Bioactive Components and Their Activities from Different Parts of Citrus aurantifolia (Christm.) Swingle for Food Development
Abstract
1. Introduction
2. Taxonomy
| Kingdom | Plantae |
| Subkingdom | Tracheobionta |
| Superdivision | Spermatophyta |
| Division | Magnoliophyta |
| Class | Magnoliopsida |
| Subclass | Rosidae |
| Order | Sapindales |
| Family | Rutaceae |
| Genus | Citrus |
| Species | Citrus aurantifolia [11] |
3. Extraction
4. Chemical Components in Parts of C. aurantifolia
4.1. Essential Oil
4.2. Flavonoids
4.3. Terpenoids
4.4. Phenolic
4.5. Limonoids
4.6. Alkaloids
5. Biological Activities
5.1. Antibacterial
5.2. Antioxidant
5.3. Anticancer
5.4. Insecticide
5.5. Anti-Inflammatory
5.6. Other Bioactivities
5.6.1. Antidiabetic
5.6.2. Leukopenia
5.6.3. Antiplasmodium
6. Microencapsulation in Food Application
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Wang, J.; Fang, L.; Zheng, Z.; Zhi, D.; Wang, S.; Li, S.; Ho, C.T.; Zhao, H. Anticancer Activities of Citrus Peel Polymethoxyflavones Related to Angiogenesis and Others. BioMed Res. Int. 2014, 2014, 453972. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.M.O.; Ayoub, S.M.H. Study of Phytochemical Screening and Antimicrobial Activity of Citrus aurantifolia Seed Extracts. Am. J. Anal. Chem. 2016, 7, 254–259. [Google Scholar] [CrossRef]
- Phucharoenrak, P.; Muangnoi, C.; Trachootham, D. A Green Extraction Method to Achieve the Highest Yield of Limonin and Hesperidin from Lime Peel Powder (Citrus aurantifolia). Molecules 2022, 27, 820. [Google Scholar] [CrossRef] [PubMed]
- Swandiny, G.F.; Nafisa, S.; Gangga, E. Standardization of 70% Ethanol Extract and 96% Lime Leaves as Antioxidants with DPPH and FRAP. J. Pharmacogn. Phytochem. 2021, 10, 47–52. [Google Scholar]
- Patil, J.R.; Chidambara Murthy, K.N.; Jayaprakasha, G.K.; Chetti, M.B.; Patil, B.S. Bioactive Compounds from Mexican Lime (Citrus aurantifolia) Juice Induce Apoptosis in Human Pancreatic Cells. J. Agric. Food Chem. 2009, 57, 10933–10942. [Google Scholar] [CrossRef]
- Galovičová, L.; Borotová, P.; Vukovic, N.L.; Vukic, M.; Kunová, S.; Hanus, P.; Kowalczewski, P.Ł.; Bakay, L.; Kačániová, M. The Potential Use of Citrus aurantifolia L. Essential Oils for Decay Control, Quality Preservation of Agricultural Products, and Anti-Insect Activity. Agronomy 2022, 12, 735. [Google Scholar] [CrossRef]
- Asmah, N.; Suniarti, D.; Margono, A.; Mas’ud, Z.; Bachtiar, E. Identification of Active Compounds in Ethyl Acetate, Chloroform, and N-Hexane Extracts from Peels of Citrus aurantifolia from Maribaya, West Java, Indonesia. J. Adv. Pharm. Technol. Res. 2020, 11, 107–112. [Google Scholar] [CrossRef]
- Julaeha, E.; Nurzaman, M.; Wahyudi, T.; Nurjanah, S.; Permadi, N.; Al Anshori, J. The Development of the Antibacterial Microcapsules of Citrus Essential Oil for the Cosmetotextile Application: A Review. Molecules 2022, 27, 8090. [Google Scholar] [CrossRef]
- Jeffrey, J.; Sudigdoadi, S.; Kurnia, D.; Satari, M.H. A Monoterpenoid Isolated from Citrus aurantifolia Peel and Its Potential as an Antibacterial for the Inhibition and Eradication of Streptococcus Mutans Biofilm. Syst. Rev. Pharm. 2020, 11, 1205–1210. [Google Scholar] [CrossRef]
- Shchérazade, O.S.F.; Pétronille, A.Z.; Joseph, F.K.Y.; Georges, A. Study of the Analgesic Effect of the Aqueous Extract of the Leaves of Citrus aurantifolia (Rutaceae) in Mice. GSC Biol. Pharm. Sci. 2021, 14, 207–214. [Google Scholar] [CrossRef]
- Al Namani, J.; Baqir, E.; Al Abri, A.; Al Hubaishi, T.; Husain, A.; Khan, S.A. Phytochemical Screening, Phenolic Content and Antioxidant Activity of Citrus aurantifolia l. Leaves Grown in Two Regions of Oman. Iran. J. Pharm. Sci. 2018, 14, 27–34. [Google Scholar]
- Kazeem, M.I.; Bankole, H.A.; Oladokun, T.I.; Bello, A.O.; Maliki, M.A. Citrus aurantifolia (Christm.) Swingle (Lime) Fruit Extract Inhibits the Activities of Polyol Pathway Enzymes. EFood 2020, 1, 310–315. [Google Scholar] [CrossRef]
- Ramadaini, K.; Azizah, Z.; Zulharmita; Rivai, H. Overview of Pharmacology and Product Development of Lime (Citrus aurantifolia) Rind. Int. J. Pharm. Sci. Med. 2020, 5, 35–45. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Bonesi, M.; Menichini, F.; De Luca, D.; Colica, C.; Menichini, F. Evaluation of Citrus aurantifolia Peel and Leaves Extracts for Their Chemical Composition, Antioxidant and Anti-Cholinesterase Activities. J. Sci. Food Agric. 2012, 92, 2960–2967. [Google Scholar] [CrossRef] [PubMed]
- Czech, A.; Malik, A.; Sosnowska, B.; Domaradzki, P. Bioactive Substances, Heavy Metals, and Antioxidant Activity in Whole Fruit, Peel, and Pulp of Citrus Fruits. Int. J. Food Sci. 2021, 2021, 6662259. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.M.; Shahgol, M.; Estevinho, B.N.; Rocha, F. Microencapsulation of Vitamin A by Spray-Drying, Using Binary and Ternary Blends of Gum Arabic, Starch and Maltodextrin. Food Hydrocoll. 2020, 108, 106029. [Google Scholar] [CrossRef]
- Joye, I.J.; Davidov-Pardo, G.; McClements, D.J. Nanotechnology for Increased Micronutrient Bioavailability. Trends Food Sci. Technol. 2014, 40, 168–182. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Ponce-Alquicira, E. The Role of Microencapsulation in Food Application. Molecules 2022, 27, 1499. [Google Scholar] [CrossRef]
- Weimer, P.; Lisbôa Moura, J.G.; Mossmann, V.; Immig, M.L.; de Castilhos, J.; Rossi, R.C. Citrus aurantifolia (Christm) Swingle: Biological Potential and Safety Profile of Essential Oils from Leaves and Fruit Peels. Food Biosci. 2021, 40, 100905. [Google Scholar] [CrossRef]
- Fagodia, S.K.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Phytotoxicity and Cytotoxicity of Citrus aurantifolia Essential Oil and Its Major Constituents: Limonene and Citral. Ind. Crops Prod. 2017, 108, 708–715. [Google Scholar] [CrossRef]
- Apraj, V.; Thakur, N.D.; Bhagwat, A.; Mallya, R.; Sawant, L.; Pandita, N. Pharmacognostic and Phytochemical Evaluation of Citrus aurantifolia (Christm) Swingle PEEL. Pharmacogn. J. 2011, 3, 70–76. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Maksoud, S.; Abdel-Massih, R.M.; Rajha, H.N.; Louka, N.; Chemat, F.; Barba, F.J.; Debs, E. Citrus aurantium l. Active Constituents, Biological Effects and Extraction Methods. An Updated Review. Molecules 2021, 26, 5832. [Google Scholar] [CrossRef] [PubMed]
- Caballero, S.; Li, Y.O.; McClements, D.J.; Davidov-Pardo, G. Encapsulation and Delivery of Bioactive Citrus Pomace Polyphenols: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8028–8044. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Ohta, H.; Nogata, Y.; Yano, M.; Hasegawa, S. Limonoids in Seeds of Iyo Tangor (Citrus Iyo Hort. Ex Tanaka). Food Sci. Technol. Res. 2003, 9, 162–164. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Gamarra, F.M.C.; Sakanaka, L.S.; Tambourgi, E.B.; Cabrai, F.A. Influence on the Quality of Essential Lemon (Citrus aurantifolia) Oil by Distillation Process. Braz. J. Chem. Eng. 2006, 23, 147–151. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A Review of Sustainable and Intensified Techniques for Extraction of Food and Natural Products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of Microwave, Ultrasound and Accelerated-Assisted Solvent Extraction for Recovery of Polyphenols from Citrus Sinensis Peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of Extraction Time, Temperature and Solvent on Concentration and Antioxidant Activity of Grape Marc Phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Yu, J.; Dandekar, D.V.; Toledo, R.T.; Singh, R.K.; Patil, B.S. Supercritical Fluid Extraction of Limonoids and Naringin from Grapefruit (Citrus Paradisi Macf.) Seeds. Food Chem. 2007, 105, 1026–1031. [Google Scholar] [CrossRef]
- Naviglio, D.; Scarano, P.; Ciaravolo, M.; Gallo, M. Rapid Solid-Liquid Dynamic Extraction (RSLDE): A Powerful and Greener Alternative to the Latest Solid-Liquid Extraction Techniques. Foods 2019, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Samadi, M.; Abidin, Z.Z.; Yunus, R.; Biak, D.R.A.; Yoshida, H.; Lok, E.H. Assessing the Kinetic Model of Hydro-Distillation and Chemical Composition of Aquilaria Malaccensis Leaves Essential Oil. Chin. J. Chem. Eng. 2017, 25, 216–222. [Google Scholar] [CrossRef]
- Dabbs, D.M.; Mulders, N.; Aksay, I.A. Solvothermal Removal of the Organic Template from L 3 (“sponge”) Templated Silica Monoliths. J. Nanoparticle Res. 2006, 8, 603–614. [Google Scholar] [CrossRef]
- Parczewska-Plesnar, B.; Brzozowski, R.; Gwardiak, H.; Białecka-Florjańczyk, E.; Bujnowski, Z. Wheat Germ Oil Extracted by Supercritical Carbon Dioxide with Ethanol: Fatty Acid Composition. Grasas Aceites 2016, 67, e144. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.; Rahaman, A.; Muhammad Aadil, R.; Wahab, A. Novel Extraction Techniques and Pharmaceutical Activities of Luteolin and Its Derivatives. J. Food Biochem. 2019, 43, e12974. [Google Scholar] [CrossRef] [PubMed]
- Mohideen, M.; Iqbal, M.; Idris, H.; Abidin, Z.; Kamaruzaman, N.A. Review on Extraction Methods of Essential Oil from Kaffir Lime (Citrus Hystrix) Leaves. J. Acad. 2021, 9, 173–184. [Google Scholar]
- Atti-Santos, A.C.; Rossato, M.; Serafini, L.A.; Cassel, E.; Moyna, P. Extraction of Essential Oils from Lime (Citrus Latifolia Tanaka) by Hydrodistillation and Supercritical Carbon Dioxide. Braz. Arch. Biol. Technol. 2005, 48, 155–160. [Google Scholar] [CrossRef]
- Haggag, E.G.; Wahab, S.A.; El-Zalabany, S.M.; Abou Moustafa, E.A.; El-Kherasy, E.M.; Mabry, T.J. Volatile Oils and Pectins from Citrus aurantifolia (Lime) and Citrus Limonia (Lemon). Asian J. Chem. 1998, 10, 828–833. [Google Scholar]
- Hasibuan, R.; Gultom, E. The Effect of Method, Type of Solvent and Extraction Time towards the Yield of Oil on Essential Oil Extraction from Lime Peel (Citrus aurantifolia). IOP Conf. Ser. Mater. Sci. Eng. 2021, 1122, 1–7. [Google Scholar] [CrossRef]
- Şeker Karatoprak, G.; Yücel Aşık, Ç.; Çakır, A.; Köngül Şafak, E. In Vitro Pharmacological Screening of Antioxidant, Cytotoxic and Enzyme Inhibitory Activities of Citrus aurantifolia Linn. Dried Fruit Extract. Int. J. Environ. Health Res. 2021, 31, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Q.; Chen, J.C.; Liu, D.H.; Ye, X.Q. Simultaneous Extraction of Phenolic Compounds of Citrus Peel Extracts: Effect of Ultrasound. Ultrason. Sonochem. 2009, 16, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Singanusong, R.; Nipornram, S.; Tochampa, W.; Rattanatraiwong, P. Low Power Ultrasound-Assisted Extraction of Phenolic Compounds from Mandarin (Citrus reticulata Blanco Cv. Sainampueng) and Lime (Citrus aurantifolia) Peels and the Antioxidant. Food Anal. Methods 2015, 8, 1112–1123. [Google Scholar] [CrossRef]
- Castillo-Herrera, G.A.; Farías-Álvarez, L.J.; García-Fajardo, J.A.; Delgado-Saucedo, J.I.; Puebla-Pérez, A.M.; Lugo-Cervantes, E. Bioactive Extracts of Citrus aurantifolia Swingle Seeds Obtained by Supercritical CO2 and Organic Solvents Comparing Its Cytotoxic Activity against L5178Y Leukemia Lymphoblasts. J. Supercrit. Fluids 2015, 101, 81–86. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.S. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef]
- Okwu, D.E. Citrus Fruits: A Rich Source of Phytochemicals and Their Roles in Human Health. Int. J. Chem. Sci. 2008, 6, 451–471. [Google Scholar]
- Dar, N.G.; Hussain, A.; Paracha, G.M.; Akhter, S. Evaluation of Different Techniques for Extraction of Antioxidants as Bioactive Compounds from Citrus Peels (Industrial by Products). J. Agric. Environ. Sci 2015, 15, 676–682. [Google Scholar] [CrossRef]
- Mary, O.B.; Josiah, I.U.J.; Jonathan, Y.; Peter, A.O. Phytochemicals and Phytodisinfectant Properties of Citrus Species (Citrus limon, Citrus aurantifolia, and Citrus sinensis) for Pond Water Purification. GSC Biol. Pharm. Sci. 2019, 8, 34–44. [Google Scholar]
- Akinnibosun, F.; Edionwe, O. Evaluation of the Phytochemical and Antimicrobial Potential of the Leaf Extracts of Bryophyllum pinnatum L. and Citrus aurantifolia Sw. and Their Synergy. J. Appl. Sci. Environ. Manag. 2016, 19, 611–619. [Google Scholar] [CrossRef]
- Esparza-Martínez, J.F.; Solis, P.G.; Miranda-López, R.; Peña-Caballero, V. Inhibition of Proliferation of Colorectal Cancer Cells by Phenolic Extracts of Mandarin (Citrus reticulata) and Lime (Citrus aurantifolia) Fruit Waste. J. Food Nutr. Res. 2019, 7, 560–567. [Google Scholar] [CrossRef]
- Malleshappa, P.; Kumaran, R.C.; Venkatarangaiah, K.; Parveen, S. Peels of Citrus Fruits: A Potential Source of Anti-Inflammatory and Anti-Nociceptive Agents. Pharmacogn. J. 2018, 10, s172–s178. [Google Scholar] [CrossRef]
- Sukmilawati, N.; Rohama, R.; Manto, O.A.D. Screening of Phytochemicals and Antioxidant Activities of Lime Root Extracts (Citrus aurantifolia (Cristm.) Swingle) Using DPPH Method. Int. Conf. Health Sci. 2021, 1, 345–357. [Google Scholar]
- Williams, B.E.T.; Julius, B.; Timothy, N. Phytochemicals, Elemental, Proimate Analysis and Anti- Nutrient Composition of Citrus aurantifolia Seeds. Glob. J. Med. Res. L Nutr. Food Sci. 2020, 20, 7–12. [Google Scholar]
- Lin, L.Y.; Chuang, C.H.; Chen, H.C.; Yang, K.M. Lime (Citrus aurantifolia (Christm.) Swingle) Essential Oils: Volatile Compounds, Antioxidant Capacity, and Hypolipidemic Effect. Foods 2019, 8, 398. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.N.; Utra, U.; Alias, A.K.; Tan, T.B.; Tan, C.P.; Yussof, N.S. Physical, Morphological and Antibacterial Properties of Lime Essential Oil Nanoemulsions Prepared via Spontaneous Emulsification Method. LWT 2020, 128, 1–8. [Google Scholar] [CrossRef]
- Singh, N.; Sheikh, J. Multifunctional Linen Fabric Obtained through Finishing with Chitosan-Gelatin Microcapsules Loaded with Cinnamon Oil. J. Nat. Fibers 2022, 19, 4780–4790. [Google Scholar] [CrossRef]
- Brah, A.S.; Armah, F.A.; Obuah, C.; Akwetey, S.A.; Adokoh, C.K. Toxicity and Therapeutic Applications of Citrus Essential Oils (CEOs): A Review. Int. J. Food Prop. 2023, 26, 301–326. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Puspita, S.; Eddy, D.R.; Wahyudi, T.; Julaeha, E. Microencapsulation of Lime Peel Essential Oils (Citrus aurantifolia) with Complex Coacervation Methods Using Gelatin/Sodium Alginate Coating. J. Kim. Val. 2020, 6, 106–112. [Google Scholar] [CrossRef]
- Spadaro, F.; Costa, R.; Circosta, C.; Occhiuto, F. Volatile Composition and Biological Activity of Key Lime Citrus aurantifolia Essential Oil. Nat. Prod. Commun. 2012, 7, 1523–1526. [Google Scholar] [CrossRef]
- Jain, S.; Arora, P.; Popli, H. A Comprehensive Review on Citrus aurantifolia Essential Oil: Its Phytochemistry and Pharmacological Aspects. Braz. J. Nat. Sci. 2020, 3, 354–364. [Google Scholar] [CrossRef]
- Lemes, R.S.; Alves, C.C.F.; Estevam, E.B.B.; Santiago, M.B.; Martins, C.H.G.; Dos Santos, T.C.L.; Crotti, A.E.M.; Miranda, M.L.D. Chemical Composition and Antibacterial Activity of Essential Oils from Citrus aurantifolia Leaves and Fruit Peel against Oral Pathogenic Bacteria. An. Acad. Bras. Cienc. 2018, 90, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Herawati, D.; Ekawati, E.R.; Yusmiati, S.N.H. Identification of Saponins and Flavonoids in Lime (Citrus aurantifolia) Peel Extract. Proc. Int. Conf. Ind. Eng. Oper. Manag. 2020, 3661–3666. [Google Scholar]
- Karimi, A.; Nasab, N.K. Effect of Garlic Extract and Citrus aurantifolia (Lime) Juice and on Blood Glucose Level and Activities of Aminotransferase Enzymes in Streptozotocin-Induced Diabetic Rats. World J. Pharm. Sci. 2014, 2, 821–827. [Google Scholar]
- Mahyuni, S. Determinasi Kadar Total Polifenol Terlarut, Hesperetin Dan Quercetin Pada Daun, Kulit dan Isi Buah Citrus aurantifolia (Christm & Panzer) Swingle. FITOFARMAKA J. Ilm. Farm. 2016, 6, 1–8. [Google Scholar] [CrossRef][Green Version]
- Pérez-Nájera, V.C.; Lugo-Cervantes, E.; Amaya-Delgado, L.; Madrigal-Pulido, J.A.; Rueda-Puente, E.O.; Borboa-Flores, J.; Del-Toro-Sánchez, C.L. Biotransformation of Hesperidin from Lime Peel (Citrus limetta Risso) in Solid Fermentation by Aspergillus saitoi. CyTA J. Food 2018, 16, 537–543. [Google Scholar] [CrossRef]
- Kasim, V.N.; Hatta, M.; Natzir, R.; Hadju, V.; Febriza, A.; Idrus, H.H. Effects of Lime (Citrus aurantifolia) Peel to the Expression of MRNA Toll-like Receptors 4 in Balb/c Mice-Infected Salmonella Typhi. J. Adv. Pharm. Technol. Res. 2020, 11, 169–173. [Google Scholar] [CrossRef]
- Shafreen, R.B.; Lubinska-Szczygeł, M.; Różańska, A.; Dymerski, T.; Namieśnik, J.; Katrich, E.; Gorinstein, S. Human Serum Interactions with Phenolic and Aroma Substances of Kaffir (Citrus hystrix) and Key Lime (Citrus aurantifolia) Juices. J. Lumin. 2018, 201, 115–122. [Google Scholar] [CrossRef]
- Ezeabara, C.A.; Okeke, C.; Aziagba, B.O. Flavonoid Content of Citrus Species Grown in Awka, Anambra State, Southeastern Nigeria. Int. J. Agric. Biosci. 2013, 2, 103–107. [Google Scholar]
- Miles, E.A.; Calder, P.C. Effects of Citrus Fruit Juices and Their Bioactive Components on Inflammation and Immunity: A Narrative Review. Front. Immunol. 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Mirnawati; Mu’min, N.; Yunus, M. Terpenoid Compounds of Lime (Citrus aurantifolia) Peel Extract Using Gas Chromatography Mass Spectrometry (GCMS) Method. Indones. Chim. Acta 2021, 14, 26–29. [CrossRef]
- Singh, A.; Maurya, S.; Singh, U.P.; Singh, K.P. Chromatographic Analysis of Phenolic Acids in the Fruit Pulp of Some Citrus Varieties and Their Therapeutic Importance in Human Health. Int. J. Appl. Sci. Rev. 2014, 1, 150–154. [Google Scholar]
- Kim, J.W.; Ko, H.C.; Jang, M.G.; Han, S.H.; Kim, H.J.; Kim, S.J. Phytochemical Content and Antioxidant Activity in Eight Citrus Cultivars Grown in Jeju Island According to Harvest Time. Int. J. Food Prop. 2023, 26, 14–23. [Google Scholar] [CrossRef]
- Ogundele, O.O.; Bolade, M.K. Biochemical Characteristics and Antioxidant Properties of Citrus Juice from Lemon (Citrus limon), Lime (Citrus aurantifolia) and Grapefruit (Citrus paradisi) as Influenced by Degree of Ripening. Asian Food Sci. J. 2021, 20, 40–51. [Google Scholar] [CrossRef]
- Sanches, V.L.; Cunha, T.A.; Viganó, J.; de Souza Mesquita, L.M.; Faccioli, L.H.; Breitkreitz, M.C.; Rostagno, M.A. Comprehensive Analysis of Phenolics Compounds in Citrus Fruits Peels by UPLC-PDA and UPLC-Q/TOF MS Using a Fused-Core Column. Food Chem. X 2022, 14, 100262. [Google Scholar] [CrossRef]
- I. Nyoman Jirna, N.M. Potency of Lime (Citrus aurantifolia) as Bio-Disinfectant Of Staphylococcus Aureus. Dama Int. 2017, 2, 63–67. [Google Scholar]
- Liu, N.; Li, X.; Zhao, P.; Zhang, X.; Qiao, O.; Huang, L.; Guo, L.; Gao, W. A Review of Chemical Constituents and Health-Promoting Effects of Citrus Peels. Food Chem. 2021, 365, 130585. [Google Scholar] [CrossRef]
- Ahmed, M.; Saeid, A. Citrus Fruits: Nutritive Value and Value-Added Products. In Citrus-Research, Development and Biotechnology; IntechOpen: London, UK, 2021; p. 13. [Google Scholar]
- Abou Baker, D.H.; Ibrahim, E.A.; Salama, Z.A.E.R. Citrus Peels as a Source of Bioactive Compounds with Industrial and Therapeutic Applications. In Phenolic Compounds—Chemistry, Synthesis, Diversity, Non-Conventional Industrial, Pharmaceutical and Therapeutic Applications; IntechOpen: London, UK, 2022; pp. 1–13. [Google Scholar]
- Mahato, N.; Sharma, K.; Koteswararao, R.; Sinha, M.; Baral, E.R.; Cho, M.H. Citrus Essential Oils: Extraction, Authentication and Application in Food Preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 611–625. [Google Scholar] [CrossRef]
- Shi, Y.-S.; Zhang, Y.; Li, H.-T.; Wu, C.-H.; El-Seedi, H.R.; Ye, W.-K.; Wang, Z.-W.; Li, C.-B.; Zhang, X.-F.; Kai, G.-Y. Limonoids from Citrus: Chemistry, Anti-Tumor Potential, and Other Bioactivities. J. Funct. Foods 2020, 75, 104213. [Google Scholar] [CrossRef]
- Raghavan, S.; Gurunathan, J. Citrus Species—A Golden Treasure Box of Metabolites That Is Beneficial against Disorders. J. Herb. Med. 2021, 28, 100438. [Google Scholar] [CrossRef]
- Wheaton, T.A.; Stewart, I. Biosynthesis of Synephrine in Citrus. Phytochemistry 1969, 8, 85–92. [Google Scholar] [CrossRef]
- He, D.; Shan, Y.; Wu, Y.; Liu, G.; Chen, B.; Yao, S. Simultaneous Determination of Flavanones, Hydroxycinnamic Acids and Alkaloids in Citrus Fruits by HPLC-DAD-ESI/MS. Food Chem. 2011, 127, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Ahmed, I. Comparative Study of Antibacterial and Phytochemical Screening of Ethanolic Extracts of Citrus aurantifolia and Psidium guajava on Some Clinical Isolates. East Afr. Sch. J. Med. Sci. 2018, 1, 70–76. [Google Scholar]
- Liew, S.S.; Ho, W.Y.; Yeap, S.K.; Bin Sharifudin, S.A. Phytochemical Composition and in Vitro Antioxidant Activities of Citrus sinensis Peel Extracts. PeerJ 2018, 2018, 953–961. [Google Scholar] [CrossRef]
- Nwankwo, I.; Osaro-Matthew, R.C.; Ekpe, I. Original Research Article Synergistic Antibacterial Potentials of Citrus aurantifolia (Lime) and Honey against Some Bacteria Isolated from Sputum of Patients Attending Federal Medical Center Umuahia. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 534–544. [Google Scholar]
- Jeffrey. Antibacterial Potential of Lime (Citrus aurantifolia) on The Growth of Streptococcus Mutans. J. Heal. Dent. Sci. 2022, 1, 185–194. [Google Scholar] [CrossRef]
- Ali, M. Antibacterial Activity of Citrus aurantifolia Leaves Extracts Against Some Enteric Bacteria of Public Health Importance. Mod. Approaches Mater. Sci. 2018, 1, 33–38. [Google Scholar] [CrossRef]
- Bisno, A.L.; Stevens, D.L. Streptococcal Infections of Skin and Soft Tissues. N. Engl. J. Med. 1996, 334, 240–246. [Google Scholar] [CrossRef]
- Julaeha, E.; Puspita, S.; Eddy, D.R.; Wahyudi, T.; Nurzaman, M.; Nugraha, J.; Herlina, T.; Al Anshori, J. Microencapsulation of Lime (Citrus aurantifolia) Oil for Antibacterial Finishing of Cotton Fabric. RSC Adv. 2021, 11, 1743–1749. [Google Scholar] [CrossRef]
- Sandoval-Montemayor, N.E.; García, A.; Elizondo-Treviño, E.; Garza-González, E.; Alvarez, L.; Del Rayo Camacho-Corona, M. Chemical Composition of Hexane Extract of Citrus aurantifolia and Anti-Mycobacterium tuberculosis Activity of Some of Its Constituents. Molecules 2012, 17, 11173–11184. [Google Scholar] [CrossRef]
- Munawaroh, R. Optimum Conditions for Extraction of Antibacterial Compounds from Citrus aurantifolia Fruit Peel Waste. Pharmacon: J. Farm. Indones. 2018, 14, 34–39. [Google Scholar] [CrossRef]
- Nata’ala, M.; Dalhat, M.; Omoye, B.; Isah, A.; Kabiru, S.; Bashiru, I.; Umar, F. Phytochemical Screening and Antibacterial Activity of Citrus sinensis (L.) Osbeck [Orange] and Citrus aurantifolia (Cristm.) Swingle [Lime] Stem from Bacteria Associated with Dental Caries. J. Adv. Microbiol. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Khan Pathan, R.; Gali, P.R.; Pathan, P.; Gowtham, T.; Pasupuleti, S. In Vitro Antimicrobial Activity of Citrus aurantifolia and Its Phytochemical Screening. Asian Pac. J. Trop. Dis. 2012, 2, S328–S331. [Google Scholar] [CrossRef]
- Pohan, D.J.; Djojosaputro, M. Antibacterial Effectiveness of Extracts of Lime (Citrus aurantifolia Swingle) And Kaffir Lime (Citrus hystrix Dc) Leaves Against Escherichia coli. Int. J. Mod. Pharm. Res. 2021, 5, 29–36. [Google Scholar]
- Gbeghebo, A.J.; Atalawei, E.A. Antibacterial Activities of Aqueous, Acetone, and Ethanolic Extracts of Lime (Citrus aurantifolia) Leaves against Some Bacteria of Public Health Importance. Int. J. Innov. Med. Med. Plants Res. 2022, 10, 43–49. [Google Scholar]
- Aibinu, I.; Adenipekun, T.; Adelowotan, T.; Ogunsanya, T.; Odugbemi, T. Evaluation of The Antimicrobial Properties of Different Parts of Citrus aurantifolia (Lime Fruit) as Used Locally. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 185–190. [Google Scholar]
- Nerdy, N.; Tarigan, P.; Elysa, E.; Lestari, P.; Nurmalisa, S. Antibacterial Activity Test of Lime Juice Extract Against Escherichia coli. J. Penelit. Farm. Herb. 2020, 3, 135–139. [Google Scholar] [CrossRef]
- Ramadhianto, A. Bioactivity Test Crude Fruit of Citrus Lime (Citrus aurantifolia) on Bacteria Escherichia coli In Vitro. Bp. Int. Res. Exact Sci. J. 2019, 1, 16–20. [Google Scholar] [CrossRef]
- Herlina, T.; Julaeha, E.; Evy Ernawati, E.; Darwati; Nurzaman, M. Antioksidan dari Jeruk Nipis (Citrus aurantifolia) Peningkat Imunitas Tubuh dalam COVID-19. J. ITEKIMA 2020, 8, 19–29. [Google Scholar]
- Kumari, S.; Sarmah, N.; Handique, A.K. Antioxidant Activities of the Unripen and Ripen Citrus aurantifolia of Assam. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 4811–4816. [Google Scholar]
- Azman, N.F.I.N.; Azlan, A.; Khoo, H.E.; Razman, M.R. Antioxidant Properties of Fresh and Frozen Peels of Citrus Species. Curr. Res. Nutr. Food Sci. 2019, 7, 331–339. [Google Scholar] [CrossRef]
- Julaeha, E.; Dewi, K.S.; Nurzaman, M.; Wahyudi, T.; Herlina, T. Chemical Compositions and Antioxidant Activities of Indonesian Citrus Essential Oils and Their Elucidation Using Principal Component Analysis. Preprints 2020, 11, 86. [Google Scholar] [CrossRef]
- Dongmo, P.M.J.; Tchoumbougnang, F.; Boyom, F.F.; Sonwa, E.T.; Zollo, P.H.A.; Menut, C. Antiradical, Antioxidant Activity and Antiinflammatory Potential of The Essential Oils of The Varieties of Citrus limon and Citrus aurantifolia Growing in Cameron. Analysis 2013, 3, 1046–1057. [Google Scholar]
- Boshtam, M.; Moshtaghian, J.; Naderi, G.; Asgary, S.; Nayeri, H. Antioxidant Effects of Citrus aurantifolia (Christm) Juice and Peel Extract on LDL Oxidation. J Res. Med. Sci. 2011, 16, 951–955. [Google Scholar] [CrossRef]
- Khadijah, K.; Soekamto, N.H.; Chalid, S.M.T.; Rafidah, N.F. Total Phenol Content and Activities of Antioxidant Extracts Methanol Lime (Citrus aurantifolia) by Uv-Vis Spectrophotometry. In E3S Web Conf. 2021, 328, 1–6. [Google Scholar] [CrossRef]
- Permadi, N.; Julaeha, E.; Rosandi, Y.; Nurzaman, M. Antioxidant Activity of Non-Volatile Lime (Citrus aurantifolia Swingle) Extract. J. Agrinika J. Agroteknologi Agribisnis 2021, 5, 122–128. [Google Scholar] [CrossRef]
- Cirmi, S.; Maugeri, A.; Ferlazzo, N.; Gangemi, S.; Calapai, G.; Schumacher, U.; Navarra, M. Anticancer Potential of Citrus Juices and Their Extracts: A Systematic Review of Both Preclinical and Clinical Studies. Front. Pharmacol. 2017, 8, 1–33. [Google Scholar] [CrossRef]
- Gyawali, R.; Kim, K.S. Anticancer Phytochemicals of Citrus Fruits—A Review. J. Anim. Res. 2014, 4, 85–95. [Google Scholar] [CrossRef]
- Kenyori, I.K.; Alamsyah, M.S.; Nurjanah, C.I.A. Studi in Silico Senyawa Bioaktif Kuesetin Kulit Jeruk Nipis (Citrus aurantifolia) Sebagai Agen Antikanker Payudara. Berk. Ilm. Mhs. Farm. Indones. 2022, 9, 1–10. [Google Scholar] [CrossRef]
- Hairunisa, I.; Normaidah, N.; Ressandy, S.S.; Azhari, F. Identifikasi dan Molecular Docking Komponen Utama Minyak Kulit Buah Jeruk Nipis Sebagai Agen Antikanker. J. Ilm. Ibnu Sina 2019, 4, 314–322. [Google Scholar] [CrossRef]
- Gharagozloo, M.; Doroudchi, M.; Ghaderi, A. Effects of Citrus aurantifolia Concentrated Extract on the Spontaneous Proliferation of MDA-MB-453 and RPMI-8866 Tumor Cell Lines. Phytomedicine 2002, 9, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.R.; Jayaprakasha, G.K.; Murthy, K.N.C.; Chetti, M.B.; Patil, B.S. Characterization of Citrus aurantifolia Bioactive Compounds and Their Inhibition of Human Pancreatic Cancer Cells through Apoptosis. Microchem. J. 2010, 94, 108–117. [Google Scholar] [CrossRef]
- Sarma, R.; Adhikari, K.; Mahanta, S.; Khanikor, B. Insecticidal Activities of Citrus aurantifolia Essential Oil against Aedes aegypti (Diptera: Culicidae). Toxicol. Rep. 2019, 6, 1091–1096. [Google Scholar] [CrossRef]
- Haumein, L.N.; Dwiana, W.G. Test Effectiveness of Nipis Orange Extract (Citrus aurantifolia) to The Death of Mosquito Aedes aegypti). 2nd Str. Int. Conf. Health 2020, 2, 229–235. [Google Scholar] [CrossRef]
- Arani, Y.; Nithiyagowry, R. Study on Bioactivity of Lime, Citrus aurantifolia (Christm.) against Larvae of Diamond Back Moth, Plutella xylostella (L.) on Cabbage Crop under Laboratory. Vingnanam J. Sci. 2021, 16, 1–5. [Google Scholar]
- Roko, O.G.; Klotoe, J.R. Anti-Infammatory, Analgesic and Antipyretic Properties of Ethanolic Extracts of Three Plants of Benin Pharmacopoeia: Euphorbia hirta, Citrus aurantifolia and Heterotis rotundifolia. Res. Sq. 2019, 1–13. [Google Scholar] [CrossRef]
- Amorim, J.L.; Simas, D.L.R.; Pinheiro, M.M.G.; Moreno, D.S.A.; Alviano, C.S.; Da Silva, A.J.R.; Fernandes, P.D. Anti-Inflammatory Properties and Chemical Characterization of the Essential Oils of Four Citrus Species. PLoS ONE 2016, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wardani, R.P.; Kholifa, M.; Yuletnawati, S.E. Pengaruh Ekstrak Etanol Kulit Jeruk Nipis (Citrus aurantifolia (Christm.) Swingle) Terhadap Penyembuhan Ulkus Traumatik Pada Rattus Norvegicus Strain Wistar. JIKG (Jurnal Ilmu Kedokt. Gigi) 2017, 1, 23–27. [Google Scholar]
- Ramya, S.; Narayanan, V.; Ponnerulan, B.; Saminathan, E.; Veeranan, U. Potential of Peel Extracts of Punica granatum and Citrus aurantifolia on Alloxan-Induced Diabetic Rats. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 1–11. [Google Scholar] [CrossRef]
- Ezeigwe, O.C.; Okpala, C.O.; Enemali, M.O.; Iloanya, E.L.; Chigbo, C.M.; Okeke, C.M.; Okeke, C.B.; Okafor, M.C. Effect of Citrus aurantifolia Juice on Bodyweight and Haematological Indices of Wistar Rats. Afr. J. Food Sci. 2022, 16, 151–159. [Google Scholar] [CrossRef]
- Ettebong, E.; Ubulom, P.; Etuk, A. Antiplasmodial Activity of Methanol Leaf Extract of Citrus aurantifolia (Christm) Swingle. J. Herbmed Pharmacol. 2019, 8, 274–280. [Google Scholar] [CrossRef]
- Dias, M.I.; Ferreira, I.C.F.R.; Barreiro, M.F. Microencapsulation of Bioactives for Food Applications. Food Funct. 2015, 6, 1035–1052. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.F.R.; Pintado, M.E.; Morais, R.M.S.C.; Morais, A.M.M.B. Valorisation of Micro/Nanoencapsulated Bioactive Compounds from Plant Sources for Food Applications Towards Sustainability. Foods 2023, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, N.; Meghwal, M.; Das, K. Microencapsulation: An Overview on Concepts, Methods, Properties and Applications in Foods. Food Front. 2021, 2, 426–442. [Google Scholar] [CrossRef]
- Wahyudi, T.; Mulyawan, A.S.; Kasipah, C.; Prayudie, U.; Julaeha, E. Pembuatan Mikrokapsul Minyak Jeruk (Citrus aurantifolia) untuk Aplikasi pada Penyempurnaan Tekstil. Arena Tekst. 2017, 32, 1–8. [Google Scholar] [CrossRef][Green Version]
- Pratiwi, L.; Eddy, D.R.; Al Anshori, J.; Harja, A.; Wahyudi, T.; Mulyawan, A.S.; Julaeha, E. Microencapsulation of Citrus aurantifolia Essential Oil with the Optimized CaCl2 Crosslinker and Its Antibacterial Study for Cosmetic Textiles. RSC Adv. 2022, 12, 30682–30690. [Google Scholar] [CrossRef]
- Van Luyn, M.J.A.; Van Wachem, P.B.; Olde Damink, L.; Dijkstra, P.J.; Feijen, J.; Nieuwenhuis, P. Relations between In Vitro Cytotoxicity and Crosslinked Dermal Sheep Collagens. J. Biomed. Mater. Res. 1992, 26, 1091–1110. [Google Scholar] [CrossRef]
- Olde Damink, L.H.H.; Dijkstra, P.J.; Van Luyn, M.J.A.; Van Wachem, P.B.; Nieuwenhuis, P.; Feijen, J. Glutaraldehyde as a Crosslinking Agent for Collagen-Based Biomaterials. J. Mater. Sci. Mater. Med. 1995, 6, 460–472. [Google Scholar] [CrossRef]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of Glutaraldehyde Crosslinked Collagen/Poly(Vinyl Alcohol) Films Is by the Mechanism of Apoptosis. J. Biomed. Mater. Res. 2002, 61, 121–130. [Google Scholar] [CrossRef]

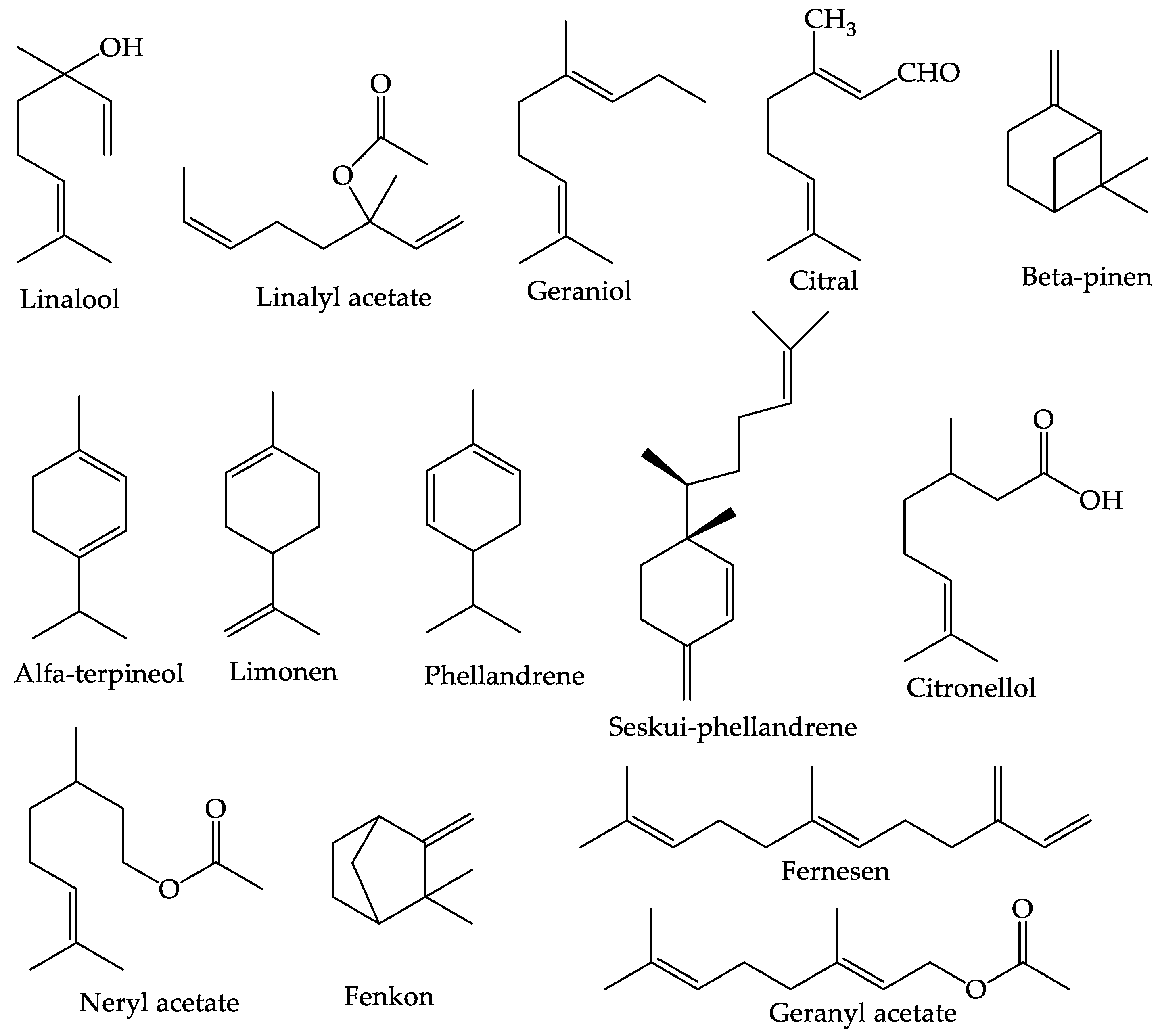

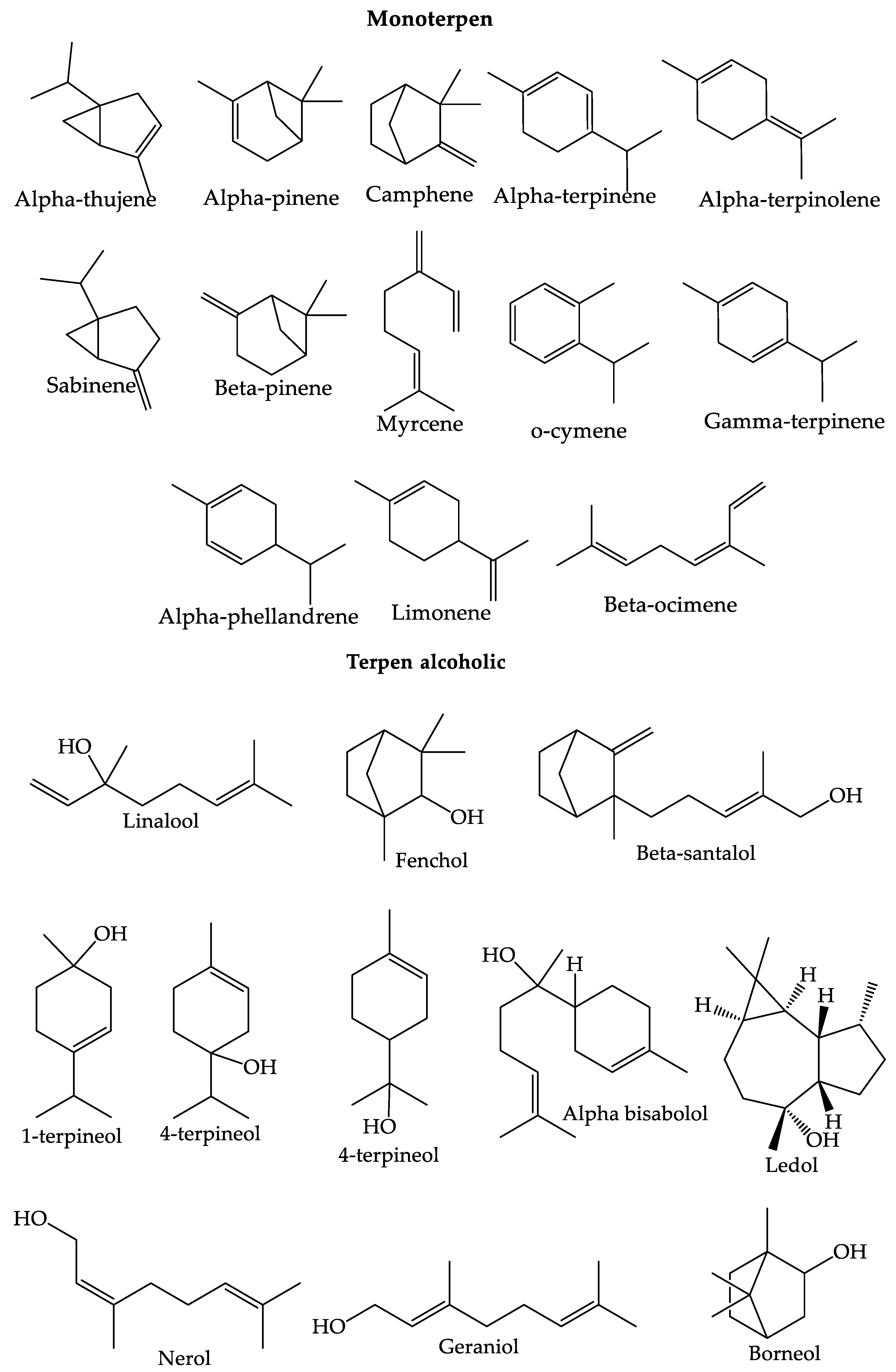
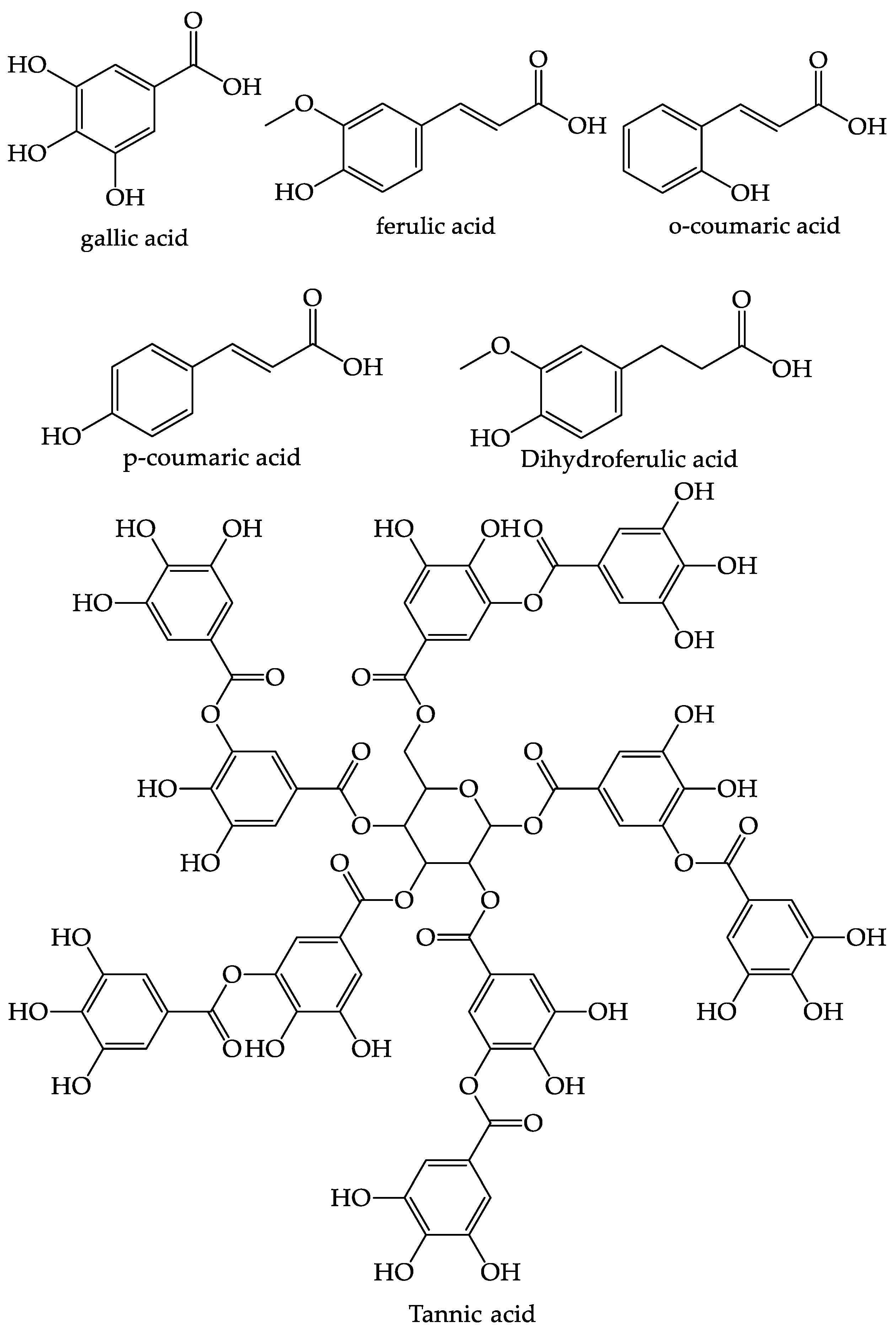
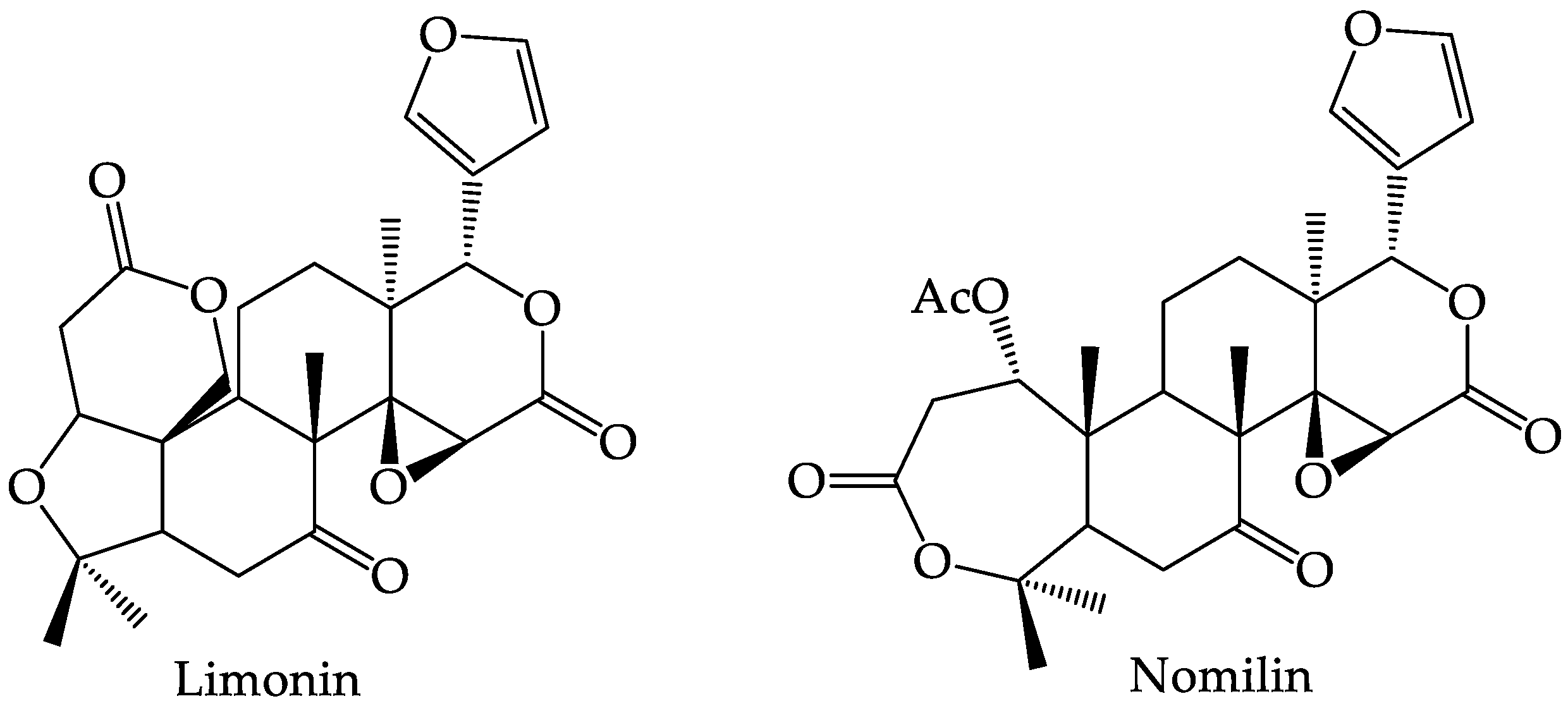


| No | Secondary Metabolite | Technique | Plant Parts | Yields | Reference |
|---|---|---|---|---|---|
| 1 | Essential Oil | Hydrodistillation | milled peel whole peels leaf | 5.45% 0.97% 0.75% | [38] [38] [39] |
| Supercritical-CO2 extraction | milled peel whole peels | 7.93% 1.98% | [38] | ||
| Steam distillation | peel leaf | 1.5% 0.75% | [39] | ||
| Maceration | peel | n-hexane = 5.24% distilled water = 1.67% ethanol = 4.20% | [40] | ||
| Soxhlation | peel | n-hexane = 6.15% ethanol = 4.89% | [40] | ||
| Cold pressed | leaf | 0.5% | [6,39] | ||
| 2 | Flavonoids | Maceration | fruit | Ethanol = 7.83 ± 2.66 mgCA/g extract Methanol 70% = 6.27 ± 0.39 mgCA/g extract Aquades = 6.52 ± 0.77 mgCA/g extract | [41] |
| Maceration with 95% ethanol | leaf | Leaf of Nakhal 64.2 ± 2.8 (μg of QE/mg dry extract) Leaves from Nizwa 41.38 ± 5.5 (μg of QE/mg dry extract) | [11] | ||
| 3 | Phenolic | Ultrasound Assisted Extraction (UAE), | peel | Yields increased when compared maceration extract | [42] |
| Low Power Ultrasound-Assisted Extraction | peel | 3083.61 mg gallic acid equivalent (eq) 100 g−1 dry weight of the total phenolic component | [43] | ||
| 4 | Limonoids | Solvent extraction, supercritical-CO2 extraction | seed | 11.39% dry base ± 1.3 for methanol 10.37% dry base ± 2.21 for acetone | [44] |
| Bioactive Components | Section C. aurantifolia | Reference | ||||||
|---|---|---|---|---|---|---|---|---|
| Leaf | Fruit | Rind | Seed | Stem | Root | Bark | ||
| Essential oil | √ | - | √ | - | - | - | - | [2,11,48,49,50,51,52,53] |
| Flavonoids | √ | √ | √ | √ | √ | √ | √ | |
| Terpenoids | √ | - | √ | √ | - | √ | √ | |
| Phenolic | √ | √ | √ | √ | √ | - | √ | |
| Limonoids | - | - | - | √ | - | - | - | |
| Alkaloids | √ | √ | √ | √ | √ | √ | √ | |
| Leaf | Fruit | Peel | Seed | Reference |
|---|---|---|---|---|
| 4.675 mg/g wet weight | 2.243 mg/g wet weight | 6.954 mg/g wet weight | 19.87 ± 0.03 mg/100 g wet weight | [53,65] |
| No | Part | Extraction Solvent | Bacteria | Method | Reference |
|---|---|---|---|---|---|
| 1 | Essential Oil | - | Azotobacter chroococcum, Serratia marcescens, Priestia megaterium | Disk diffusion method | [6] |
| Micrococcus luterus | |||||
| Staphylococcus aureus, Staphylococcus epidermidis, E. coli, Klabsiella pneumoniae | [91] | ||||
| 2 | Peel | Hexane | Mycobacterium tuberculosis H37rv | Microplate Alamar Blue Assay (MABA) | [92] |
| Ethanol 48%, 72%, 96% Ethyl acetate | S. aureus ATCC 25923 and E. coli ATCC 25922 | Disk diffusion method | [93] | ||
| Ethanol | Bacillus cereus, E. Coli | Disk diffusion method | [71] | ||
| 3 | Stem | Ethanol, Aquades | S. aureus, Pseudomonas aeruginosa, Pseudomonas mirabilis and K. Pneumoniae | Disk diffusion method | [94] |
| E. coli, Bacillus megaterium, P. aeruginosa, Enterobacter aerogenes, Salmonella spp., Proteus myxofaciens, K. pneumoniae, Kluyvera ascorbata, S. aureus | Dilution | ||||
| 4 | Leaf | S. aureus | Disk diffusion method | [76] | |
| Higher in ethanol than distilled water | Shigella, Klebsiella, E. coli, S. typhi | Disk diffusion method | [89] | ||
| Hydroalcoholic | S. aureus, E. coli, K. pneumoniae, Pseudomonas spp. | Disk diffusion method | [95] | ||
| Aquades, Ethanol, Methanol, Acetone | S. aureus, E. coli, K. pneumoniae, P. aeruginosa, Aspergillus niger, Mucor mucedo, Penicillium notatum, Candida albicans | [49] | |||
| E. coli ATCC 25922 | The Kirby–Bauer | [96] | |||
| Aqueous, acetone, ethanol | E. coli, Pseudomonas, S. aureus, Klebsiella | Disk diffusion method | [97] | ||
| 5 | Fruit | Aquades, Ethanol, Tuak, Seamann’s Schnapps, Fermented Water from 3 days Soaking Corn Powder/Corn Paste (Ekan-Ogi/Omi-Ogi) | Gram-negative Serratia spp., Salmonella paratyphi, Shigella flexnerri, P. aeruginosa, K. pneumoniae, Citrobacter spp., and E. coli Gram-positive (S. aureus, Enterococcus feacalis) fungi (Aspergillus niger, Candida albicans) three anaerobic bacteria (Clostridium spp., Bacteroides spp. and Porphyromonas spp.) | Disk diffusion method | [98] |
| 6 | Juice | Aquades | E. coli | Disk diffusion method | [99] |
| 7 | Seed | Ethanol, Chloroform, Methanol | Bacillus subtilis NCTC 8236. S. aureus ATCC 25923. E. coli ATCC 25922. Proteus vulgaris ATCC 6380. Klebsiellas pp. ATCC 53657. Shigella spp. NCTC 4837. | Disk diffusion method | [2] |
| 8 | Crude | E. coli | Kirby–Bauer susceptibility test method | [100] |
| No | Source | Method | Antioxidant Activity | Reference |
|---|---|---|---|---|
| 1 | Essential oil | DPPH | 74.5 ± 0.5%, with a corresponding 442 ± 2.3 TEAC | [6] |
| DPPH | IC50 2.19 mg/mL | [54] | ||
| DPPH | IC50 12.85 µL/mL, ascorbic acid 5.28 µL/mL | [104] | ||
| DPPH | IC50 C. aurantifolia var. Bearss 4.32 mg/L, var. Mexican 1.62 mg/L and var. sans epines 0.26 mg/L | [105] | ||
| ABTS | IC50 2.00 mg/mL | [54] | ||
| 2 | Juice | FRAP | 4.98 µmol Fe(II)/g (in immature C. aurantifolia) | [74] |
| DPPH | Ripe and unripe C. aurantifolia juices showed 91.02% and 96.14% scavenging against DPPH at a concentration of 100 µL | [102] | ||
| 3 | Fruit juices and peel | Low-density lipoprotein (LDL) | 10 µL of juice inhibited LDL oxidation and increased with increasing concentration | [106] |
| 4 | Leaves and bark (methanolic extract) | DPPH & FRAP | Reducing ability ranges from 112.1–146.0 µmol L−1 Fe(II) g−1 IC50 ranges from 91.4–107.4 µgmL−1 | [14] |
| 5 | Leaves (ethanolic extract) | DPPH | Both extracts of lime leaves from Nakhal and Nizwa showed moderate antioxidant activity depending on the concentration range (11.79–56.89 and 10.11–51.91%) | [11] |
| DPPH & FRAP | IC50 = 83.89 ppm 70% ethanolic extract and IC50 = 88.02 ppm 96% ethanolic extract FRAP test 188.74 mg AaE/g 96% ethanolic extract and 181.034 mg AaE/g 70% ethanolic extract. | [4] | ||
| 6 | Fruit (methanolic extract) | DPPH | IC50 = 1793.06 g/mL | [107] |
| 7 | Peel | DPPH | The ethyl acetate fraction has the largest IC50 which is 457.6 ppm | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Indriyani, N.N.; Anshori, J.A.; Permadi, N.; Nurjanah, S.; Julaeha, E. Bioactive Components and Their Activities from Different Parts of Citrus aurantifolia (Christm.) Swingle for Food Development. Foods 2023, 12, 2036. https://doi.org/10.3390/foods12102036
Indriyani NN, Anshori JA, Permadi N, Nurjanah S, Julaeha E. Bioactive Components and Their Activities from Different Parts of Citrus aurantifolia (Christm.) Swingle for Food Development. Foods. 2023; 12(10):2036. https://doi.org/10.3390/foods12102036
Chicago/Turabian StyleIndriyani, Nastiti Nur, Jamaludin Al Anshori, Nandang Permadi, Sarifah Nurjanah, and Euis Julaeha. 2023. "Bioactive Components and Their Activities from Different Parts of Citrus aurantifolia (Christm.) Swingle for Food Development" Foods 12, no. 10: 2036. https://doi.org/10.3390/foods12102036
APA StyleIndriyani, N. N., Anshori, J. A., Permadi, N., Nurjanah, S., & Julaeha, E. (2023). Bioactive Components and Their Activities from Different Parts of Citrus aurantifolia (Christm.) Swingle for Food Development. Foods, 12(10), 2036. https://doi.org/10.3390/foods12102036








