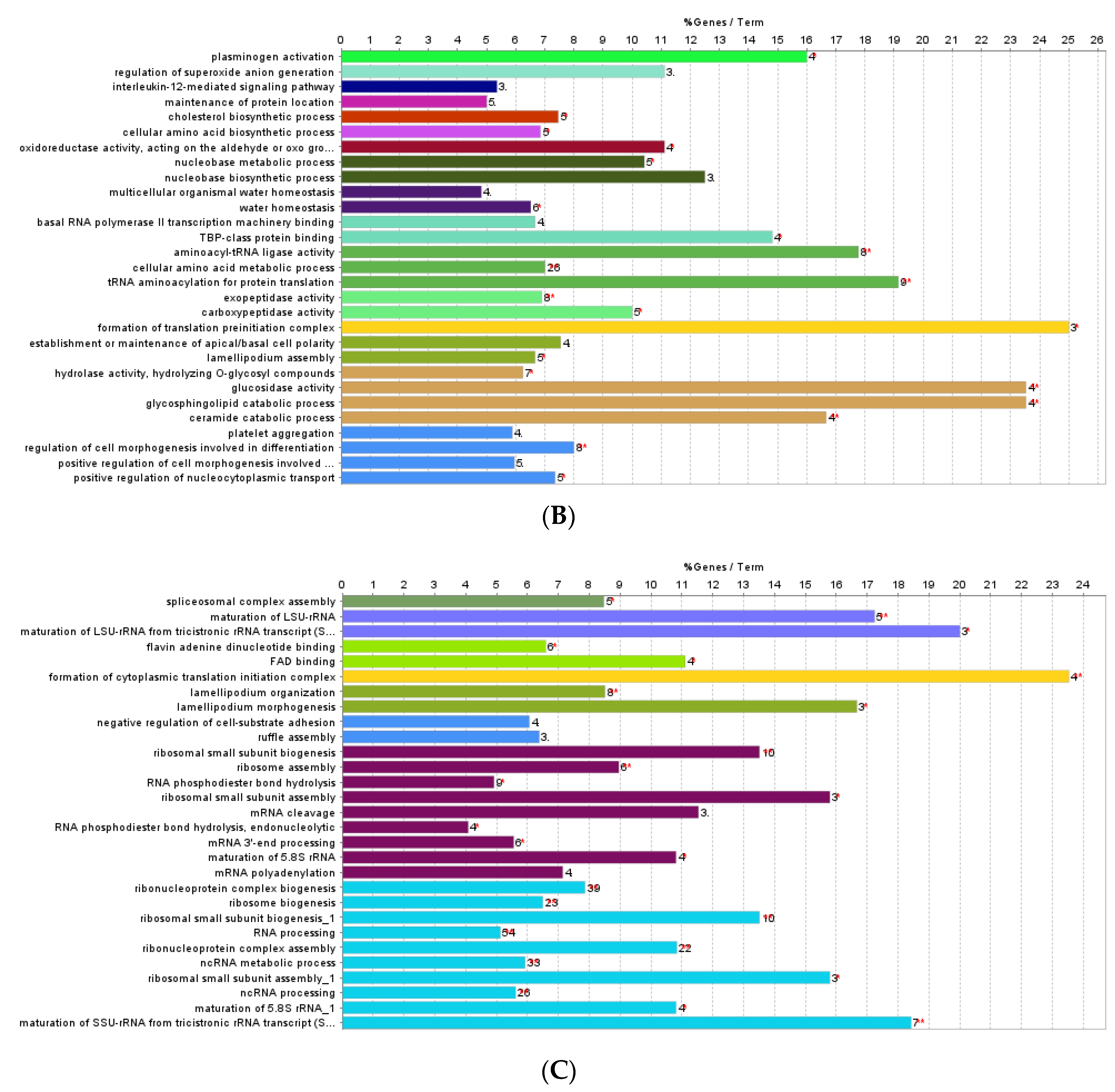
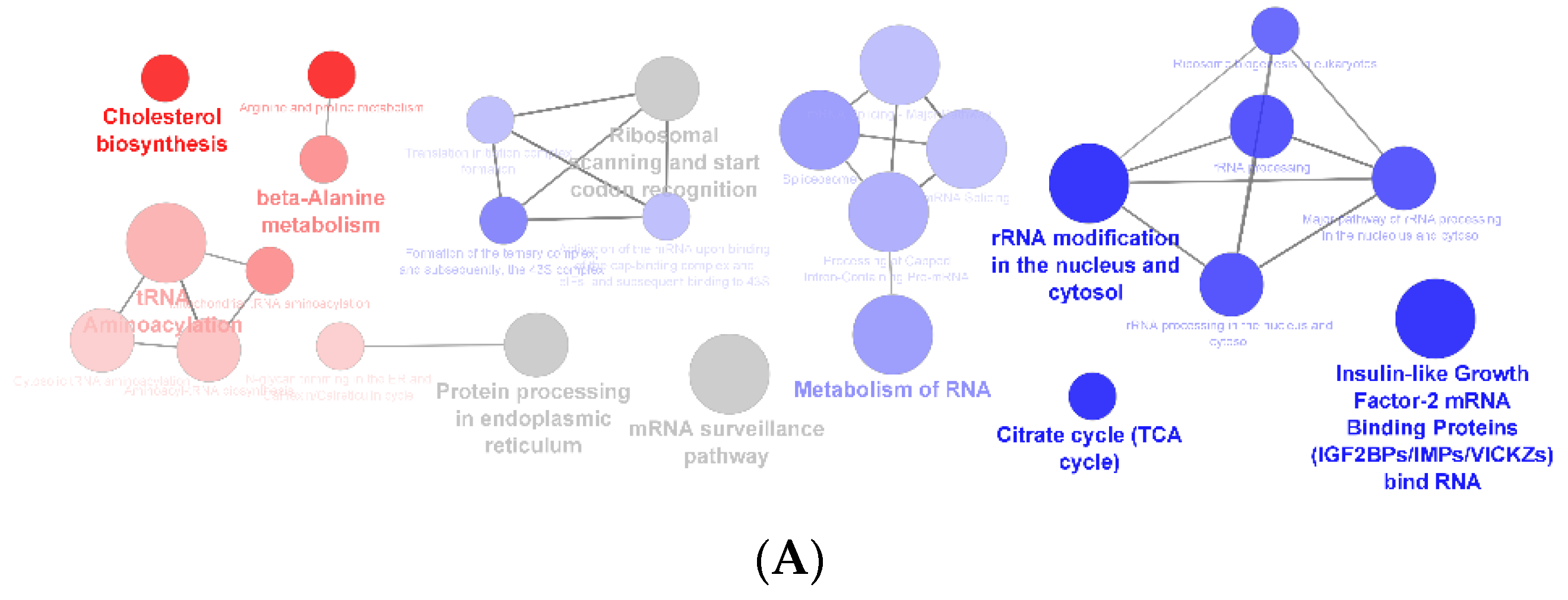
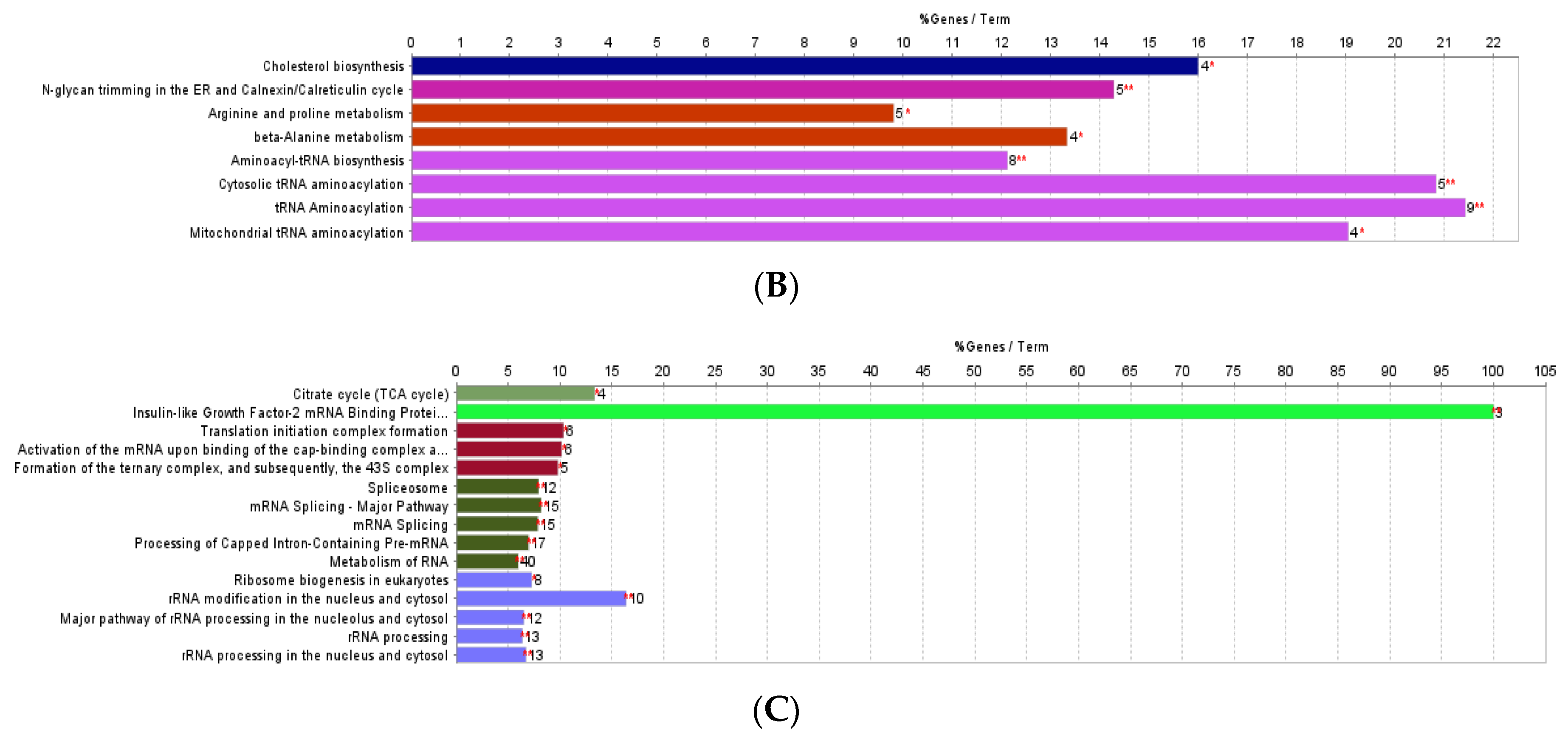
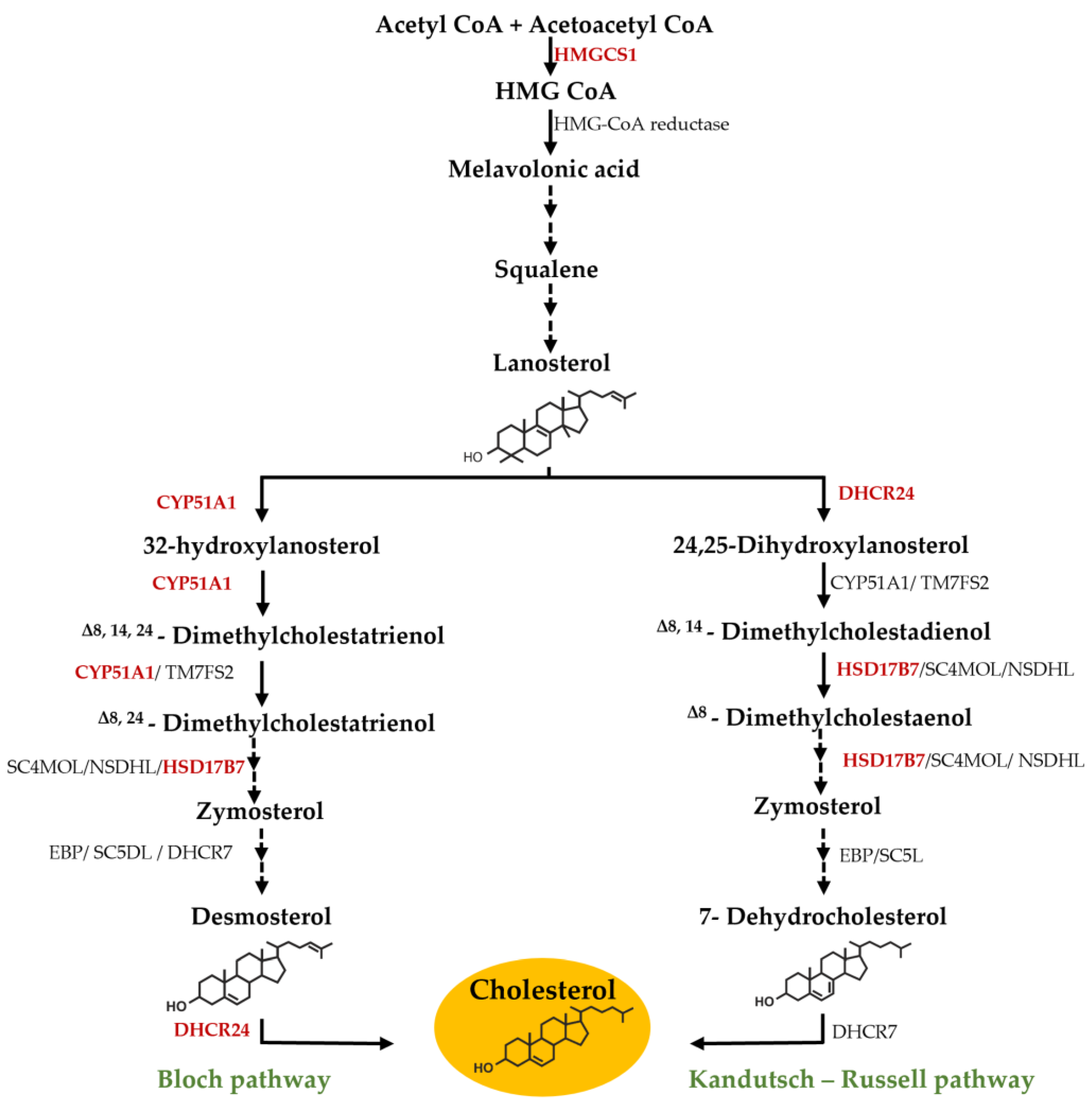
3.1. Effect of F. vesiculosus on Hepatic Proteins
Proteomic Analysis
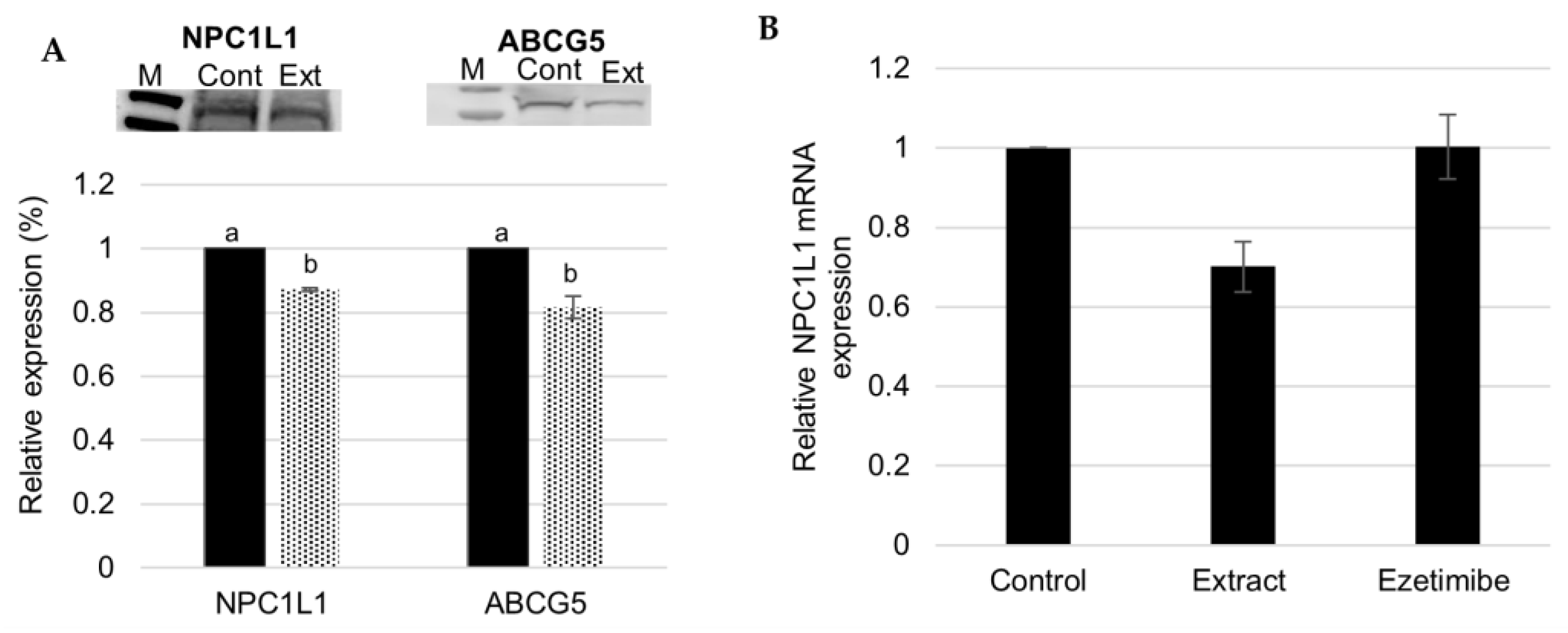
3.2. Effect of Fucus vesiculosus on Cholesterol Transporters
F. vesiculosus Aqueous Extract Decrease Hepatic Expression of NPC1L1 and ABCG5
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Yamanashi, Y.; Takada, T.; Mu, S.; Tanaka, Y.; Komine, T.; Suzuki, H. Hepatic expression of Niemann-Pick C1-like 1, a cholesterol reabsorber from bile, exacerbates western diet-induced atherosclerosis in LDL receptor mutant mice S. Mol. Pharmacol. 2019, 96, 47–55. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, E.; Sayavedra, L. 3.07—Diet, Microbiota and the Gut-Brain Axis. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Wilkins, E.; Wilson, I.; Wickramasinghe, K.; Bhatnagar, P.; Leal, J.; Luengo-Fernandez, R.; Burns, R.; Rayner, M.; Townsend, N. European Cardiovascular Disease Statistics 2017 edition. Eur. Hear. Netw. Bruss. 2017, 192. [Google Scholar]
- Taddei, C.; Zhou, B.; Bixby, H.; Carrillo-Larco, R.M.; Danaei, G.; Jackson, R.T.; Farzadfar, F.; Sophiea, M.K.; Di Cesare, M.; Iurilli, M.L.C.; et al. Repositioning of the global epicentre of non-optimal cholesterol. Nature 2020, 582, 73–77. [Google Scholar] [CrossRef]
- Singh, P.; Saxena, R.; Srinivas, G.; Pande, G.; Chattopadhyay, A. Cholesterol biosynthesis and homeostasis in regulation of the cell cycle. PLoS ONE 2013, 8, e58833. [Google Scholar] [CrossRef] [PubMed]
- Mitsche, M.A.; McDonald, J.G.; Hobbs, H.H.; Cohen, J.C. Flux analysis of cholesterol biosynthesis in vivo reveals multiple tissue and cell-type specific pathways. Elife 2015, 4, e07999. [Google Scholar] [CrossRef] [PubMed]
- Jessup, W.; Gelissen, I.C.; Gaus, K.; Kritharides, L. Roles of ATP binding cassette transporters A1 and G1, scavenger receptor BI and membrane lipid domains in cholesterol export from macrophages. Curr. Opin. Lipidol. 2006, 17, 247–257. [Google Scholar] [CrossRef]
- Tang, W.; Jia, L.; Ma, Y.; Xie, P.; Haywood, J.; Dawson, P.A.; Li, J.; Yu, L. Ezetimibe restores biliary cholesterol excretion in mice expressing Niemann-Pick C1-Like 1 only in liver. Biochim. Biophys. Acta 2011, 1811, 549–555. [Google Scholar] [CrossRef]
- Huff, M.W.; Pollex, R.L.; Hegele, R.A. NPC1L1: Evolution from pharmacological target to physiological sterol transporter. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2433–2438. [Google Scholar] [CrossRef]
- Ge, L.; Wang, J.; Qi, W.; Miao, H.-H.; Cao, J.; Qu, Y.-X.; Li, B.-L.; Song, B.-L. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 2008, 7, 508–519. [Google Scholar] [CrossRef]
- Landis, M.N.; Adams, D.R. Drugs for the Skinternist. In Comprehensive Dermatologic Drug Therapy, 4th ed.; Wolverton, S.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 430–444.e3. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Murillo, A.G. Is There a Correlation between Dietary and Blood Cholesterol? Evidence from Epidemiological Data and Clinical Interventions. Nutrients 2022, 14, 2168. [Google Scholar] [CrossRef]
- André, R.; Pacheco, R.; Bourbon, M.; Serralheiro, M.L. Brown Algae Potential as a Functional Food against Hypercholesterolemia: Review. Foods 2021, 10, 234. [Google Scholar] [CrossRef]
- Ososki, A.L.; Lohr, P.; Reiff, M.; Balick, M.J.; Kronenberg, F.; Fugh-Berman, A.; O’Connor, B. Ethnobotanical literature survey of medicinal plants in the Dominican Republic used for women’s health conditions. J. Ethnopharmacol. 2002, 79, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Geukens, K.; Wijnhoven, L. Assessment Report on Fucus vesiculosus L., Thallus Herbal Preparations in Solid Dosage Form for Oral Use; European Medicines Agency: London, UK, 2014; Volume 44.
- Skibola, C.F. The effect of Fucus vesiculosus, an edible brown seaweed, upon menstrual cycle length and hormonal status in three pre-menopausal women: A case report. BMC Complement. Altern. Med. 2004, 4, 10. [Google Scholar] [CrossRef]
- Romm, A.; Hardy, M.L.; Mills, S. Endocrine Disorders and Adrenal Support. In Botanical Medicine for Women’s Health, 2nd ed.; Elsevier: St. Louis, MO, USA, 2017; pp. 186–210. [Google Scholar]
- Yoon, N.Y.; Kim, H.R.; Chung, H.Y.; Choi, J.S. Anti-hyperlipidemic effect of an edible brown algae, Ecklonia stolonifera, and its constituents on poloxamer 407-induced hyperlipidemic and cholesterol-fed rats. Arch. Pharm. Res. 2008, 31, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-C.; Kim, S.H.; Park, Y.; Lee, B.H.; Hwang, H.J. Effects of 12-week oral supplementation of Ecklonia cava polyphenols on anthropometric and blood lipid parameters in overweight Korean individuals: A double-blind randomized clinical trial. Phytother. Res. 2012, 26, 363–368. [Google Scholar] [CrossRef]
- Yeo, A.-R.; Lee, J.; Tae, I.H.; Park, S.-R.; Cho, Y.H.; Lee, B.H.; Shin, H.C.; Kim, S.H.; Yoo, Y.C. Anti-hyperlipidemic Effect of Polyphenol Extract (Seapolynol(TM)) and Dieckol Isolated from Ecklonia cava in in vivo and in vitro Models. Prev. Nutr. food Sci. 2012, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- André, R.; Guedes, L.; Melo, R.; Ascensão, L.; Pacheco, R.; Vaz, P.D.; Serralheiro, M.L. Effect of food preparations on in vitro bioactivities and chemical components of Fucus vesiculosus. Foods 2020, 9, 955. [Google Scholar] [CrossRef]
- André, R.; Guedes, R.; López, J.; Serralheiro, M.L. Untargeted metabolomic study of HepG2 cells under the effect of Fucus vesiculosus aqueous extract. Rapid Commun. Mass Spectrom. 2021, 35, e9197. [Google Scholar] [CrossRef]
- Oliveira, E.; Araújo, J.E.; Gómez-Meire, S.; Lodeiro, C.; Perez-Melon, C.; Iglesias-Lamas, E.; Otero-Glez, A.; Capelo, J.L.; Santos, H.M. Proteomics analysis of the peritoneal dialysate effluent reveals the presence of calciumregulation proteins and acute inflammatory response. Clin. Proteom. 2014, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Jorge, S.; Capelo, J.L.; Laframboise, W.; Dhir, R.; Lodeiro, C.; Santos, H.M. Development of a Robust Ultrasonic-Based Sample Treatment to Unravel the Proteome of OCT-Embedded Solid Tumor Biopsies. J. Proteome Res. 2019, 18, 2979–2986. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A Peptide Search Engine Integrated into the MaxQuant Environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef]
- Tyanova, S.; Cox, J. Perseus: A Bioinformatics Platform for Integrative Analysis of Proteomics Data in Cancer Research. In Methods in Molecular Biology; von Stechow, L., Ed.; Springer: New York, NY, USA, 2018; pp. 133–148. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Ressaissi, A.; Attia, N.; Pacheco, R.; Falé, P.L.; Serralheiro, M.L.M. Cholesterol transporter proteins in HepG2 cells can be modulated by phenolic compounds present in Opuntia ficus-indica aqueous solutions. J. Funct. Foods 2020, 64, 103674. [Google Scholar] [CrossRef]
- Zou, J.; Feng, D. Lycopene reduces cholesterol absorption through the downregulation of Niemann-Pick C1-like 1 in Caco-2 cells. Mol. Nutr. Food Res. 2015, 59, 2225–2230. [Google Scholar] [CrossRef] [PubMed]
- El-Darzi, N.; Astafev, A.; Mast, N.; Saadane, A.; Lam, M.; Pikuleva, I.A. N,N-Dimethyl-3β-hydroxycholenamide Reduces Retinal Cholesterol via Partial Inhibition of Retinal Cholesterol Biosynthesis Rather Than its Liver X Receptor Transcriptional Activity. Front. Pharmacol. 2018, 9, 827. [Google Scholar] [CrossRef]
- Jokela, H.; Rantakari, P.; Lamminen, T.; Strauss, L.; Ola, R.; Mutka, A.-L.; Gylling, H.; Miettinen, T.; Pakarinen, P.; Sainio, K.; et al. Hydroxysteroid (17beta) dehydrogenase 7 activity is essential for fetal de novo cholesterol synthesis and for neuroectodermal survival and cardiovascular differentiation in early mouse embryos. Endocrinology 2010, 151, 1884–1892. [Google Scholar] [CrossRef]
- Wang, Y.; Rogers, P.M.; Su, C.; Varga, G.; Stayrook, K.R.; Burris, T.P. Regulation of cholesterologenesis by the oxysterol receptor, LXRalpha. J. Biol. Chem. 2008, 283, 26332–26339. [Google Scholar] [CrossRef]
- Song, B.-L.; Javitt, N.B.; DeBose-Boyd, R.A. Insig-mediated degradation of HMG CoA reductase stimulated by lanosterol, an intermediate in the synthesis of cholesterol. Cell Metab. 2005, 1, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Ercal, B.; Crawford, P.A. Ketone Body Metabolism in the Neonate. In Fetal and Neonatal Physiology, 5th ed.; Polin, R.A., Abman, S.H., Rowitch, D.H., Benitz, W.E., Fox, W.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 370–379. [Google Scholar] [CrossRef]
- Falé, P.L.; Amaral, F.; Amorim Madeira, P.J.; Sousa Silva, M.; Florêncio, M.H.; Frazão, F.N.; Serralheiro, M.L.M. Acetylcholinesterase inhibition, antioxidant activity and toxicity of Peumus boldus water extracts on HeLa and Caco-2 cell lines. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2012, 50, 2656–2662. [Google Scholar] [CrossRef]
- Xie, P.; Jia, L.; Ma, Y.; Ou, J.; Miao, H.; Wang, N.; Guo, F.; Yazdanyar, A.; Jiang, X.-C.; Yu, L. Ezetimibe inhibits hepatic Niemann-Pick C1-Like 1 to facilitate macrophage reverse cholesterol transport in mice. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 920–925. [Google Scholar] [CrossRef]
- Kawase, A.; Hata, S.; Takagi, M.; Iwaki, M. Pravastatin modulate niemann-pick C1-like 1 and ATP-binding cassette G5 and G8 to influence intestinal cholesterol absorption. J. Pharm. Pharm. Sci. 2015, 18, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, A.J.; Lamarche, B.; Lemelin, V.; Hoos, L.; Benjannet, S.; Seidah, N.G.; Davis, H.R.; Couture, P. Atorvastatin increases intestinal expression of NPC1L1 in hyperlipidemic men. J. Lipid Res. 2011, 52, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Ma, Y.; Yu, L. Plasma cholesterol is hyperresponsive to statin in ABCG5/ABCG8 transgenic mice. Hepatology 2006, 44, 1259–1266. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
André, R.; Pacheco, R.; Alves, A.C.; Santos, H.M.; Bourbon, M.; Serralheiro, M.L. The Hypocholesterolemic Potential of the Edible Algae Fucus vesiculosus: Proteomic and Quantitative PCR Analysis. Foods 2023, 12, 2758. https://doi.org/10.3390/foods12142758
André R, Pacheco R, Alves AC, Santos HM, Bourbon M, Serralheiro ML. The Hypocholesterolemic Potential of the Edible Algae Fucus vesiculosus: Proteomic and Quantitative PCR Analysis. Foods. 2023; 12(14):2758. https://doi.org/10.3390/foods12142758
Chicago/Turabian StyleAndré, Rebeca, Rita Pacheco, Ana Catarina Alves, Hugo M. Santos, Mafalda Bourbon, and Maria Luísa Serralheiro. 2023. "The Hypocholesterolemic Potential of the Edible Algae Fucus vesiculosus: Proteomic and Quantitative PCR Analysis" Foods 12, no. 14: 2758. https://doi.org/10.3390/foods12142758
APA StyleAndré, R., Pacheco, R., Alves, A. C., Santos, H. M., Bourbon, M., & Serralheiro, M. L. (2023). The Hypocholesterolemic Potential of the Edible Algae Fucus vesiculosus: Proteomic and Quantitative PCR Analysis. Foods, 12(14), 2758. https://doi.org/10.3390/foods12142758







