Use of Essential Oil Emulsions to Control Escherichia coli O157:H7 in the Postharvest Washing of Lettuce
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of EO Emulsion
2.2. Preparation of Bacteria Culture
2.3. Minimum Inhibitory and Minimum Bactericidal Concentration
2.4. Inoculation of Bacteria Culture on Lettuce Leaves
2.5. Preparation of Washing Treatments
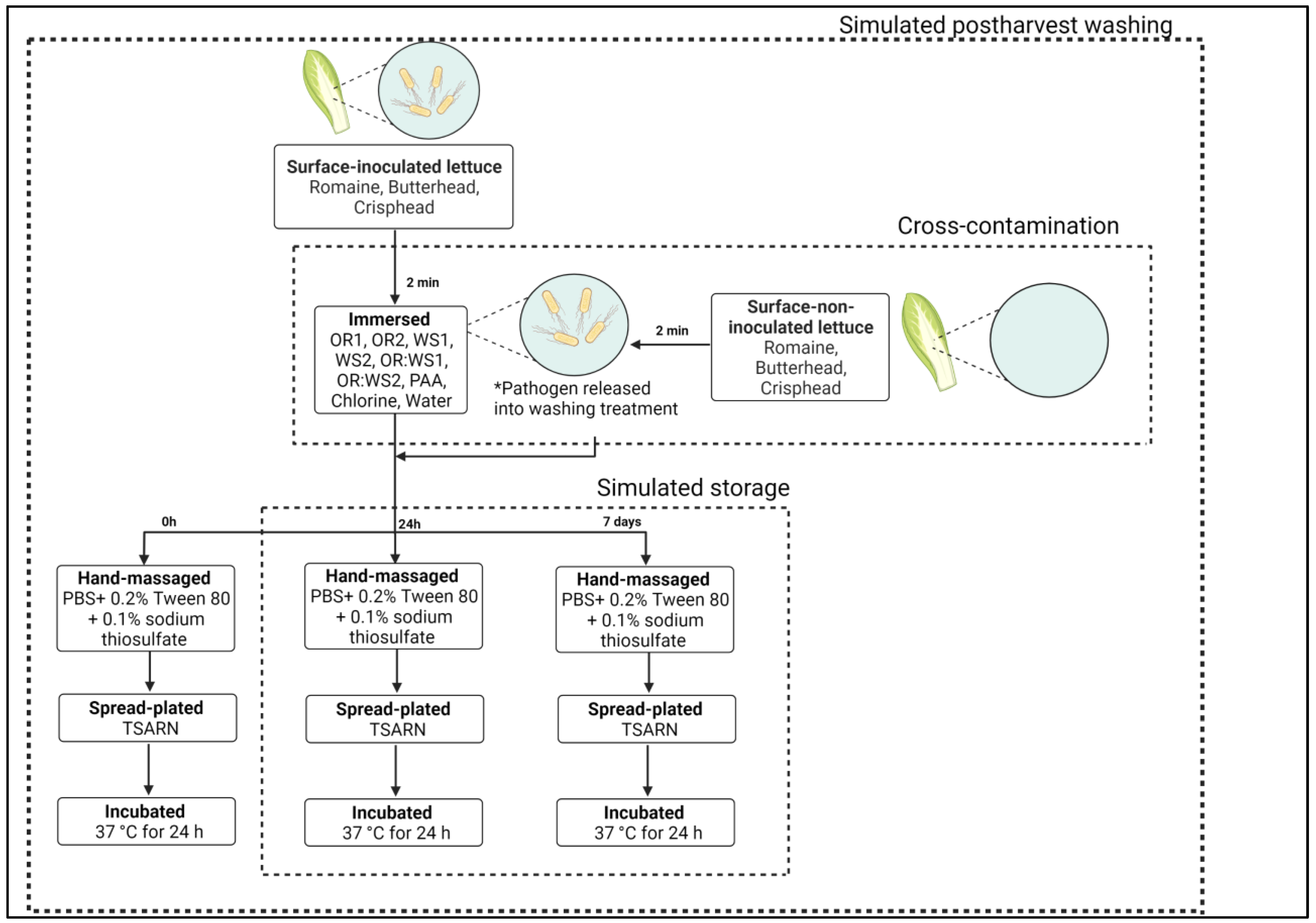
2.6. Simulated Postharvest Washing
2.7. Simulated Storage
2.8. Cross-Contamination in Non-Inoculated Lettuce Leaves
2.9. Statistical Analysis
3. Results
3.1. Minimum Inhibitory and Minimum Bactericidal Concentration
3.2. Effects of Treatments on Inoculated Lettuce Leaves over Time
3.3. Cross-Contamination on Non-Inoculated Lettuce Leaves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC Lettuce, Other Leafy Greens, and Food Safety. Available online: https://www.cdc.gov/foodsafety/communication/leafy-greens.html (accessed on 9 October 2022).
- Coulombe, G.; Catford, A.; Martinez-Perez, A.; Buenaventura, E. Outbreaks of Escherichia Coli O157:H7 Infections Linked to Romaine Lettuce in Canada from 2008 to 2018: An Analysis of Food Safety Context. J. Food Prot. 2020, 83, 1444–1462. [Google Scholar] [CrossRef] [PubMed]
- Pahariya, P.; Fisher, D.J.; Choudhary, R. Comparative Analyses of Sanitizing Solutions on Microbial Reduction and Quality of Leafy Greens. LWT 2022, 154, 112696. [Google Scholar] [CrossRef]
- Cimowsky, S.; Kumar, G.D.; Biscaia Ribeiro da Silva, A.L.; White, E.; Kerr, W.L.; Rodrigues, C.; Juneja, V.K.; Dunn, L.L. Postharvest Control of Escherichia Coli O157:H7 on Romaine Lettuce Using a Novel Pelargonic Acid Sanitizer. LWT 2022, 154, 112168. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, M.H.; Song, K.B. Efficacy of Aqueous Chlorine Dioxide and Fumaric Acid for Inactivating Pre-Existing Microorganisms and Escherichia Coli O157:H7, Salmonella Typhimurium, and Listeria Monocytogenes on Broccoli Sprouts. Food Control 2009, 20, 1002–1005. [Google Scholar] [CrossRef]
- Neo, S.Y.; Lim, P.Y.; Phua, L.K.; Khoo, G.H.; Kim, S.J.; Lee, S.C.; Yuk, H.G. Efficacy of Chlorine and Peroxyacetic Acid on Reduction of Natural Microflora, Escherichia Coli O157:H7, Listeria Monocyotgenes and Salmonella Spp. on Mung Bean Sprouts. Food Microbiol. 2013, 36, 475–480. [Google Scholar] [CrossRef]
- Dunn, L.L.; Harness, M.L.; Smith, D.M.; Gorman, S.J.; Zhong, Q.; Davidson, P.M.; Critzer, F.J. Essential Oil Emulsions as Postharvest Sanitizers to Mitigate Salmonella Cross-Contamination on Peppers. J. Food Prot. 2019, 82, 159–163. [Google Scholar] [CrossRef]
- Banach, J.L.; Sampers, I.; Van Haute, S.; Van Der Fels-Klerx, I.; Uyttendaele, M.; Franz, E.; Schlüter, O. Effect of Disinfectants on Preventing the Cross-Contamination of Pathogens in Fresh Produce Washing Water. Int. J. Environ. Res. Public Health 2015, 12, 8658–8677. [Google Scholar] [CrossRef]
- Chen, C.H.; Yin, H.B.; Teng, Z.I.; Byun, S.; Guan, Y.; Luo, Y.; Upadhyay, A.; Patel, J. Nanoemulsified Carvacrol as a Novel Washing Treatment Reduces Escherichia Coli O157:H7 on Spinach and Lettuce. J. Food Prot. 2021, 84, 2163–2173. [Google Scholar] [CrossRef]
- Park, J.B.; Kang, J.H.; Bin Song, K. Clove Bud Essential Oil Emulsion Containing Benzethonium Chloride Inactivates Salmonella Typhimurium and Listeria Monocytogenes on Fresh-Cut Pak Choi during Modified Atmosphere Storage. Food Control 2019, 100, 17–23. [Google Scholar] [CrossRef]
- Park, J.B.; Kang, J.H.; Song, K. Bin Geranium Essential Oil Emulsion Containing Benzalkonium Chloride as a Wash Solution on Fresh-Cut Vegetables. Food Bioproc. Tech. 2018, 11, 2164–2171. [Google Scholar] [CrossRef]
- Dávila-Rodríguez, M.; López-Malo, A.; Palou, E.; Ramírez-Corona, N.; Jiménez-Munguía, M.T. Antimicrobial Activity of Nanoemulsions of Cinnamon, Rosemary, and Oregano Essential Oils on Fresh Celery. LWT 2019, 112, 108247. [Google Scholar] [CrossRef]
- Pellegrini, M.; Rossi, C.; Palmieri, S.; Maggio, F.; Chaves-López, C.; Lo Sterzo, C.; Paparella, A.; De Medici, D.; Ricci, A.; Serio, A. Salmonella Enterica Control in Stick Carrots Through Incorporation of Coriander Seeds Essential Oil in Sustainable Washing Treatments. Front. Sustain. Food Syst. 2020, 4, 14. [Google Scholar] [CrossRef]
- Bhargava, K.; Conti, D.S.; da Rocha, S.R.P.; Zhang, Y. Application of an Oregano Oil Nanoemulsion to the Control of Foodborne Bacteria on Fresh Lettuce. Food Microbiol. 2015, 47, 69–73. [Google Scholar] [CrossRef]
- Mouatcho, J.C.; Tzortzakis, N.; Sivakumar, D. Bio-Sanitation Treatment Using Essential Oils against E. Coli O157:H7 on Fresh Lettuce. N. Zealand J. Crop Hortic. Sci. 2017, 45, 165–174. [Google Scholar] [CrossRef]
- Olijhoek, D.W.; Hellwing, A.L.F.; Grevsen, K.; Haveman, L.S.; Chowdhury, M.R.; Løvendahl, P.; Weisbjerg, M.R.; Noel, S.J.; Højberg, O.; Wiking, L.; et al. Effect of Dried Oregano (Origanum Vulgare L.) Plant Material in Feed on Methane Production, Rumen Fermentation, Nutrient Digestibility, and Milk Fatty Acid Composition in Dairy Cows. J. Dairy Sci. 2019, 102, 9902–9918. [Google Scholar] [CrossRef] [PubMed]
- Béjaoui, A.; Chaabane, H.; Jemli, M.; Boulila, A.; Boussaid, M. Essential Oil Composition and Antibacterial Activity of Origanum Vulgare Subsp. Glandulosum Desf. at Different Phenological Stages. J. Med. Food 2013, 16, 1115. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, Z.; Kladar, N.; Čabarkapa, I.; Radinović, M.; Maletić, M.; Erdeljan, M.; Božin, B. New Perspective of Origanum Vulgare L. and Satureja Montana L. Essential Oils as Bovine Mastitis Treatment Alternatives. Antibiotics 2021, 10, 1460. [Google Scholar] [CrossRef]
- Kosakowska, O.; Węglarz, Z.; Bączek, K. Yield and Quality of ‘Greek Oregano’ (Origanum Vulgare L. Subsp. Hirtum) Herb from Organic Production System in Temperate Climate. Ind. Crop. Prod. 2019, 141, 111782. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Simin, N.; Anackov, G. Characterization of the Volatile Composition of Essential Oils of Some Lamiaceae Spices and the Antimicrobial and Antioxidant Activities of the Entire Oils. J. Agric. Food Chem. 2006, 54, 1822–1828. [Google Scholar] [CrossRef]
- Pizzo, J.S.; Visentainer, J.V.; da Silva, A.L.B.R.; Rodrigues, C. Application of Essential Oils as Sanitizer Alternatives on the Postharvest Washing of Fresh Produce. Food Chem. 2023, 407, 135101. [Google Scholar] [CrossRef]
- Miladi, H.; ben Slama, R.; Mili, D.; Zouari, S.; Bakhrouf, A.; Ammar, E. Chemical Composition and Cytotoxic and Antioxidant Activities of Satureja Montana L. Essential Oil and Its Antibacterial Potential against Salmonella Spp. Strains. J. Chem. 2013, 2013, 275698. [Google Scholar] [CrossRef]
- Ben Lagha, A.; Vaillancourt, K.; Huacho, P.M.; Grenier, D. Effects of Labrador Tea, Peppermint, and Winter Savory Essential Oils on Fusobacterium Nucleatum. Antibiotics 2020, 9, 794. [Google Scholar] [CrossRef]
- Pino-Otín, M.R.; Gan, C.; Terrado, E.; Sanz, M.A.; Ballestero, D.; Langa, E. Antibiotic Properties of Satureja Montana L. Hydrolate in Bacteria and Fungus of Clinical Interest and Its Impact in Non-Target Environmental Microorganisms. Sci. Rep. 2022, 12, 18460. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, G.; Lupidi, G.; Maggi, F. Comparison of Chemical Composition and Antioxidant Activities of Two Winter Savory Subspecies (Satureja Montana Subsp. Variegata and Satureja Montana Subsp. Montana) Cultivated in Northern Italy. Nat. Prod. Res. 2019, 33, 3143–3147. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Ibrahim, S.A. Natural Products as Antimicrobial Agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial Mechanisms of Cinnamon and Its Constituents: A Review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef]
- Ziaee, E.; Razmjooei, M.; Shad, E.; Eskandari, M.H. Antibacterial Mechanisms of Zataria Multiflora Boiss. Essential Oil against Lactobacillus Curvatus. LWT 2018, 87, 406–412. [Google Scholar] [CrossRef]
- Saad, N.Y.; Muller, C.D.; Lobstein, A. Major Bioactivities and Mechanism of Action of Essential Oils and Their Components. Flavour. Fragr. J. 2013, 28, 269–279. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, H.; Beuchat, L.R.; Ryu, J.H. Synergistic Activities of Gaseous Oregano and Thyme Thymol Essential Oils against Listeria Monocytogenes on Surfaces of a Laboratory Medium and Radish Sprouts. Food Microbiol. 2020, 86, 103357. [Google Scholar] [CrossRef]
- Milagres de Almeida, J.; Crippa, B.L.; Martins Alencar de Souza, V.V.; Perez Alonso, V.P.; da Motta Santos Júnior, E.; Siqueira Franco Picone, C.; Prata, A.S.; Cirone Silva, N.C. Antimicrobial Action of Oregano, Thyme, Clove, Cinnamon and Black Pepper Essential Oils Free and Encapsulated against Foodborne Pathogens. Food Control 2023, 144, 109356. [Google Scholar] [CrossRef]
- Nguefack, J.; Tamgue, O.; Dongmo, J.B.L.; Dakole, C.D.; Leth, V.; Vismer, H.F.; Amvam Zollo, P.H.; Nkengfack, A.E. Synergistic Action between Fractions of Essential Oils from Cymbopogon Citratus, Ocimum Gratissimum and Thymus Vulgaris against Penicillium Expansum. Food Control 2012, 23, 377–383. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, D.; Cao, Y.; Zhang, Y.; Xiao, X.; Liu, F.; Yu, Y. Synergistic Inactivation of Escherichia Coli O157:H7 and Staphylococcus Aureus by Gallic Acid and Thymol and Its Potential Application on Fresh-Cut Tomatoes. Food Microbiol. 2022, 102, 103925. [Google Scholar] [CrossRef] [PubMed]
- Miladi, H.; Zmantar, T.; Kouidhi, B.; Al Qurashi, Y.M.A.; Bakhrouf, A.; Chaabouni, Y.; Mahdouani, K.; Chaieb, K. Synergistic Effect of Eugenol, Carvacrol, Thymol, p-Cymene and γ-Terpinene on Inhibition of Drug Resistance and Biofilm Formation of Oral Bacteria. Microb. Pathog. 2017, 112, 156–163. [Google Scholar] [CrossRef] [PubMed]
- FDA. Code of Federal Regulations Title 21 Chapter 1. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=182.20 (accessed on 11 January 2023).
- Zhang, L.; Critzer, F.; Davidson, P.M.; Zhong, Q. Formulating Essential Oil Microemulsions as Washing Solutions for Organic Fresh Produce Production. Food Chem. 2014, 165, 113–118. [Google Scholar] [CrossRef]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Ultrasonic Emulsification of Food-Grade Nanoemulsion Formulation and Evaluation of Its Bactericidal Activity. Ultrason. Sonochem. 2013, 20, 338–344. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—CLSI Document M07-A10, 10th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; ISBN 610.688.0700. [Google Scholar]
- Chazotte, B. Labeling Golgi with Fluorescent Ceramides. Cold Spring Harb. Protoc. 2012, 8. [Google Scholar] [CrossRef]
- RStudio Team RStudio: Integrated Development for, R. Available online: http://www.rstudio.com/ (accessed on 14 February 2023).
- Vitanza, L.; Maccelli, A.; Marazzato, M.; Scazzocchio, F.; Comanducci, A.; Fornarini, S.; Crestoni, M.E.; Filippi, A.; Fraschetti, C.; Rinaldi, F.; et al. Satureja Montana L. Essential Oil and Its Antimicrobial Activity Alone or in Combination with Gentamicin. Microb. Pathog. 2019, 126, 323–331. [Google Scholar] [CrossRef]
- Memar, M.Y.; Raei, P.; Alizadeh, N.; Aghdam, M.A.; Kafil, H.S. Carvacrol and Thymol: Strong Antimicrobial Agents against Resistant Isolates. Rev. Res. Med. Microbiol. 2017, 28, 63–68. [Google Scholar] [CrossRef]
- Guarda, A.; Rubilar, J.F.; Miltz, J.; Galotto, M.J. The Antimicrobial Activity of Microencapsulated Thymol and Carvacrol. Int. J. Food Microbiol. 2011, 146, 144–150. [Google Scholar] [CrossRef]
- García-García, R.; López-Malo, A.; Palou, E. Bactericidal Action of Binary and Ternary Mixtures of Carvacrol, Thymol, and Eugenol against Listeria Innocua. J. Food Sci. 2011, 76, M95–M100. [Google Scholar] [CrossRef]
- Sokolik, C.G.; Ben-Shabat-Binyamini, R.; Gedanken, A.; Lellouche, J.P. Proteinaceous Microspheres as a Delivery System for Carvacrol and Thymol in Antibacterial Applications. Ultrason. Sonochem. 2018, 41, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Miladi, H.; Zmantar, T.; Chaabouni, Y.; Fedhila, K.; Bakhrouf, A.; Mahdouani, K.; Chaieb, K. Antibacterial and Efflux Pump Inhibitors of Thymol and Carvacrol against Food-Borne Pathogens. Microb. Pathog. 2016, 99, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Assessment of Factors Influencing Antimicrobial Activity of Carvacrol and Cymene against Vibrio Cholerae in Food. J. Biosci. Bioeng. 2010, 110, 614–619. [Google Scholar] [CrossRef]
- Churklam, W.; Chaturongakul, S.; Ngamwongsatit, B.; Aunpad, R. The Mechanisms of Action of Carvacrol and Its Synergism with Nisin against Listeria Monocytogenes on Sliced Bologna Sausage. Food Control 2020, 108, 106864. [Google Scholar] [CrossRef]
- Nowotarska, S.W.; Nowotarski, K.; Grant, I.R.; Elliott, C.T.; Friedman, M.; Situ, C. Mechanisms of Antimicrobial Action of Cinnamon and Oregano Oils, Cinnamaldehyde, Carvacrol, 2,5-Dihydroxybenzaldehyde, and 2-Hydroxy-5-Methoxybenzaldehyde against Mycobacterium Avium Subsp. Paratuberculosis (Map). Foods 2017, 6, 72. [Google Scholar] [CrossRef]
- Landry, K.S.; Micheli, S.; McClements, D.J.; McLandsborough, L. Effectiveness of a Spontaneous Carvacrol Nanoemulsion against Salmonella Enterica Enteritidis and Escherichia Coli O157: H7 Oncontaminated Broccoli and Radish Seeds. Food Microbiol 2015, 51, 10–17. [Google Scholar] [CrossRef]
- Arsi, K.; Donoghue, A.M.; Venkitanarayanan, K.; Kollanoor-Johny, A.; Fanatico, A.C.; Blore, P.J.; Donoghue, D.J. The Efficacy of the Natural Plant Extracts, Thymol and Carvacrol against Campylobacter Colonization in Broiler Chickens. J. Food Saf. 2014, 34, 321–325. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J.E. A Study of the Minimum Inhibitory Concentration and Mode of Action of Oregano Essential Oil, Thymol and Carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- Burt, S.A.; Vlielander, R.; Haagsman, H.P.; Veldhuizen, E.J.A. Increase in Activity of Essential Oil Components Carvacrol and Thymol against Escherichia Coli O157:H7 by Addition of Food Stabilizers. J. Food Prot. 2005, 68, 919–926. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The Phenolic Hydroxyl Group of Carvacrol Is Essential for Action against the Food-Borne Pathogen Bacillus Cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Hossain, F.; Follett, P.; Dang Vu, K.; Harich, M.; Salmieri, S.; Lacroix, M. Evidence for Synergistic Activity of Plant-Derived Essential Oils against Fungal Pathogens of Food. Food Microbiol. 2016, 53, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, L.N.; Alves, F.C.B.; Andrade, B.F.M.T.; Albano, M.; Rall, V.L.M.; Fernandes, A.A.H.; Buzalaf, M.A.R.; de Lima Leite, A.; de Pontes, L.G.; dos Santos, L.D.; et al. Proteomic Analysis and Antibacterial Resistance Mechanisms of Salmonella Enteritidis Submitted to the Inhibitory Effect of Origanum Vulgare Essential Oil, Thymol and Carvacrol. J. Proteom. 2020, 214, 103625. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Shankar, S.; Fernandez, J.; Juillet, E.; Salmieri, S.; Lacroix, M. A Rapid Way of Formulation Development Revealing Potential Synergic Effects on Numerous Antimicrobial Combinations against Foodborne Pathogens. Microb. Pathog. 2021, 158, 105047. [Google Scholar] [CrossRef]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical Profile, Antioxidant and Antibacterial Activity of Thyme and Oregano Essential Oils, Thymol and Carvacrol and Their Possible Synergism. J. Essent. Oil-Bear. Plants JEOP 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Žitek, T.; Borjan, D.; Golle, A.; Knez, Ž.; Knez, M. Optimization of Extraction of Phenolic Compounds with Antimicrobial Properties from Origanum vulgare. Processes 2021, 9, 1032. [Google Scholar] [CrossRef]
- Gonzalez, R.J.; Luo, Y.; Ruiz-Cruz, S.; McEvoy, J.L. Efficacy of Sanitizers to Inactivate Escherichia Coli O157:H7 on Fresh-Cut Carrot Shreds under Simulated Process Water Conditions. J. Food Prot. 2004, 67, 2375–2380. [Google Scholar] [CrossRef]
- Guerra-Rosas, M.I.; Morales-Castro, J.; Cubero-Márquez, M.A.; Salvia-Trujillo, L.; Martín-Belloso, O. Antimicrobial Activity of Nanoemulsions Containing Essential Oils and High Methoxyl Pectin during Long-Term Storage. Food Control 2017, 77, 131–138. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martín-Belloso, O. Antimicrobial Activity of Essential Oils on Salmonella Enteritidis, Escherichia Coli, and Listeria Innocua in Fruit Juices. J. Food Prot. 2006, 69, 1579–1586. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Q.; Critzer, F.; Davidson, P.M.; Zhong, Q. Organic Thyme Oil Emulsion as an Alternative Washing Solution to Enhance the Microbial Safety of Organic Cantaloupes. Food Control 2016, 67, 31–38. [Google Scholar] [CrossRef]
- Todd, J.; Friedman, M.; Patel, J.; Jaroni, D.; Ravishankar, S. The Antimicrobial Effects of Cinnamon Leaf Oil against Multi-Drug Resistant Salmonella Newport on Organic Leafy Greens. Int. J. Food Microbiol. 2013, 166, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Bin Song, K. Antibacterial Activity of the Noni Fruit Extract against Listeria Monocytogenes and Its Applicability as a Natural Sanitizer for the Washing of Fresh-Cut Produce. Food Microbiol. 2019, 84, 103260. [Google Scholar] [CrossRef] [PubMed]
- Truschi, S.; Baldi, A.; Bruschi, P.; Cacciari, I.; Marvasi, M.; Lenzi, A. Foliar Roughness and Water Content Impact on Escherichia Coli Attachment in Baby Leafy Greens. Biology 2023, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H. Understanding Inactivation of Listeria Monocytogenes and Escherichia Coli O157:H7 Inoculated on Romaine Lettuce by Emulsified Thyme Essential Oil. Food Microbiol. 2022, 105, 104013. [Google Scholar] [CrossRef]
- Rodgers, S.L.; Cash, J.N.; Siddiq, M.; Ryser, E.T. A Comparison of Different Chemical Sanitizers for Inactivating Escherichia Coli O157:H7 and Listeria Monocytogenes in Solution and on Apples, Lettuce, Strawberries, and Cantaloupe. J. Food Prot. 2004, 67, 721–731. [Google Scholar] [CrossRef]
- Reygaert, W.C. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482. [Google Scholar] [CrossRef]
- Sousa, V.I.; Parente, J.F.; Marques, J.F.; Forte, M.A.; Tavares, C.J. Microencapsulation of Essential Oils: A Review. Polymers 2022, 14, 1730. [Google Scholar] [CrossRef]
| Sample | MIC (µL/mL) | MBC (µL/mL) |
|---|---|---|
| WS | 1.88 | >30 |
| OR | 0.94 | 0.94 |
| OR:WS (1:1) | 0.94 | 7.50 |
| OR:WS (3:1) | 0.94 | 7.50 |
| OR:WS (1:3) | 0.94 | 3.75 |
| OR:WS (2:3) | 0.94 | 1.88 |
| OR:WS (3:2) | 0.94 | 7.50 |
| Treatments | Storage Times | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Romaine (Log CFU/g) | Butterhead (Log CFU/g) | Crisphead (Log CFU/g) | |||||||
| No Treatment | 0 h | 24 h | 7 Days | 0 h | 24 h | 7 Days | 0 h | 24 h | 7 Days |
| NR | 4.56 ± 0.28 | 4.61 ± 0.03 | 4.45 ± 0.09 | 5.71 ± 0.27 | 5.65 ± 0.10 | 5.64 ± 0.10 | 5.80 ± 0.22 | 5.69 ± 0.06 | 5.44 ± 0.02 |
| Water | 3.04 ± 0.01 | 3.93 ± 0.07 | 4.64 ± 0.22 | 5.20 ± 0.06 | 5.48 ± 0.42 | 5.79 ± 0.14 | 4.24 ± 0.21 | 4.07 ± 0.11 | 2.94 ± 0.50 |
| Sanitizers | |||||||||
| OR1 | 3.64 ± 0.03 b,A | 3.22 ± 0.15 c,d,B | 3.09 ± 0.17 b,c,B | 5.56 ± 0.21 a,A | 5.42 ± 0.37 a,A | 5.47 ± 0.26 a,A | 4.35 ± 0.18 a,A | 4.05 ± 0.21 a,A | 3.69 ± 0.01 a,B |
| OR2 | 4.05 ± 0.07 a,A | 2.64 ± 0.12 e,C | 3.36 ± 0.09 b,B | 5.07 ± 0.22 a,A | 4.85 ± 0.22 b,A | 3.83 ± 0.13 c,d,B | 3.03 ± 0.06 c,A | 2.66 ± 0.05 d,B | 2.46 ± 0.15 d,B |
| WS1 | 3.67 ± 0.03 a,b,A | 3.26 ± 0.08 c,d,B | 2.96 ± 0.08 c,C | 5.57 ± 0.18 a,A | 5.76 ± 0.30 a,A | 5.78 ± 0.16 a,A | 4.17 ± 0.07 a,b,A | 3.51 ± 0.06 b,B | 2.76 ± 0.07 b,c,C |
| WS2 | 3.80 ± 0.11 a,b,A | 3.80 ± 0.34 a,b,A | 3.84 ± 0.33 a,A | 5.04 ± 0.17 a,A | 5.22 ± 0.20 a,b,A | 4.24 ± 0.29 b,c,B | 3.87 ± 0.20 b,A | 3.80 ± 0.05 a,b,A | 2.84 ± 0.05 b,c,B |
| OR:WS1 | 3.88 ± 0.05 a,b,A | 3.25 ± 0.00 c,d,B | 3.07 ± 0.04 b,c,B | 5.25 ± 0.41 a,B | 5.38 ± 0.05 a,B | 5.88 ± 0.23 a,A | 3.29 ± 0.04 c,A | 2.17 ± 0.23 e,C | 2.58 ± 0.09 c,d,B |
| OR:WS2 | 2.99 ± 0.13 c,B | 3.56 ± 0.15 b,c,A | 3.38 ± 0.16 b,A | 4.17 ± 0.05 b,B | 4.80 ± 0.05 b,A | 4.33 ± 0.29 b,B | 3.15 ± 0.05 c,A | 2.28 ± 0.12 e,C | 2.69 ± 0.05 b,c,d,B |
| 80 ppm PAA | 3.70 ± 0.14 a,b,A | 3.99 ± 0.24 a,A | 2.57 ± 0.13 c,B | 3.89 ± 0.18 b,A | 3.56 ± 0.29 d,B | 3.50 ± 0.06 d,B | 3.18 ± 0.02 c,A | 3.14 ± 0.07 c,A | 2.97 ± 0.16 b,A |
| 200 ppm Chlorine | 2.95 ± 0.06 c,A | 3.00 ± 0.12 d,A | 2.18 ± 0.06 d,B | 4.09 ± 0.05 b,A | 4.14 ± 0.38 c,A | 4.27 ± 0.17 b,A | 3.10 ± 0.18 c,A | 3.01 ± 0.01 c,A | 2.90 ± 0.12 b,A |
| Contrasts | |||||||||
| Sanitizers vs. Water | |||||||||
| OR1 X Water | *** | *** | *** | ns | ns | ns | ns | ns | *** |
| OR2 X Water | *** | *** | *** | ns | * | *** | *** | *** | ns |
| WS1 X Water | *** | *** | *** | ns | ns | ns | ns | *** | ns |
| WS2 X Water | *** | ns | *** | ns | ** | *** | ns | ns | ns |
| OR:WS1 X Water | *** | *** | *** | ns | ns | ns | *** | *** | ns |
| OR:WS2 X Water | ns | ns | *** | *** | ns | *** | *** | *** | ns |
| 80 ppm PAA X Water | *** | ns | *** | *** | *** | *** | *** | *** | ns |
| 200 ppm Chlorine X Water | ns | *** | *** | *** | *** | *** | *** | *** | ns |
| Sanitizers vs. NR | |||||||||
| OR1 X NR | *** | *** | *** | ns | ns | ns | *** | *** | *** |
| OR2 X NR | *** | *** | *** | ns | ** | *** | *** | *** | *** |
| WS1 X NR | *** | *** | *** | ns | ns | ns | *** | *** | *** |
| WS2 X NR | *** | *** | *** | * | ** | *** | *** | *** | *** |
| OR:WS1 X NR | *** | *** | *** | ns | ns | ns | *** | *** | *** |
| OR:WS2 X NR | *** | *** | *** | *** | ** | *** | *** | *** | *** |
| 80 ppm PAA X NR | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| 200 ppm Chlorine X NR | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| No treatment | |||||||||
| Water X NR | *** | *** | ns | ns | ns | ns | *** | *** | *** |
| Treatments | Storage Times | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Romaine (Log CFU/g) | Butterhead (Log CFU/g) | Crisphead (Log CFU/g) | |||||||
| No Treatment | 0 h | 24 h | 7 Days | 0 h | 24 h | 7 Days | 0 h | 24 h | 7 Days |
| Water | 2.88 ± 0.16 | 1.75 ± 0.09 | 1.80 ± 0.02 | 1.98 ± 0.50 | 2.33 ± 0.03 | 2.81 ± 0.06 | 3.40 ± 0.30 | 2.17 ± 0.13 | 1.66 ± 0.07 |
| Sanitizers | |||||||||
| OR1 | 0.24 ± 0.05 c,d,A | 0.26 ± 0.02 d,e,A | 0.18 ± 0.15 c,d,A | 1.69 ± 0.04 c,d,B | 2.02 ± 0.08 b,A,B | 2.36 ± 0.25 a,b,A | 1.85 ± 0.24 b,A | 1.45 ± 0.03 b,B | 1.46 ± 0.05 b,B |
| OR2 | 0.74 ± 0.26 c,A | 0.97 ± 0.28 c,A | <LD d,B | 0.91 ± 0.08 d,A | <LD d,B | 0.13 ± 0.10 d,B | <LD e,A | <LD d,A | <LD d,A |
| WS1 | 2.15 ± 0.53 a,A | 1.95 ± 0.03 a,A | 0.42 ± 0.19 b,c,B | 4.12 ± 0.27 a,A | 2.91 ± 0.27 a,B | 2.81 ± 0.48 a,B | 2.64 ± 0.17 a,A | 1.64 ± 0.00 b,B | 1.29 ± 0.07 b,C |
| WS2 | 1.42 ± 0.20 b,A | 0.45 ± 0.22 d,B | <LD d,C | 1.54 ± 0.43 c,d,A | 1.24 ± 0.34 c,A | 1.18 ± 0.17 c,A | 2.56 ± 0.07 a,A | 2.23 ± 0.15 a,A,B | 1.93 ± 0.19 a,B |
| OR:WS1 | 1.83 ± 0.07 a,b,A | 1.15 ± 0.15 b,c,B | 0.56 ± 0.15 b,C | 2.91 ± 0.21 b,A | 2.59 ± 0.25 a,A,B | 2.12 ± 0.12 a,b,B | 1.07 ± 0.13 c,A | 0.76 ± 0.30 c,A | 0.60 ± 0.18 c,A |
| OR:WS2 | 0.57 ± 0.13 c,d,A | <LD e,B | <LD d,B | <LD e,A | <LD d,A | <LD d,A | 0.45 ± 0.01 d,A | 0.14 ± 0.09 d,B | <LD d,C |
| 80 ppm PAA | <LD d,A | <LD e,A | <LD d,A | <LD e,A | <LD d,A | <LD d,A | <LD e,A | <LD d,A | <LD d,A |
| 200 ppm Chlorine | 1.55 ± 0.14 a,b,A | 1.46 ± 0.06 b,A | 1.09 ± 0.05 a,B | 1.88 ± 0.65 c,A | 2.42 ± 0.12 a,b,A | 1.60 ± 0.06 b,c,A | <LD e,A | <LD d,A | <LD d,A |
| Contrasts | |||||||||
| Sanitizers vs. Water | |||||||||
| OR1 X Water | *** | *** | *** | ns | ns | ns | *** | ns | ns |
| OR2 X Water | *** | * | *** | ** | *** | *** | *** | *** | *** |
| WS1 X Water | ns | ns | *** | ** | ns | ns | * | ns | ns |
| WS2 X Water | ** | *** | *** | ns | *** | *** | * | ns | ns |
| OR:WS1 XWater | ns | ns | *** | ns | ns | ns | *** | *** | *** |
| OR:WS2 X Water | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| 80 ppm PAA X Water | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| 200 ppm Chlorine X Water | * | ns | ns | ns | ns | * | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizzo, J.S.; Pelvine, R.A.; da Silva, A.L.B.R.; Mikcha, J.M.G.; Visentainer, J.V.; Rodrigues, C. Use of Essential Oil Emulsions to Control Escherichia coli O157:H7 in the Postharvest Washing of Lettuce. Foods 2023, 12, 2571. https://doi.org/10.3390/foods12132571
Pizzo JS, Pelvine RA, da Silva ALBR, Mikcha JMG, Visentainer JV, Rodrigues C. Use of Essential Oil Emulsions to Control Escherichia coli O157:H7 in the Postharvest Washing of Lettuce. Foods. 2023; 12(13):2571. https://doi.org/10.3390/foods12132571
Chicago/Turabian StylePizzo, Jessica Santos, Raira Andrade Pelvine, Andre Luiz Biscaia Ribeiro da Silva, Jane Martha Graton Mikcha, Jesui Vergilio Visentainer, and Camila Rodrigues. 2023. "Use of Essential Oil Emulsions to Control Escherichia coli O157:H7 in the Postharvest Washing of Lettuce" Foods 12, no. 13: 2571. https://doi.org/10.3390/foods12132571
APA StylePizzo, J. S., Pelvine, R. A., da Silva, A. L. B. R., Mikcha, J. M. G., Visentainer, J. V., & Rodrigues, C. (2023). Use of Essential Oil Emulsions to Control Escherichia coli O157:H7 in the Postharvest Washing of Lettuce. Foods, 12(13), 2571. https://doi.org/10.3390/foods12132571








