Quality and Nutritional Changes of Traditional Cupcakes in the Processing and Storage as a Result of Sunflower Oil Replacements with Refined Olive Pomace Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Oils and Cupcakes
2.2. Oil Characterisation
2.3. Cupcake Characterisation
2.4. Fat Extraction
2.5. Oxidative Changes in the Processing
2.6. Storage Assay
2.7. Consumer Tests
2.8. Statistical Analyses
3. Results and Discussion
3.1. Oil Characterisation
3.2. Characterisation of the Fresh Cupcakes
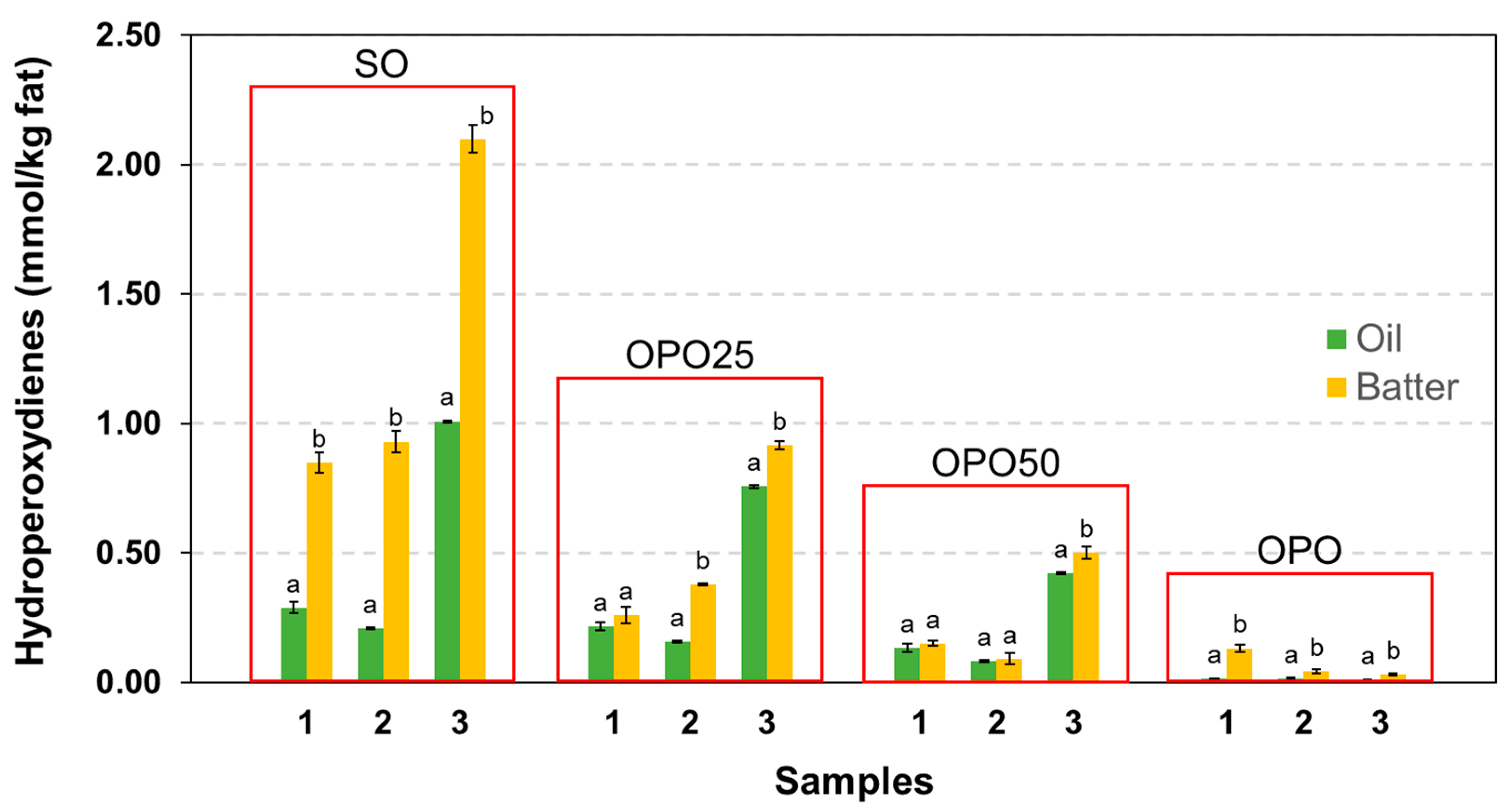
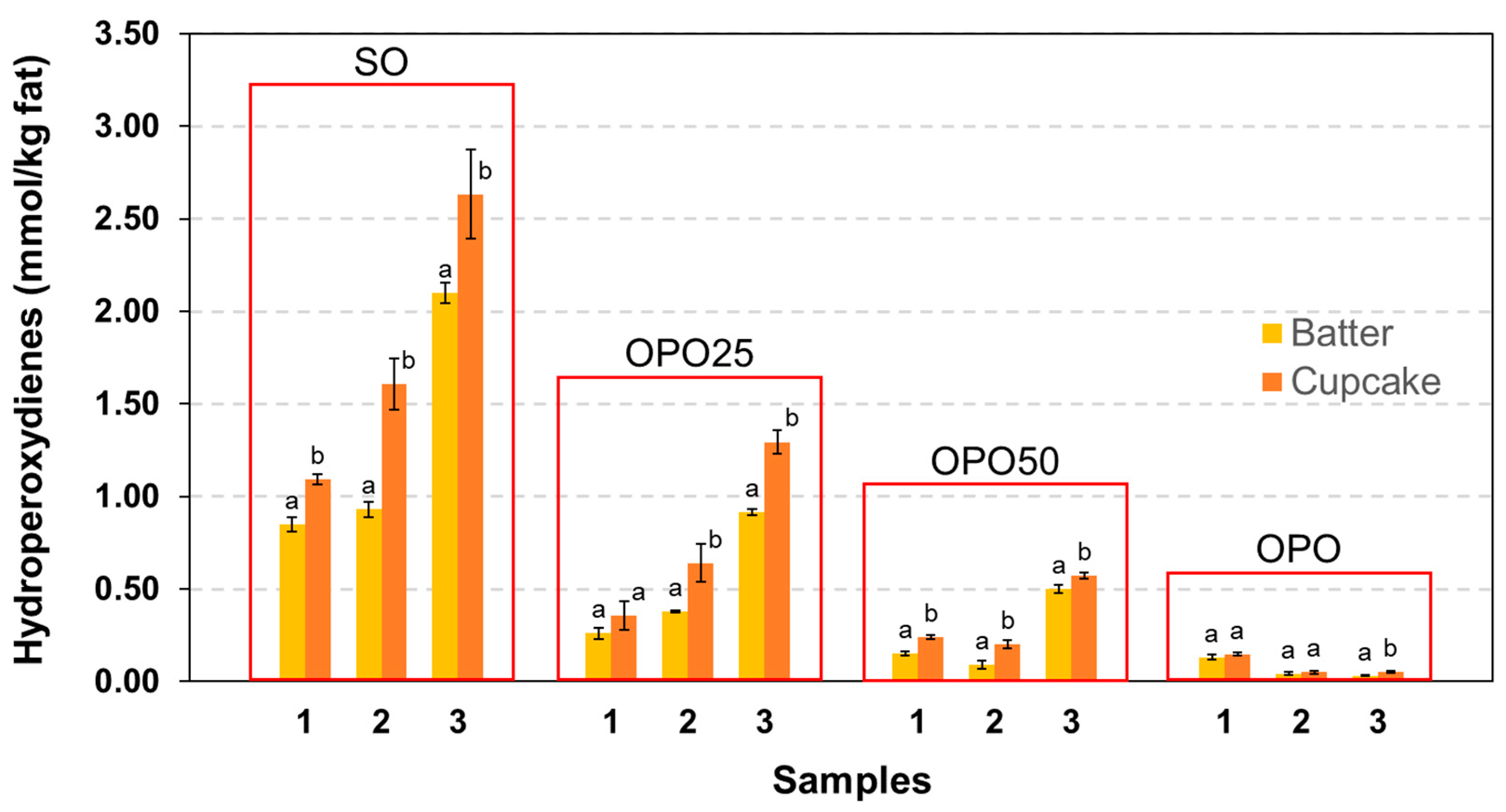
3.3. Oxidative Deterioration and Losses of Oil Bioactive Components in the Processing
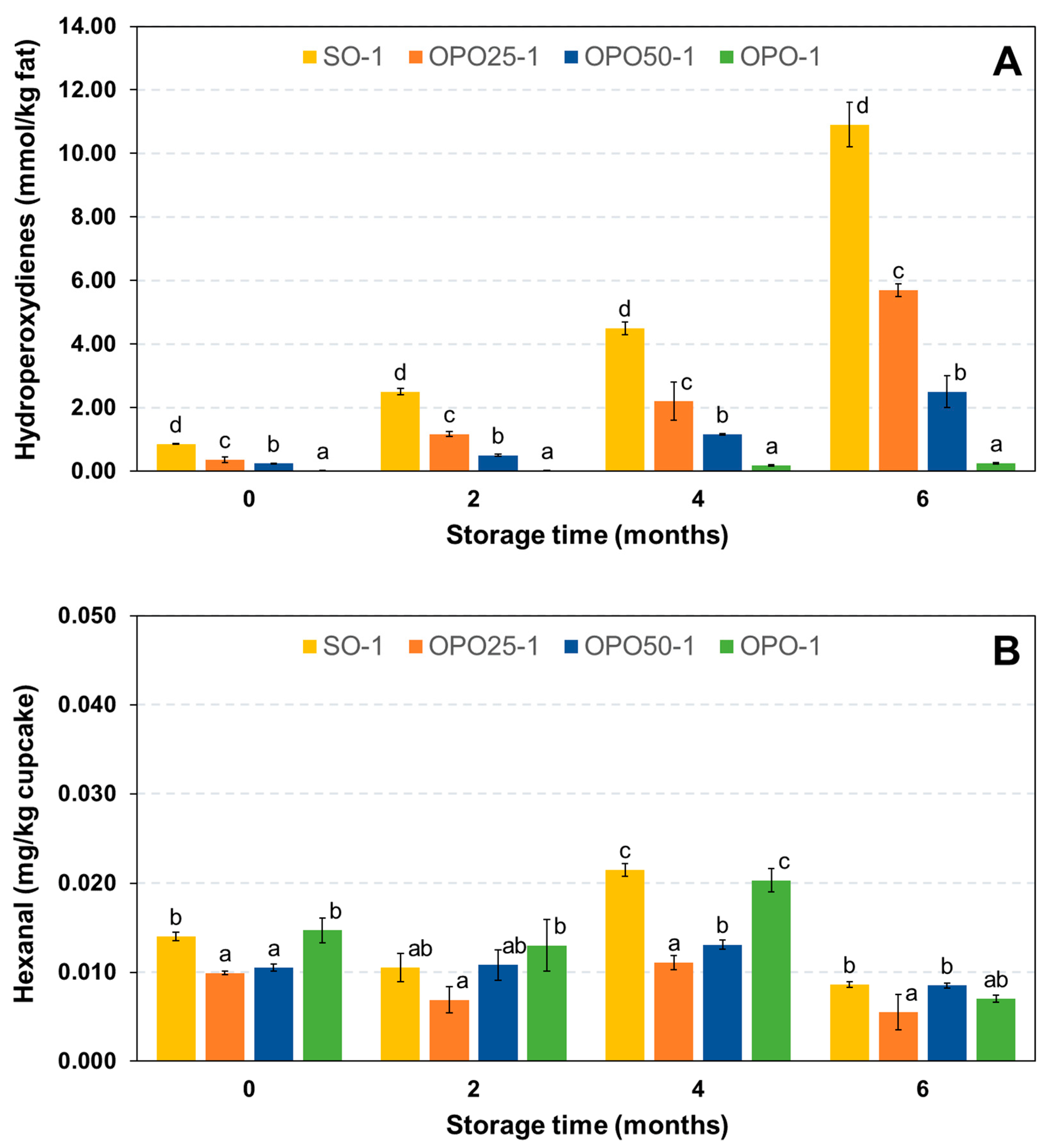
3.4. Oxidative Changes and Losses of Oil Bioactive Components during the Shelf-Life
3.4.1. Oxidative Degradation
3.4.2. Contents of Oil Bioactive Components
3.5. Acceptability and Consumer Preferences
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- COI/T.15/NC No 3/Rev.19/2022; Trade Standard Applying to Olive Oils and Olive Pomace Oils. International Olive Council: Madrid, Spain, 2022. Available online: https://www.internationaloliveoil.org/what-we-do/chemistry-standardisation-unit/standards-and-methods/ (accessed on 3 May 2023).
- FAO-WHO. Fats and fatty acids in human nutrition. In Report of an Expert Consultation; FAO: Geneva, Switzerland, 2008; ISBN 978-92-5-106733-8. Available online: https://www.fao.org/3/i1953e/i1953e00.pdf (accessed on 3 May 2023).
- EFSA. Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. Eur. Food Saf. Auth. J. 2010, 8, 1461–1568. [Google Scholar] [CrossRef][Green Version]
- Mateos, R.; Sarria, B.; Bravo, L. Nutritional and other health properties of olive pomace oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 3506–3521. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Ruiz, G.; Holgado, F. Frying performance of olive-extracted oils. Grasas Aceites 2018, 69, e264. [Google Scholar] [CrossRef]
- González-Rámila, S.; Mateos, R.; García-Cordero, J.; Seguido, M.A.; Bravo-Clemente, L.; Sarriá, B. Olive pomace oil versus high oleic sunflower oil and sunflower oil: A comparative study in healthy and cardiovascular risk humans. Foods 2022, 11, 2186. [Google Scholar] [CrossRef]
- González-Rámila, S.; Sarriá, B.; Seguido, M.A.; García-Cordero, J.; Bravo-Clemente, L.; Mateos, R. Effect of olive pomace oil on cardiovascular health and associated pathologies. Nutrients 2022, 14, 3927. [Google Scholar] [CrossRef] [PubMed]
- González-Rámila, S.; Sarriá, B.; Seguido, M.A.; García-Cordero, J.; Mateos, R.; Bravo, L. Olive pomace oil can improve blood lipid profile: A randomized, blind, crossover, controlled clinical trial in healthy and at-risk volunteers. Eur. J. Nutr. 2022, 62, 589–603. [Google Scholar] [CrossRef]
- Holgado, F.; Ruiz-Méndez, M.V.; Velasco, J.; Márquez-Ruiz, G. Performance of olive-pomace oils in discontinuous and continuous frying. Comparative behavior with sunflower oils and high-oleic sunflower oils. Foods 2021, 10, 3081. [Google Scholar] [CrossRef]
- Ruiz-Méndez, M.V.; Márquez-Ruiz, G.; Holgado, F.; Velasco, J. Stability of bioactive compounds in olive-pomace oil at frying temperature and incorporation into fried foods. Foods 2021, 10, 2906. [Google Scholar] [CrossRef]
- Lin, S.; Chi, W.; Hu, J.; Pan, Q.; Zheng, B.; Zeng, S. Sensory and nutritional properties of Chinese olive pomace based high fibre biscuit. Emir. J. Food Agric. 2017, 29, 495–501. [Google Scholar] [CrossRef][Green Version]
- Trindade, P.C.O.; Dalfolo, A.D.C.; Monteiro, C.S.; Wagner, R.; dos Santos, B.A.; Dalla Nora, F.M.; Verruck, S.; da Rosa, C.S. Development and characterization of biscuits with olive pomace. Food Sci. Technol. 2023, 43, e99922. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Picone, G.; Pasini, F.; Chiarello, E.; Caboni, M.F.; Capozzi, F.; Gianotti, A.; Bordoni, A. Olive oil by-products as functional ingredient in bakery products. Influence of processing and evaluation of biological effects. Food Res. Int. 2020, 131, 108940. [Google Scholar] [CrossRef][Green Version]
- Simsek, M.; Süfer, Ö. Olive pomace from olive oil processing as partial flour substitute in breadsticks: Bioactive, textural, sensorial and nutritional properties. J. Food Process. Preserv. 2022, 46, e15705. [Google Scholar] [CrossRef]
- Caponio, F.; Giarnetti, M.; Paradiso, V.M.; Summo, C.; Gomes, T. Potential use of extra virgin olive oil in bakery products rich in fats: A comparative study with refined oils. Int. J. Food Sci. Technol. 2013, 48, 82–88. [Google Scholar] [CrossRef]
- Frankel, E.N. Foods. In Lipid Oxidation, 2nd ed.; Frankel, E.N., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2005; pp. 299–354. [Google Scholar]
- CXS 210-1999; Vegetable Oils. World Health Organization, Food and Agriculture Organization of the United Nations: Rome, Italy, 1999. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B210-1999%252FCXS_210e.pdf (accessed on 3 May 2023).
- Jackson, V.; Penumetcha, M. Dietary oxidised lipids, health consequences and novel food technologies that thwart food lipid oxidation: An update. Int. J. Food Sci. Technol. 2019, 54, 1981–1988. [Google Scholar] [CrossRef][Green Version]
- Grootveld, M.; Percival, B.C.; Leenders, J.; Wilson, P.B. Potential adverse public health effects afforded by the ingestion of dietary lipid oxidation product toxins: Significance of fried food sources. Nutrients 2020, 12, 974. [Google Scholar] [CrossRef][Green Version]
- Cake Market Size, Share & Trends Analysis Report by Product (Cupcakes, Dessert Cakes, Sponge Cakes), by Distribution Channel, by Region, and Segment Forecasts, 2020–2027. Report GVR-4-68038-883-1. Grand View Research. Available online: https://www.grandviewresearch.com/industry-analysis/cakes-market (accessed on 3 May 2023).
- Lassoued, N. Cellular Structure of Baked Cereal Product Linked with Rheological and Thermal Properties of Dough: Effect of Formulation. Food and Agriculture Organization of the United Nations. ENSIA (AgroParisTech). 2005. Available online: https://agris.fao.org/agris-search/search.do?recordID=FR20210188868 (accessed on 3 May 2023).
- Guillemin, I.; Marrel, A.; Arnould, B.; Capuron, L.; Dupuy, A.; Ginon, E.; Layé, S.; Lecerf, J.-M.; Prost, M.; Rogeaux, M.; et al. How French subjects describe well-being from food and eating habits? Development, item reduction and scoring definition of the Well-Being related to Food Questionnaire (Well-BFQ©). Appetite 2016, 96, 333–346. [Google Scholar] [CrossRef]
- ISO 7251:2005; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection and Enumeration of Presumptive Escherichia Coli—Most Probable Number Technique; Standard ISO/TC 34/SC 9. International Organization for Standardization: Geneva, Switzerland, 2005. Available online: https://www.iso.org/standard/34568.html (accessed on 3 May 2023).
- ISO 6888-3:2003; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus Aureus and Other Species)—Part 3: Detection and MNP Technique for Low Numbers; Standard ISO/TC 34/SC 9. International Organization for Standardization: Geneva, Switzerland, 2003. Available online: https://www.iso.org/standard/33147.html (accessed on 3 May 2023).
- ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp.; Standard ISO/TC 34/SC 9. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/56712.html (accessed on 3 May 2023).
- ISO 15213:2003; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Sulphite-Reducing Bacteria Growing under Anaerobic Conditions; Standard ISO/TC 34/SC 9. International Organization for Standardization: Geneva, Switzerland, 2003. Available online: https://www.iso.org/standard/26852.html (accessed on 3 May 2023).
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique; Standard ISO/TC 34/SC 9. International Organization for Standardization: Geneva, Switzerland, 2013. Available online: https://www.iso.org/standard/53728.html (accessed on 3 May 2023).
- ISO 21527-1:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeast and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95; Standard ISO/TC 34/SC 9. International Organization for Standardization: Geneva, Switzerland, 2008. Available online: https://www.iso.org/standard/38275.html (accessed on 3 May 2023).
- ISO 21527-2:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeast and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less than or Equal to 0.95; Standard ISO/TC 34/SC 9. International Organization for Standardization: Geneva, Switzerland, 2008. Available online: https://www.iso.org/standard/38276.html (accessed on 3 May 2023).
- EEC. Commission Regulation (EEC) No. 2568/91 of 11 July 1991 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. Official Journal of the European Union 1991. Volume 248, pp. 1–83. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A31991R2568 (accessed on 3 May 2023).
- Pérez-Camino, M.C.; Cert, A. Quantitative determination of hydroxy pentacyclic triterpene acids in vegetable oils. J. Agric. Food Chem. 1999, 47, 1558–1562. [Google Scholar] [CrossRef] [PubMed]
- ISO 9936:2016; Animal and Vegetable Fats and Oils—Determination of Tocopherol and Tocotrienol Contents by High-Performance Liquid Chromatography; Standard ISO/TC 34/SC 11. International Organization for Standardization: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/69595.html (accessed on 3 May 2023).
- ISO 660:2020; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity; Standard ISO/TC 34/SC 11. International Organization for Standardization: Geneva, Switzerland, 2020. Available online: https://www.iso.org/standard/75594.html (accessed on 3 May 2023).
- ISO 3960:2017; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint determination; Standard ISO/TC 34/SC 11. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/71268.html (accessed on 3 May 2023).
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 7th ed.; AOCS Press: Champaign, IL, USA, 2022. [Google Scholar]
- ISO 16931:2009; Animal and Vegetable Fats and Oils—Determination of Polymerized Triacylglycerols by High-Performance Size-Exclusion Chromatography (HPSEC); Standard ISO/TC 34/SC 11. International Organization for Standardization: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/44881.html (accessed on 3 May 2023).
- Velasco, J.; Morales-Barroso, A.; Ruiz-Méndez, M.V.; Márquez-Ruiz, G. Quantitative determination of major oxidation products in edible oils by direct NP-HPLC-DAD analysis. J. Chromatogr. A 2018, 1547, 62–70. [Google Scholar] [CrossRef]
- ISO 734:2015; Oil Seed Meals—Determination of Oil Content—Extraction Method with Hexane (or Light Petroleum); Standard ISO/TC 34/SC 6. International Organization for Standardization: Geneva, Switzerland, 2015. Available online: https://www.iso.org/standard/66393.html (accessed on 3 May 2023).
- Castellano, J.M.; García, J.M.; Morilla, A.; Perdiguero, S.; Gutiérrez, F. Quality of Picual olive fruits stored under controlled atmospheres. J. Agric. Food Chem. 1993, 41, 537–539. [Google Scholar] [CrossRef]
- Dobarganes, M.C.; Velasco, J.; Dieffenbacher, A. Determination of polar compounds, polymerized and oxidized triacylglycerols, and diacylglycerols in oils and fats. Pure Appl. Chem. 2000, 72, 1563–1575. [Google Scholar] [CrossRef]
- ISO 8420:2002; Animal and Vegetable Fats and Oils—Determination of Content of Polar Compounds; Standard ISO/TC 34/SC 11. International Organization for Standardization: Geneva, Switzerland, 2002. Available online: https://www.iso.org/standard/33289.html (accessed on 3 May 2023).
- Trypidis, D.; García-González, D.L.; Lobo-Prieto, A.; Nenadis, N.; Tsimidou, M.Z.; Tena, N. Real time monitoring of the combined effect of chlorophyll content and light filtering packaging on virgin olive oil photo-stability using mesh cell-FTIR spectroscopy. Food Chem. 2019, 295, 94–100. [Google Scholar] [CrossRef]
- Morales, A.; Marmesat, S.; Dobarganes, C.; Márquez-Ruiz, G.; Velasco, J. Quantitative analysis of hydroperoxy-, keto- and hydroxy-dienes in refined vegetable oils. J. Chromatogr. A 2012, 1229, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.; Keller, S.; Hashemi, A.; Descharles, N.; Bonazzi, C.; Rega, B. From flours to cakes: Reactivity potential of pulse ingredients to generate volatile compounds impacting the quality of processed foods. Food Chem. 2022, 371, 131379. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.; Asamoah, E.A.; Moulin, G.; Bonazzi, C.; Rega, B. Lipid oxidation during the beating of cake batter containing yellow pea (Pisum sativum L.) flour. LWT 2022, 154, 112770. [Google Scholar] [CrossRef]
- Hrncirik, K.; Zeelenberg, M. Stability of essential fatty acids and formation of nutritionally undesirable compounds in baking and shallow frying. J. Am. Oil Chem. Soc. 2014, 91, 591–598. [Google Scholar] [CrossRef]
- Maire, M.; Rega, B.; Cuvelier, M.-E.; Soto, P.; Giampaoli, P. Lipid oxidation in baked products: Impact of formula and process on the generation of volatile compounds. Food Chem. 2013, 141, 3510–3518. [Google Scholar] [CrossRef]
- Dugo, P.; Ragonese, C.; Russo, M.; Sciarrone, D.; Santi, L.; Cotroneo, A.; Mondello, L. Sicilian lemon oil: Composition of volatile and oxygen heterocyclic fractions and enantiomeric distribution of volatile components. J. Sep. Sci. 2010, 33, 3374–3385. [Google Scholar] [CrossRef]
- Rastrelli, L.; Passi, S.; Ippolito, F.; Vacca, G.; De Simone, F. Rate of degradation of α-tocopherol, squalene, phenolics, and polyunsaturated fatty acids in olive oil during different storage conditions. J. Agric. Food Chem. 2002, 50, 5566–5570. [Google Scholar] [CrossRef]
- Mousavi, S.; Mariotti, R.; Stanzione, V.; Pandolfi, S.; Mastio, V.; Baldoni, L.; Cultrera, N.G.M. Evolution of extra virgin olive oil quality under different storage conditions. Foods 2021, 10, 1945. [Google Scholar] [CrossRef]
- Nhouchi, Z.; Botosoa, E.P.; Karoui, R. Critical assessment of formulation, processing and storage conditions on the quality of alveolar baked products determined by different analytical techniques: A review. Trends Food Sci. Technol. 2018, 81, 159–171. [Google Scholar] [CrossRef]
| Cupcake | SO | OPO | Manufacture Date |
|---|---|---|---|
| SO-1 | SO-1 | - | 29 September 2021 |
| SO-2 | SO-2 | - | 21 September 2021 |
| SO-3 | SO-3 | - | 21 September 2021 |
| OPO25-1 | SO-1 | OPO-1 | 30 September 2021 |
| OPO25-2 | SO-2 | OPO-2 | 21 September 2021 |
| OPO25-3 | SO-3 | OPO-3 | 21 September 2021 |
| OPO50-1 | SO-1 | OPO-1 | 30 September 2021 |
| OPO50-2 | SO-2 | OPO-2 | 22 September 2021 |
| OPO50-3 | SO-3 | OPO-3 | 22 September 2021 |
| OPO-1 | - | OPO-1 | 29 September 2021 |
| OPO-2 | - | OPO-2 | 22 September 2021 |
| OPO-3 | - | OPO-3 | 22 September 2021 |
| SO-1 | SO-2 | SO-3 | OPO-1 | OPO-2 | OPO-3 | |
|---|---|---|---|---|---|---|
| Sterols (%) | ||||||
| Cholesterol | 0.43 | 0.40 | 0.38 | 0.47 | 0.70 | 0.64 |
| ±0.11 a | ±0.01 a | ±0.05 a | ±0.25 a | ±0.17 a | ±0.16 a | |
| Brassicasterol | 0.44 | 0.52 | 0.60 | nd | nd | 0.22 |
| ±0.08 a | ±0.09 a | ±0.55 a | ±0.38 | |||
| 24-Me-Cholesterol | 0.27 | 0.22 | 0.20 | nd | nd | nd |
| ±0.01 b | ±0.02 a | ±0.01 a | ||||
| Campesterol | 7.85 | 7.90 | 7.95 | 3.13 | 3.03 | 3.25 |
| ±0.10 a | ±0.09 a | ±0.40 a | ±0.09 a | ±0.33 a | ±0.30 a | |
| Stigmasterol | 7.03 | 7.18 | 6.37 | 1.49 | 1.71 | 1.80 |
| ±0.11 a | ±0.16 a | ±0.67 a | ±0.04 a | ±0.09 ab | ±0.20 b | |
| Δ7-Campesterol | 2.74 | 2.71 | 2.35 | nd | nd | nd |
| ±0.21 a | ±0.14 a | ±0.33 a | ||||
| Δ5,23-Stigmastadienol | 0.82 | 0.88 | 0.86 | 0.64 | 0.54 | 0.49 |
| ±0.01 a | ±0.15 a | ±0.61 a | ±0.03 a | ±0.47 a | ±0.20 a | |
| Clerosterol | 0.13 | 0.14 | 0.16 | nd | nd | nd |
| ±0.01 a | ±0.05 a | ±0.10 a | ||||
| β-Sitosterol | 57.79 | 57.55 | 53.67 | 85.20 | 81.70 | 84.39 |
| ±1.74 a | ±0.90 a | ±5.93 a | ±0.43 a | ±7.89 a | ±1.00 a | |
| Sitostanol | 1.40 | 1.56 | 1.76 | 4.92 | 4.44 | 5.74 |
| ±0.28 a | ±0.12 a | ±0.50 a | ±0.87 ab | ±0.47 a | ±0.23 b | |
| Δ5-Avenasterol | 1.33 | 1.28 | 1.13 | 1.97 | 1.93 | 1.28 |
| ±0.10 a | ±0.07 a | ±0.60 a | ±0.40 a | ±0.38 a | ±0.38 a | |
| Δ5,24-Stigmastadienol | 3.20 | 3.22 | 2.96 | 2.18 | 1.15 | 1.71 |
| ±0.29 a | ±0.14 a | ±0.40 a | ±0.95 a | ±0.29 a | ±0.49 a | |
| Δ7-Stigmastenol | 13.58 | 13.54 | 13.57 | nd | nd | nd |
| ±1.02 a | ±0.56 a | ±1.50 a | ||||
| Δ7-Avenasterol | 2.95 | 2.89 | 3.83 | nd | nd | nd |
| ±0.30 a | ±0.13 a | ±0.53 b | ||||
| Total sterols (mg/kg) | 2451 | 2144 | 2811 | 2525 | 2495 | 2683 |
| ±105 a | ±155 a | ±622 a | ±51 a | ±114 a | ±284 a | |
| Triterpenic alcohols (%) | - | - | - | 18.5 | 22.7 | 21.7 |
| ±0.7 a | ±1.3 b | ±1.1 b | ||||
| α-Tocopherol (mg/kg) | 748 | 732 | 694 | 334 | 394 | 412 |
| ±7 b | ±27 b | ±13 a | ±5 a | ±3 b | ±6 c | |
| Squalene (mg/kg) | 62 | 63 | 115 | 1451 | 1674 | 1439 |
| ±3 a | ±5 a | ±6 b | ±51 a | ±67 b | ±60 a | |
| Triterpenic acids (mg/kg) | - | - | - | 60 | 64 | 59 |
| ±7 a | ±15 a | ±9 a | ||||
| Fatty alcohols (mg/kg) | - | - | - | 1733 | 1774 | 1145 |
| ±67 b | ±61 b | ±111 a |
| Sample | Moisture Content (%) | Fat Content (%) | Colour Index | OSI * (h) |
|---|---|---|---|---|
| SO-1 | 13.5 ± 0.9 abcA | 26.1 ± 0.5 eA | 8.3 ± 5.0 aA | 13.0 ± 0.3 bA |
| SO-2 | 14.9 ± 0.6 cA | 24.3 ± 0.3 abA | 13.3 ± 4.6 deA | 11.6 ± 0.1 abA |
| SO-3 | 12.4 ± 0.1 aA | 24.3 ± 0.1 abA | 13.3 ± 3.9 deA | 10.0 ± 0.6 aA |
| OPO25-1 | 14.6 ± 0.3 cA | 25.5 ± 0.3 deA | 10.0 ± 4.2 abcA | 16.7 ± 0.1 cB |
| OPO25-2 | 14.0 ± 0.5 bcA | 24.9 ± 0.2 bcdA | 13.8 ± 3.6 deA | 16.1 ± 0.7 cB |
| OPO25-3 | 13.1 ± 0.2 abA | 24.3 ± 0.4 abA | 12.5 ± 3.5 cdeA | 13.2 ± 0.3 bB |
| OPO50-1 | 14.8 ± 0.4 cA | 25.1 ± 0.2 cdA | 9.2 ± 4.3 abA | 22.6 ± 1.3 eC |
| OPO50-2 | 13.8 ± 0.9 bcA | 24.1 ± 0.4 aA | 13.1 ± 4.5 deA | 20.9 ± 1.2 eC |
| OPO50-3 | 13.9 ± 0.8 bcA | 24.1 ± 0.5 aA | 14.4 ± 2.8 eA | 18.9 ± 0.4 dC |
| OPO-1 | 14.4 ± 1.0 bcA | 25.5 ± 0.2 deA | 11.6 ± 4.3 bcdA | 51.8 ± 1.5 hD |
| OPO-2 | 14.2 ± 1.1 bcA | 24.4 ± 0.6 abA | 11.3 ± 5.0 bcdA | 48.8 ± 3.0 gD |
| OPO-3 | 14.5 ± 0.9 cA | 24.8 ± 0.3 bcA | 11.8 ± 5.1 cdA | 46.3 ± 1.1 fD |
| Sample | TGD (%) | oxTGM (%) | DG (%) | MG (%) | FFA (%) | TPC (%) | |
|---|---|---|---|---|---|---|---|
| SO-1 | Batter | 0.60 ± 0.01 a | 1.28 ± 0.04 a | 1.28 ± 0.02 a | 0.22 ± 0.01 a | 0.51 ± 0.01 a | 3.89 ± 0.05 a |
| Cupcake | 0.57 ± 0.02 a | 1.28 ± 0.09 a | 1.30 ± 0.03 a | 0.19 ± 0.02 a | 0.66 ± 0.03 b | 4.00 ± 0.07 a | |
| SO-2 | Batter | 0.56 ± 0.01 a | 1.12 ± 0.02 a | 1.20 ± 0.02 a | 0.22 ± 0.01 a | 0.41 ± 0.04 a | 3.52 ± 0.04 a |
| Cupcake | 0.53 ± 0.04 a | 1.29 ± 0.06 b | 1.19 ± 0.03 a | 0.20 ± 0.02 a | 0.53 ± 0.01 b | 3.74 ± 0.12 b | |
| SO-3 | Batter | 0.91 ± 0.04 a | 1.19 ± 0.10 b | 1.03 ± 0.04 a | 0.17 ± 0.01 a | 0.45 ± 0.04 a | 3.76 ± 0.17 a |
| Cupcake | 0.89 ± 0.04 a | 0.99 ± 0.06 a | 1.07 ± 0.03 a | 0.17 ± 0.01 a | 0.62 ± 0.02 b | 3.73 ± 0.11 a | |
| OPO-1 | Batter | 1.05 ± 0.05 a | 1.41 ± 0.05 a | 5.99 ± 0.26 a | 0.66 ± 0.02 b | 0.84 ± 0.04 a | 9.94 ± 0.28 a |
| Cupcake | 1.05 ± 0.08 a | 1.59 ± 0.13 a | 5.72 ± 0.23 a | 0.57 ± 0.02 a | 1.02 ± 0.05 b | 9.94 ± 0.44 a | |
| OPO-2 | Batter | 0.91 ± 0.04 a | 1.41 ± 0.09 a | 6.77 ± 0.42 a | 0.71 ± 0.02 a | 0.86 ± 0.10 a | 10.66 ± 0.55 a |
| Cupcake | 0.86 ± 0.03 a | 1.32 ± 0.07 a | 6.45 ± 0.16 a | 0.69 ± 0.01 a | 1.13 ± 0.02 b | 10.44 ± 0.16 a | |
| OPO-3 | Batter | 1.01 ± 0.13 a | 1.18 ± 0.18 a | 6.80 ± 0.09 a | 0.68 ± 0.01 b | 0.81 ± 0.02 a | 10.48 ± 0.17 a |
| Cupcake | 0.94 ± 0.02 a | 1.24 ± 0.03 a | 6.73 ± 0.07 a | 0.62 ± 0.01 a | 1.12 ± 0.03 b | 10.65 ± 0.02 a | |
| Sample | α-Tocopherol (mg/kg Fat) | Squalene (mg/kg Fat) | ||
|---|---|---|---|---|
| Batter | Cupcake | Batter | Cupcake | |
| SO-1 | 792 ± 5 a | 762 ± 9 b | 65 ± 8 a | 76 ± 11 a |
| SO-2 | 732 ± 32 a | 665 ± 25 b | 63 ± 1 a | 63 ± 1 a |
| SO-3 | 653 ± 30 a | 646 ± 15 a | 102 ± 5 a | 107 ± 2 a |
| OPO25-1 | 727 ± 22 a | 678 ± 12 b | 391 ± 18 a | 359 ± 12 a |
| OPO25-2 | 636 ± 21 a | 665 ± 3 a | 386 ± 20 a | 367 ± 18 a |
| OPO25-3 | 612 ± 17 a | 633 ± 6 a | 367 ± 6 a | 344 ± 9 b |
| OPO50-1 | 607 ± 8 a | 572 ± 19 b | 678 ± 15 a | 621 ± 8 b |
| OPO50-2 | 567 ± 41 a | 608 ± 4 a | 665 ± 11 a | 610 ± 7 b |
| OPO50-3 | 569 ± 17 a | 578 ± 22 a | 587 ± 23 a | 555 ± 9 a |
| OPO-1 | 392 ± 4 a | 377 ± 15 a | 1404 ± 23 a | 1286 ± 52 b |
| OPO-2 | 417 ± 15 a | 449 ± 26 a | 1374 ± 42 a | 1304 ± 27 a |
| OPO-3 | 419 ± 4 b | 435 ± 6 a | 1237 ± 10 a | 1121 ± 89 a |
| Sample | Total Sterols (mg/kg Fat) | Total Phytosterols (mg/kg Fat) | ||
|---|---|---|---|---|
| Batter | Cupcake | Batter | Cupcake | |
| SO-1 | 2969 ± 124 a | 4207 ± 173 b | 2598 ± 110 a | 2346 ± 164 a |
| OPO25-1 | 3361 ± 159 a | 4872 ± 125 b | 2833 ± 211 a | 2628 ± 155 a |
| OPO50-1 | 3085 ± 203 a | 4029 ± 221 b | 2692 ± 212 a | 2370 ± 154 a |
| OPO-1 | 3202 ± 63 a | 4219 ± 106 b | 2750 ± 206 a | 2448 ± 121 a |
| Batter | Cupcake | |
|---|---|---|
| Triterpenic alcohols | 607 ± 19 a | 610 ± 21 a |
| Erythrodiol | 521 ± 16 a | 532 ± 17 a |
| Uvaol | 86 ± 3 a | 78 ± 6 a |
| Triterpenic acids | 59 ± 12 a | 66 ± 9 a |
| Oleanolic | 49 ± 8 a | 54 ± 6 a |
| Ursolic | 10 ± 4 a | 12 ± 3 a |
| Maslinic | nd | nd |
| Sample | Time (Months) | |||
|---|---|---|---|---|
| 0 | 2 | 4 | 6 | |
| SO-1 | 762 ± 9 a | 716 ± 28 b | 674 ± 6 c | 638 ± 6 d |
| OPO25-1 | 678 ± 12 a | 616 ± 4 b | 584 ± 5 c | 580 ± 5 c |
| OPO50-1 | 572 ± 19 a | 523 ± 5 b | 499 ± 3 c | 468 ± 3 d |
| OPO-1 | 377 ± 15 a | 341 ± 6 b | 339 ± 3 b | 328 ± 2 b |
| Attributes | Samples | ANOVA/ p-Value | |||
|---|---|---|---|---|---|
| SO-1 | OPO25-1 | OPO50-1 | OPO-1 | ||
| Global appraisal | 6.5 ± 1.5 a | 6.7 ± 1.3 a | 6.5 ± 1.3 a | 6.6 ± 1.4 a | 0.813 |
| Appearance | 6.9 ± 1.4 a | 7.0 ± 1.3 a | 6.9 ± 1.5 a | 6.7 ± 1.4 a | 0.817 |
| Fresh appearance | 6.6 ± 1.6 a | 6.7 ± 1.3 a | 6.7 ± 1.3 a | 6.7 ± 1.3 a | 0.995 |
| Colour | 6.7 ± 1.6 a | 6.9 ± 1.5 a | 7.0 ± 1.4 a | 7.0 ± 1.4 a | 0.656 |
| Aroma | 6.7 ± 1.7 a | 6.7 ± 1.5 a | 6.7 ± 1.7 a | 7.0 ± 1.4 a | 0.800 |
| Flavour | 6.3 ± 1.6 a | 6.9 ± 1.3 a | 6.8 ± 1.4 a | 6.8 ± 1.2 a | 0.180 |
| Taste intensity * | 2.7 ± 0.7 a | 2.8 ± 0.7 a | 2.7 ± 0.7 a | 2.9 ± 0.7 a | 0.214 |
| Sweet taste * | 2.9 ± 0.7 a | 3.0 ± 0.6 a | 3.0 ± 0.6 a | 3.1 ± 0.7 a | 0.550 |
| Texture | 5.8 ± 1.6 a | 6.0 ± 1.6 a | 5.8 ± 1.5 a | 6.0 ± 1.7 a | 0.699 |
| Sponginess | 5.5 ± 1.9 a | 5.8 ± 1.6 a | 5.4 ± 1.7 a | 5.8 ± 2.0 a | 0.564 |
| Hard–soft texture | 5.3 ± 1.6 a | 5.7 ± 1.5 ab | 5.5 ± 1.4 ab | 6.0 ± 1.6 b | 0.093 |
| Dry–juicy texture | 5.1 ± 1.6 a | 5.6 ± 1.7 a | 5.2 ± 1.5 a | 5.5 ± 1.8 a | 0.332 |
| Freshness in mouth | 5.7 ± 1.8 a | 5.9 ± 1.4 a | 5.9 ± 1.4 a | 6.0 ± 1.6 a | 0.802 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velasco, J.; García-González, A.; Zamora, R.; Hidalgo, F.J.; Ruiz-Méndez, M.-V. Quality and Nutritional Changes of Traditional Cupcakes in the Processing and Storage as a Result of Sunflower Oil Replacements with Refined Olive Pomace Oil. Foods 2023, 12, 2125. https://doi.org/10.3390/foods12112125
Velasco J, García-González A, Zamora R, Hidalgo FJ, Ruiz-Méndez M-V. Quality and Nutritional Changes of Traditional Cupcakes in the Processing and Storage as a Result of Sunflower Oil Replacements with Refined Olive Pomace Oil. Foods. 2023; 12(11):2125. https://doi.org/10.3390/foods12112125
Chicago/Turabian StyleVelasco, Joaquín, Aída García-González, Rosario Zamora, Francisco J. Hidalgo, and María-Victoria Ruiz-Méndez. 2023. "Quality and Nutritional Changes of Traditional Cupcakes in the Processing and Storage as a Result of Sunflower Oil Replacements with Refined Olive Pomace Oil" Foods 12, no. 11: 2125. https://doi.org/10.3390/foods12112125
APA StyleVelasco, J., García-González, A., Zamora, R., Hidalgo, F. J., & Ruiz-Méndez, M.-V. (2023). Quality and Nutritional Changes of Traditional Cupcakes in the Processing and Storage as a Result of Sunflower Oil Replacements with Refined Olive Pomace Oil. Foods, 12(11), 2125. https://doi.org/10.3390/foods12112125







