The Influence of Storage and Cooking on the Vitamin D Content of 25-Hydroxyvitamin D3-Enriched Eggs
Abstract
1. Introduction
2. Materials and Methods
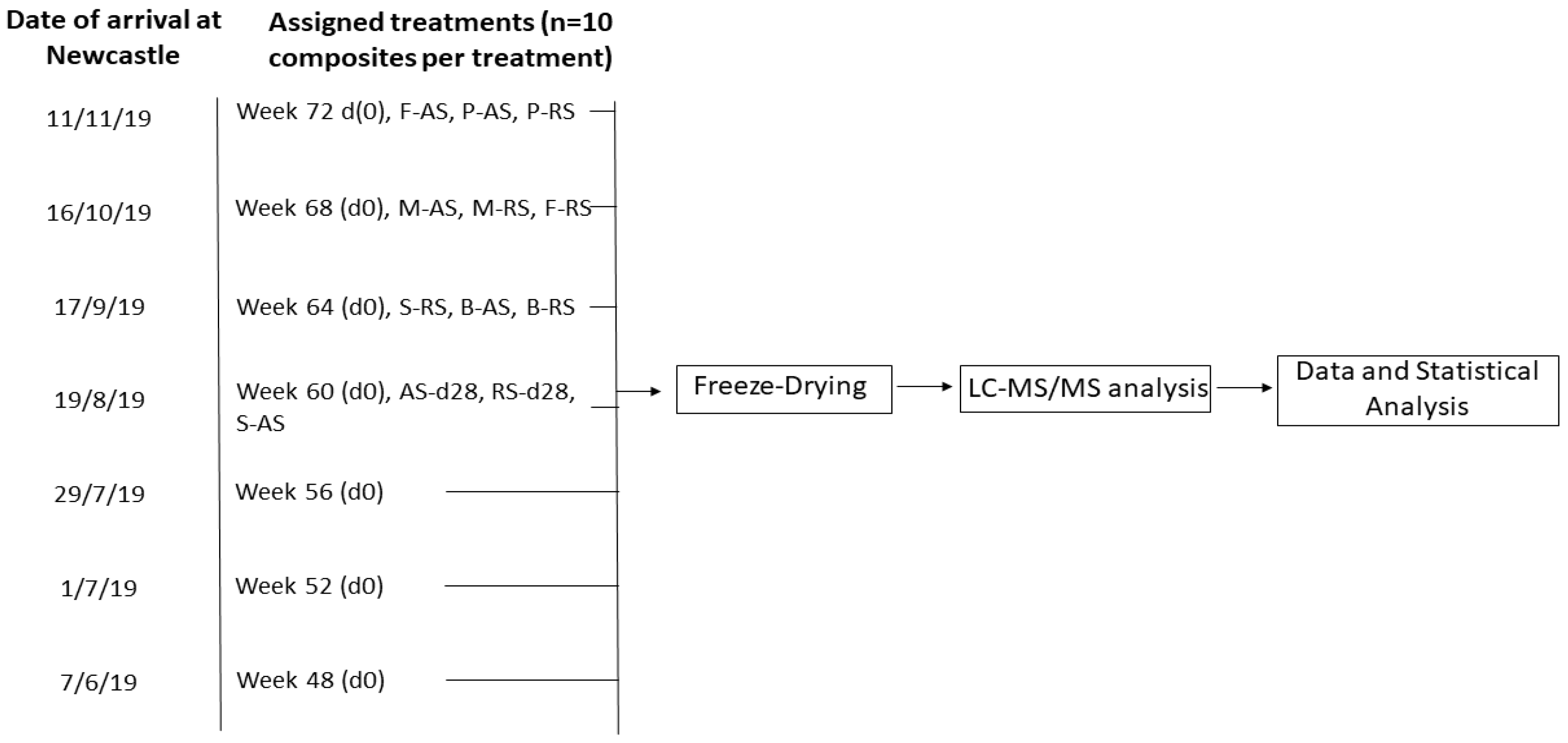
2.1. Obtainment of 25-D3-Enriched Eggs and Experimental Design
2.2. Egg Processing and Cooking
2.3. Extraction and Analysis of Vitamin D3 and 25-Hydroxyvitamin D3
2.4. Data Calculations
2.5. Statistical Analysis
3. Results
3.1. Effects of Time of Lay on D3, 25-D3 and Calculated Total VD Content of Enriched Eggs
3.2. Effects of Storage on D3, 25-D3 and Calculated Total VD Content of Enriched Eggs
3.3. Effects of Cooking on D3, 25-D3 and Calculated Total VD Content of Enriched Eggs
3.4. Comparison of Raw Values to Current UK Food Compositional Data for Eggs [5]
4. Discussion
4.1. Effect of Time of Lay on VD Metabolites
4.2. Effect of Storage on VD Metabolites
4.3. Effect of Cooking on VD Metabolites
4.4. Use of 25-D3-Enriched Eggs as a Source of VD
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buttriss, J.L.; Lanham-New, S.A. Is a Vitamin D Fortification Strategy Needed? Nutr. Bull. 2020, 45, 115–122. [Google Scholar] [CrossRef]
- Lanham-New, S.A.; Buttriss, J.L.; Miles, L.M.; Ashwell, M.; Berry, J.L.; Boucher, B.J.; Cashman, K.D.; Cooper, C.; Darling, A.L.; Francis, R.M.; et al. Proceedings of the Rank Forum on Vitamin D. Br. J. Nutr. 2011, 105, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Public Health England NDNS: Results from years 9 to 11 (combined). Available online: https://www.gov.uk/government/statistics/ndns-results-from-years-9-to-11-2016-to-2017-and-2018-to-2019. (accessed on 2 March 2022).
- Scientific Advisory Committee on Nutrition. Vitamin D and Health. 2016. Available online: https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report (accessed on 4 April 2022).
- Department of Health. Nutrient Analysis of Eggs. 2012. Available online: https://www.gov.uk/government/publications/nutrient-analysis-of-eggs (accessed on 5 January 2021).
- Cashman, K.D.; Seamans, K.M.; Lucey, A.J.; Stöcklin, E.; Weber, P.; Kiely, M.; Hill, T.R. Relative Effectiveness of Oral 25-hydroxyvitamin D3 and Vitamin D3 in Raising Wintertime Serum 25-hydroxyvitamin D in Older Adults. Am. J. Clin. Nutr. 2012, 95, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Moulas, A.N.; Vaiou, M. Vitamin D Fortification of Foods and Prospective Health Outcomes. J. Biotechnol. 2018, 285, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Browning, L.C.; Cowieson, A.J. Vitamin D Fortification of Eggs for Human Health. J. Sci. Food Agric. 2014, 94, 1389–1396. [Google Scholar] [CrossRef]
- Mattila, P.; Valaja, J.; Rossow, L.; Venäläinen, E.; Tupasela, T. Effect of Vitamin D2- and D3-Enriched Diets on Egg Vitamin D Content, Production, and Bird Condition during an Entire Production Period. Poult. Sci. 2004, 83, 433–440. [Google Scholar] [CrossRef]
- Mattila, P.H.; Valkonen, E.; Valaja, J. Effect of Different Vitamin D Supplementations in Poultry Feed on Vitamin D Content of Eggs and Chicken Meat. J. Agric. Food Chem. 2011, 59, 8298–8303. [Google Scholar] [CrossRef]
- Clark, A.; Kuznesof, S.; Davies, S.; Waller, A.; Ritchie, A.; Wilson, S.; Harbord, L.; Hill, T. Egg Enrichment with Vitamin D: The Sunshine Eggs Projects. Nutr. Bull. 2021, 46, 332–338. [Google Scholar] [CrossRef]
- Mattila, P.; Ronkainen, R.; Lehikoinen, K.; Piironen, V. Effect of Household Cooking on the Vitamin D Content in Fish, Eggs, and Wild Mushrooms. J. Food Compos. Anal. 1999, 12, 153–160. [Google Scholar] [CrossRef]
- Jakobsen, J.; Knuthsen, P. Stability of Vitamin D in Foodstuffs during Cooking. Food Chem. 2014, 148, 170–175. [Google Scholar] [CrossRef]
- Bajaj, S.R.; Singhal, R.S. Fortification of Wheat Flour and Oil with Vitamins B12 and D3: Effect of Processing and Storage. J. Food Compos. Anal. 2021, 96, 103703. [Google Scholar] [CrossRef]
- Szlinder-Richert, J.; Malesa-Ciećwierz, M. Effect of Household Cooking Methods on Nutritional Value of Cod and Salmon—Twin Fillet Approach. Carpathian J. Food Sci. Technol. 2018, 10, 142–157. [Google Scholar]
- European Commision. Concerning the Authorisation of Cholecalciferol as a Feed Additive for All Animal Species. Off. J. Eur. Union 2017, 60, 19–22. [Google Scholar]
- Food Standards Scotland. Food Safety—Eggs. Available online: https://www.foodstandards.gov.scot/consumers/food-safety/at-home/eggs (accessed on 3 March 2022).
- Schadt, H.S.; Gössl, R.; Seibel, N.; Aebischer, C.P. Quantification of Vitamin D3 in Feed, Food, and Pharmaceuticals Using High-Performance Liquid Chromatography/Tandem Mass Spectrometry. J. AOAC Int. 2012, 95, 1487–1494. [Google Scholar] [CrossRef]
- Murphy, E.W.; Criner, P.E.; Gray, B.C. Comparisons of Methods for Calculating Retentions of Nutrients in Cooked Foods. J. Agric. Food Chem. 1975, 23, 1153–1157. [Google Scholar] [CrossRef]
- Croghan, C.; Egeghy, P. Methods of Dealing with Values Below the Limit of Detection using SAS. United States Environmental Protection Agency. Available online: https://www.semanticscholar.org/paper/Methods-of-Dealing-with-Values-Below-the-Limit-of-Croghan-Egeghy/98ec87a1208e06a9f4e08136d245a986c5ff5019 (accessed on 28 July 2022).
- R Core Team. R: A Language and Environment for Statistical Computing. 2022. Available online: https://www.r-project.org/ (accessed on 1 May 2023).
- Herv, M. RVAideMemoire. 2022. Available online: https://cran.r-project.org/web/packages/RVAideMemoire/index.html (accessed on 1 May 2023).
- Mangiafico, S. Rcompanion. 2023. Available online: https://cran.r-project.org/web/packages/rcompanion/index.html (accessed on 1 May 2023).
- Lešková, E.; Kubíková, J.; Kováčiková, E.; Košická, M.; Porubská, J.; Holčíková, K. Vitamin Losses: Retention during Heat Treatment and Continual Changes Expressed by Mathematical Models. J. Food Compos. Anal. 2006, 19, 252–276. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Schieber, A.; Wu, J. Effects of Storage and Cooking on the Antioxidant Capacity of Laying Hen Eggs. Food Chem. 2016, 194, 111–116. [Google Scholar] [CrossRef]
- Ragni, L.; Al-Shami, A.; Mikhaylenko, G.; Tang, J. Dielectric Characterization of Hen Eggs during Storage. J. Food Eng. 2007, 82, 450–459. [Google Scholar] [CrossRef]
- Feddern, V.; de Prá, M.C.; Mores, R.; Nicoloso, R.d.S.; Coldebella, A.; de Abreu, P.G. Egg Quality Assessment at Different Storage Conditions, Seasons and Laying Hen Strains. Cienc. Agrotecnologia 2017, 41, 322–333. [Google Scholar] [CrossRef]
- Oliveira, G.E.; Figueiredo, T.C.; Souza, M.R.; Oliveira, A.L.; Cançado, S.V.; Gloria, M.B.A. Bioactive Amines and Quality of Egg from Dekalb Hens under Different Storage Conditions. Poult. Sci. 2009, 88, 2428–2434. [Google Scholar] [CrossRef]
- Sowa, M.; Yu, J.; Palacios-Rojas, N.; Goltz, S.R.; Howe, J.A.; Davis, C.R.; Rocheford, T.; Tanumihardjo, S.A. Retention of Carotenoids in Biofortified Maize Flour and β-Cryptoxanthin-Enhanced Eggs after Household Cooking. ACS Omega 2017, 2, 7320–7328. [Google Scholar] [CrossRef]
- US Department of Agriculture. Food Data Central. Available online: https://fdc.nal.usda.gov/index.html (accessed on 8 April 2022).
- Dunlop, E.; Cunningham, J.; Sherriff, J.L.; Lucas, R.M.; Greenfield, H.; Arcot, J.; Strobel, N.; Black, L.J. Vitamin D3 and 25-hydroxyvitamin D3 Content of Retail White Fish and Eggs in Australia. Nutrients 2017, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, H.M.M.; Santos, V.V.A.; Medeiros, V.P.Q.; Silva, K.H.D.; Dimenstein, R. Effect of Thermal Processing on Retinol Levels of Free-Range and Caged Hen Eggs. Int. J. Food Sci. Nutr. 2009, 57, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Egg Info. Industry Data. Available online: https://www.egginfo.co.uk/egg-facts-and-figures/industry-information/data (accessed on 10 November 2021).
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock—A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Guo, J.; Kliem, K.E.; Lovegrove, J.A.; Givens, D.I. Effect of Production System, Supermarket and Purchase Date on the Vitamin D Content of Eggs at Retail. Food Chem. 2016, 221, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
| Batch 1 | Batch 2 | Batch 3 | Batch 4 | Batch 5 | Batch 6 | Batch 7 | |
|---|---|---|---|---|---|---|---|
| Date received at Newcastle University | 07/06/2019 | 01/07/2019 | 29/07/2019 | 19/08/2019 | 17/09/2019 | 16/10/2019 | 11/11/2019 |
| Age of Laying Hens | Week 48 | Week 52 | Week 56 | Week 60 | Week 64 | Week 68 | Week 72 |
| Assigned Treatments (n = 10 composites each) | Week 48 | Week 52 | Week 56 | Week 60 (d0) AS-d28 RS-28 S-AS | Week 64 (d0) S-RS B-AS B-RS | Week 68 (d0) M-AS M-RS F-RS | Week 72 (d0) F-AS P-AS P-RS |
| Metabolite 2 | Week | ||||||
|---|---|---|---|---|---|---|---|
| 48 | 52 | 56 | 60 | 64 | 68 | 72 | |
| Vitamin D3 (µg/100 g whole egg) | A 0.41 (0.11) | A 0.48 (0.20) | AB 0.45 (0.21) | AB 0.39 (0.16) | A 0.41 (0.11) | BC 0.21 (0.09) | C 0.12 (0.01) |
| 25-hydroxyvitamin D3 (µg/100 g whole egg) | BC 1.01 (0.11) | ABE 1.18 (0.16) | AB 1.25 (0.19) | CD 0.90 (0.07) | CD 0.87 (0.13) | D 0.80 (0.06) | CDE 0.93 (0.09) |
| Total VD (µg/100 g whole egg) | BC 5.45 (0.61) | AB 6.36 (0.89) | AB 6.70 (0.91) | CD 4.88 (0.42) | CD 4.77 (0.76) | D 4.22 (0.29) | CD 4.76 (0.47) |
| d0 (n = 10) | AS-d28 (n = 10) | RS-d28 (n = 10) | AS-d28 (n = 10) | RS-d28 (n = 10) | |
|---|---|---|---|---|---|
| Metabolite | µg/100 g Egg | µg/100 g Egg | µg/100 g Egg | TR% | TR% |
| Vitamin D3 | 0.39 (0.16) | 0.43 (0.07) | 0.30 (0.11) | A 111.91 (19.14) | B 83.13 (29.53) |
| 25-hydroxyvitamin D3 | 0.90 (0.07) | 0.92 (0.06) | 0.86 (0.05) | 103.68 (9.01) | 103.09 (6.50) |
| Total VD | 4.88 (0.42) | A 5.02 (0.34) | B 4.62 (0.26) | 104.34 (9.06) | 101.48 (6.78) |
| Condition 2 | Vitamin D3 TR% | 25-Hydroxyvitamin D3 TR% | Total VD TR% | |
|---|---|---|---|---|
| Scrambled | Ambient (n = 10) | BC 152.36 (59.73) | B 91.88 (7.61) | A 109.55 (6.44) |
| Refrigerated (n = 10) | B 93.42 (24.91) | BC 94.94 (9.37) | B 94.81 (9.83) | |
| Hard-Boiled | Ambient (n = 10) | AB 90.62 (21.14) | D 79.66 (7.18) | C 80.61 (7.18) |
| Refrigerated (n = 10) | AB 79.43 (33.57) | D 85.01 (5.99) | C 84.53 (5.58) | |
| Microwaved | Ambient (n = 10) | A 70.10 (12.74) | A 111.40 (7.22) | A 109.50 (7.37) |
| Refrigerated (n = 10) | A 56.58 (5.88) | B 101.78 (7.54) | B 98.60 (6.23) | |
| Fried | Ambient (n = 10) | C 127.41 (13.83) | DE 75.93 (5.79) | C 78.11 (5.78) |
| Refrigerated (n = 10) | A 50.54 (5.35) | E 72.64 (5.89) | D 70.71 (5.85) | |
| Poached | Ambient (n = 10) | B 95.59 (11.20) | BC 92.51 (4.85) | B 92.56 (5.37) |
| Refrigerated (n = 10) | B 96.35 (10.26) | CD 91.11 (4.33) | BC 91.16 (4.91) | |
| Storage | <0.01 | 0.491 | <0.01 | |
| Method | <0.01 | <0.01 | <0.01 | |
| Storage × Method | <0.01 | 0.013 | <0.01 | |
| Condition | D3 (µg/100 g Whole Egg) | 25-Hydroxyvitamin D3 (µg/100 g Whole Egg) | Total VD (µg/100 g Whole Egg) | Total VD UK Reference Values (µg/100 g Whole Egg) 2 | |
|---|---|---|---|---|---|
| Scrambled | AS | 0.61 (0.25) | 0.96 (0.05) | 5.43 (0.41) | N/A |
| RS | 0.40 (0.11) | 0.86 (0.10) | 4.72 (0.59) | ||
| Hard-Boiled | AS | 0.38 (0.09) | 0.71 (0.05) | 3.92 (0.28) | 3.2 |
| RS | 0.32 (0.13) | 0.72 (0.06) | 3.89 (0.31) | ||
| Microwaved | AS | 0.16 (0.03) | 0.97 (0.05) | 5.02 (0.29) | N/A |
| RS | 0.12 (0.01) | 0.83 (0.05) | 4.27 (0.23) | ||
| Fried | AS | 0.18 (0.02) | 0.85 (0.05) | 4.41 (0.24) | 1.9 |
| RS | 0.12 (0.01) | 0.67 (0.06) | 3.45 (0.28) | ||
| Poached | AS | 0.12 (0.02) | 0.91 (0.05) | 4.69 (0.27) | 2.9 |
| RS | 0.11 (0.01) | 0.87 (0.04) | 4.46 (0.21) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, A.; Kuznesof, S.; Waller, A.; Davies, S.; Wilson, S.; Ritchie, A.; Duesterloh, A.; Harbord, L.; Hill, T.R. The Influence of Storage and Cooking on the Vitamin D Content of 25-Hydroxyvitamin D3-Enriched Eggs. Foods 2023, 12, 2522. https://doi.org/10.3390/foods12132522
Clark A, Kuznesof S, Waller A, Davies S, Wilson S, Ritchie A, Duesterloh A, Harbord L, Hill TR. The Influence of Storage and Cooking on the Vitamin D Content of 25-Hydroxyvitamin D3-Enriched Eggs. Foods. 2023; 12(13):2522. https://doi.org/10.3390/foods12132522
Chicago/Turabian StyleClark, Adam, Sharron Kuznesof, Anthony Waller, Sarah Davies, Simon Wilson, Avril Ritchie, Andre Duesterloh, Lance Harbord, and Thomas Robert Hill. 2023. "The Influence of Storage and Cooking on the Vitamin D Content of 25-Hydroxyvitamin D3-Enriched Eggs" Foods 12, no. 13: 2522. https://doi.org/10.3390/foods12132522
APA StyleClark, A., Kuznesof, S., Waller, A., Davies, S., Wilson, S., Ritchie, A., Duesterloh, A., Harbord, L., & Hill, T. R. (2023). The Influence of Storage and Cooking on the Vitamin D Content of 25-Hydroxyvitamin D3-Enriched Eggs. Foods, 12(13), 2522. https://doi.org/10.3390/foods12132522






