Structural Properties of Lotus Seed Starch Nanocrystals Prepared Using Ultrasonic-Assisted Acid Hydrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Starch Extraction
2.2.2. Preparation of Lotus Seed Starch Nanocrystals (LS-SNCs)
2.2.3. Preparation of Lotus Seed Starch Nanocrystals (U-LS-SNCs)
2.3. Scanning Electron Microscopy (SEM)
2.4. Particle Size Measurement
2.5. Molecular Weight (Mw) Distribution
2.6. X-ray Diffraction (XRD) Spectral Measurement
2.7. Fourier Transform Infrared (FT-IR) Spectral Measurement
2.8. Statistical Analysis
3. Results and Discussion
3.1. Morphological Structure of LS-SNPs and U-LS-SNCs
3.2. Particle Sizes of LS-SNPs and U-LS-SNCs
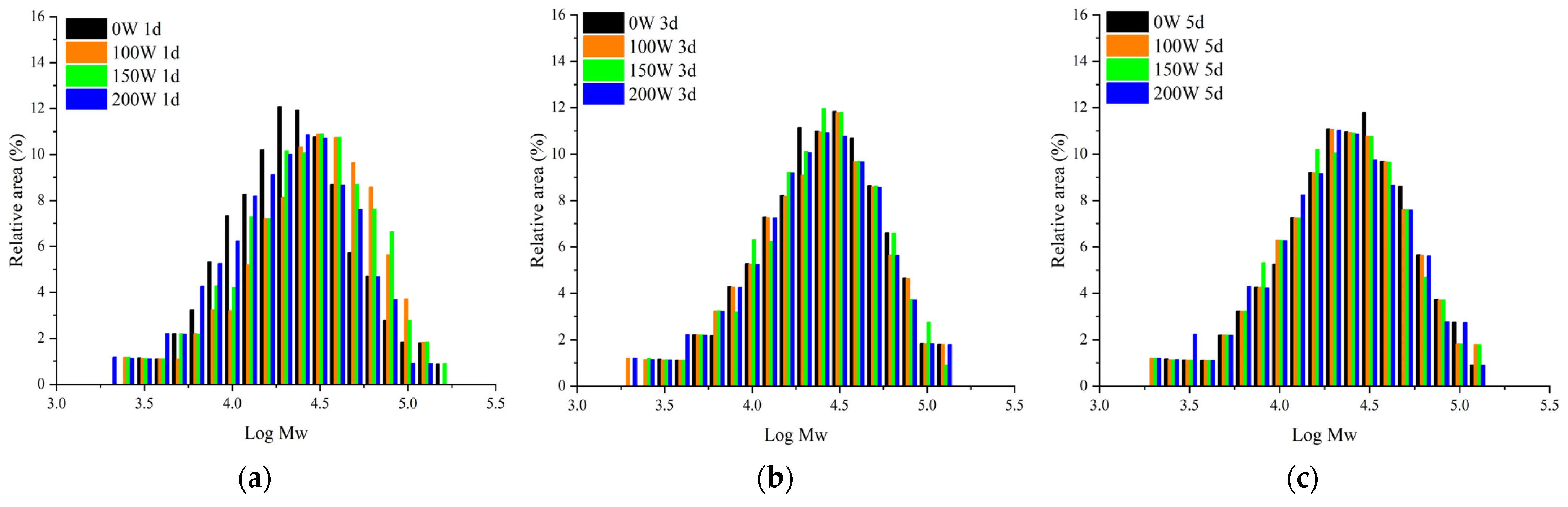
3.3. Mw Distribution
3.4. X-ray Diffraction
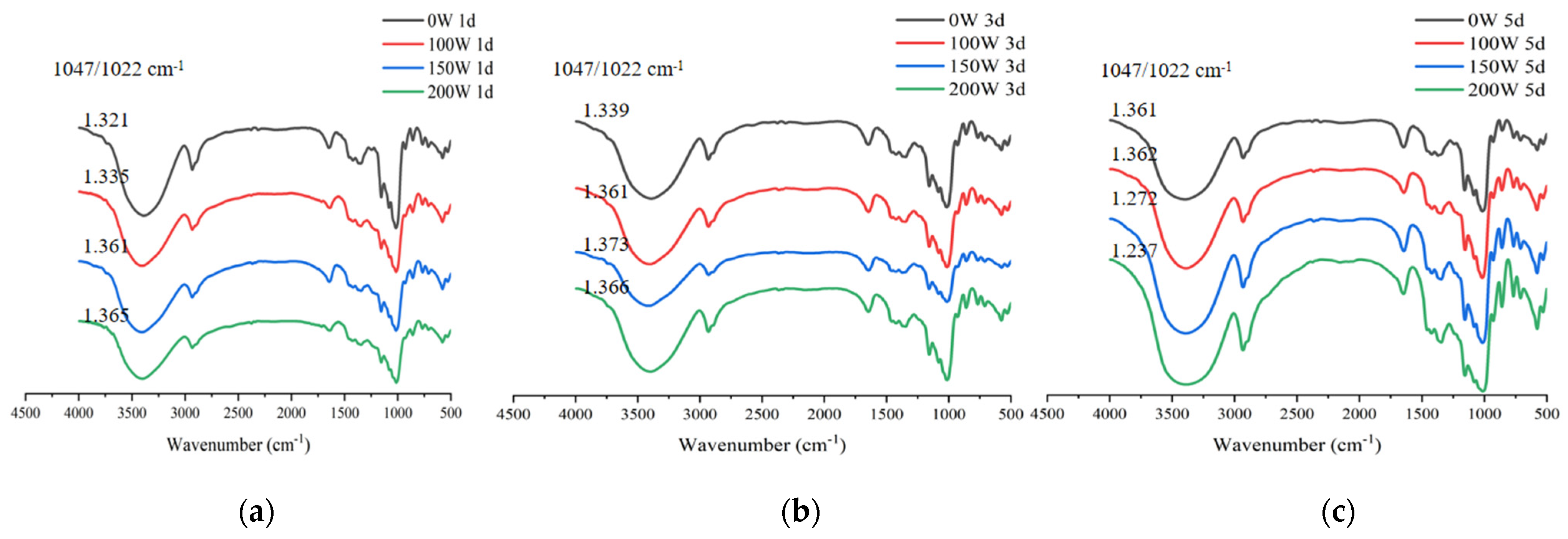
3.5. FT-IR Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukherjee, P.K.; Mukherjee, D.; Maji, A.K.; Rai, S.; Heinrich, M. The Sacred Lotus (Nelumbo nucifera)—Phytochemical and Therapeutic Profile. J. Pharm. Pharmacol. 2010, 61, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Punia Bangar, S.; Dunno, K.; Kumar, M.; Mostafa, H.; Maqsood, S. A Comprehensive Review on Lotus Seeds (Nelumbo nucifera Gaertn.): Nutritional Composition, Health-Related Bioactive Properties, and Industrial Applications. J. Funct. Foods 2022, 89, 104937. [Google Scholar] [CrossRef]
- Huang, G. Characteristics and Value of Traditional Lotus Culture System in Guangchang Jiangxi Province. Agric. Biotechnol. 2021, 10, 87–93. [Google Scholar] [CrossRef]
- Jia, X.; Sun, S.; Chen, B.; Zheng, B.; Guo, Z. Understanding the Crystal Structure of Lotus Seed Amylose–Long-Chain Fatty Acid Complexes Prepared by High Hydrostatic Pressure. Food Res. Int. 2018, 111, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, H.; Wang, Y.; Zeng, S.; Zheng, B. Structural Characteristics and Crystalline Properties of Lotus Seed Resistant Starch and Its Prebiotic Effects. Food Chem. 2014, 155, 311–318. [Google Scholar] [CrossRef]
- Matveev, Y.I.; van Soest, J.J.G.; Nieman, C.; Wasserman, L.A.; Protserov, V.A.; Ezernitskaja, M.; Yuryev, V.P. The Relationship between Thermodynamic and Structural Properties of Low and High Amylose Maize Starches. Carbohydr. Polym. 2001, 44, 151–160. [Google Scholar] [CrossRef]
- Zhu, F. Structures, Properties, and Applications of Lotus Starches. Food Hydrocoll. 2017, 63, 332–348. [Google Scholar] [CrossRef]
- Guo, Z.; Zeng, S.; Lu, X.; Zhou, M.; Zheng, M.; Zheng, B. Structural and Physicochemical Properties of Lotus Seed Starch Treated with Ultra-High Pressure. Food Chem. 2015, 186, 223–230. [Google Scholar] [CrossRef]
- Liu, C.; Li, K.; Li, X.; Zhang, M.; Li, J. Formation and Structural Evolution of Starch Nanocrystals from Waxy Maize Starch and Waxy Potato Starch. Int. J. Biol. Macromol. 2021, 180, 625–632. [Google Scholar] [CrossRef]
- Kumari, S.; Yadav, B.S.; Yadav, R.B. Synthesis and Modification Approaches for Starch Nanoparticles for Their Emerging Food Industrial Applications: A Review. Food Res. Int. 2020, 128, 108765. [Google Scholar] [CrossRef]
- Martins, P.C.; Latorres, J.M.; Martins, V.G. Impact of Starch Nanocrystals on the Physicochemical, Thermal and Structural Characteristics of Starch-Based Films. Lwt-Food Sci. Technol. 2022, 156, 113041. [Google Scholar] [CrossRef]
- Angellier, H.; Choisnard, L.; Molina-Boisseau, S.; Ozil, P.; Dufresne, A. Optimization of the Preparation of Aqueous Suspensions of Waxy Maize Starch Nanocrystals Using a Response Surface Methodology. Biomacromolecules 2004, 5, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Castillo, L.E.; Leite, M.A.; Ditchfield, C.; Sobral, P.J.D.A.; Moraes, I.C.F. Quinoa Starch Nanocrystals Production by Acid Hydrolysis: Kinetics and Properties. Int. J. Biol. Macromol. 2020, 143, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Amini, A.; Razavi, S.M.A. A Fast and Efficient Approach to Prepare Starch Nanocrystals from Normal Corn Starch. Food Hydrocoll. 2016, 57, 132–138. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Park, S.S.; Lim, S.-T. Preparation, Characterization and Utilization of Starch Nanoparticles. Colloids Surf. B-Biointerfaces 2015, 126, 607–620. [Google Scholar] [CrossRef]
- Hu, A.; Zhang, Z.; Zheng, J.; Shen, S.; Li, Q.; Yang, L. Effect of Ultrasonic Treatment on Acid Hydrolysis and Oxidation of Corn Starch. In Chemical Engineering and Material Properties, Pts 1 and 2; Zhang, H.M., Wu, B., Eds.; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2012; Volume 391–392, pp. 1105–1108. [Google Scholar] [CrossRef]
- Bonto, A.P.; Tiozon, R.N.; Sreenivasulu, N.; Camacho, D.H. Impact of Ultrasonic Treatment on Rice Starch and Grain Functional Properties: A Review. Ultrason. Sonochem. 2021, 71, 105383. [Google Scholar] [CrossRef]
- Wang, R.; Wang, F.; Kang, X.; Wang, J.; Li, M.; Liu, J.; Strappe, P.; Zhou, Z. Ultrasonication Enhanced the Multi-Scale Structural Characteristics of Rice Starch Following Short-Chain Fatty Acids Acylation. Int. J. Biol. Macromol. 2021, 190, 333–342. [Google Scholar] [CrossRef]
- Shabana, S.; Prasansha, R.; Kalinina, I.; Potoroko, I.; Bagale, U.; Shirish, S.H. Ultrasound Assisted Acid Hydrolyzed Structure Modification and Loading of Antioxidants on Potato Starch Nanoparticles. Ultrason. Sonochem. 2019, 51, 444–450. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, S.; Lin, H.; Chen, L.; Qin, S.; Wu, W.; Zheng, B.; Guo, Z. Physicochemical Properties and Digestion of the Lotus Seed Starch-Green Tea Polyphenol Complex under Ultrasound-Microwave Synergistic Interaction. Ultrason. Sonochem. 2019, 52, 50–61. [Google Scholar] [CrossRef]
- Lin, X.; Sun, S.; Wang, B.; Zheng, B. Structural and physicochemical properties of lotus seed starch nanoparticles prepared using ultrasonic-assisted enzymatic hydrolysis. Ultrason. Sonochem. 2020, 68, 105199. [Google Scholar] [CrossRef]
- Huang, M.; Zeng, M.; Liu, S.; Lin, J.; Qiu, Y.; Xie, J.; Guo, Z. Preparation and Structural Properties of Lotus Seed Starch Nanocrystals. J. Chin. Cereals Oils Assoc. 2022, 37, 163–170. [Google Scholar]
- Sun, S.; Lin, X.; Zhao, B.; Wang, B.; Guo, Z. Structural Properties of Lotus Seed Starch Prepared by Octenyl Succinic Anhydride Esterification Assisted by High Hydrostatic Pressure Treatment. LWT 2020, 117, 108698. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, Y.; Li, Q.; Gao, Q. Preparation of Starch Nanocrystals through Enzymatic Pretreatment from Waxy Potato Starch. Carbohydr. Polym. 2018, 184, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Sujka, M.; Jamroz, J. Ultrasound-Treated Starch: SEM and TEM Imaging, and Functional Behaviour. Food Hydrocoll. 2013, 31, 413–419. [Google Scholar] [CrossRef]
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the Crystallinity Degree of Starch Having Different Types of Crystal Structure after Mechanical Pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef]
- Jamalabadi, M.; Saremnezhad, S.; Bahrami, A.; Jafari, S.M. The Influence of Bath and Probe Sonication on the Physicochemical and Microstructural Properties of Wheat Starch. Food Sci. Nutr. 2019, 7, 2427–2435. [Google Scholar] [CrossRef]
- Li, W.; Zhang, F.; Zheng, J. Influence of Ultrasonic Treatment on the Physiochemical Properties and Feature Structure of Pea Starch in Acid and Salt Systems. Starch-Stärke 2019, 71, 1900064. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, Y.; Yu, K.; Ding, X.; Hou, H.; Wang, W.; Zhang, H.; Li, X.; Dong, H. Preparation of Octenyl Succinic Anhydride-Modified Cassava Starch by the Ultrasonic-Assisted Method and Its Influence Mechanism. J. Food Process. Preserv. 2019, 43, e14222. [Google Scholar] [CrossRef]
- Hu, A.; Li, L.; Zheng, J.; Lu, J.; Meng, X.; Liu, Y.; Rehman, R. Different-Frequency Ultrasonic Effects on Properties and Structure of Corn Starch. J. Sci. Food Agric. 2014, 94, 2929–2934. [Google Scholar] [CrossRef]
- Singh, R.; Sharanagat, V.S. Physico-Functional and Structural Characterization of Ultrasonic-Assisted Chemically Modified Elephant Foot Yam Starch. Int. J. Biol. Macromol. 2020, 164, 1061–1069. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Lee, J.H.; Kim, J.-Y.; Lim, W.-J.; Lim, S.-T. Characterization of Nanoparticles Prepared by Acid Hydrolysis of Various Starches. Starch-Starke 2012, 64, 367–373. [Google Scholar] [CrossRef]
- Pourmohammadi, K.; Abedi, E. The Effect of Pre and Post-Ultrasonication on the Aggregation Structure and Physicochemical Characteristics of Tapioca Starch Containing Sucrose, Isomalt and Maltodextrin. Int. J. Biol. Macromol. 2020, 163, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Li, Y.; Zheng, J. Dual-Frequency Ultrasonic Effect on the Structure and Properties of Starch with Different Size. Lwt-Food Sci. Technol. 2019, 106, 254–262. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Pickering Emulsions Stabilized by Monodisperse Latex Particles: Effects of Particle Size. Langmuir 2001, 17, 4540–4547. [Google Scholar] [CrossRef]
- Kristo, E.; Biliaderis, C.G. Physical Properties of Starch Nanocrystal-Reinforced Pullulan Films. Carbohydr. Polym. 2007, 68, 146–158. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Chang, P.R.; Feng, L.; Yu, J. Effect of Polysaccharide Nanocrystals on Structure, Properties, and Drug Release Kinetics of Alginate-Based Microspheres. Colloids Surf. B Biointerfaces 2011, 85, 270–279. [Google Scholar] [CrossRef] [PubMed]

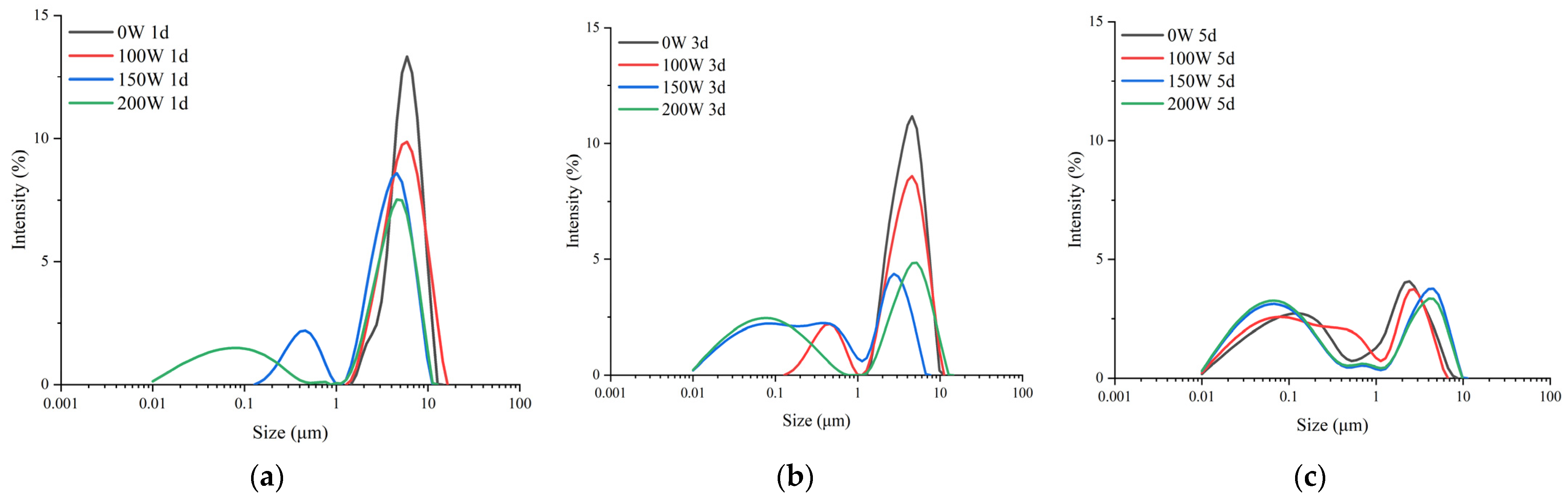
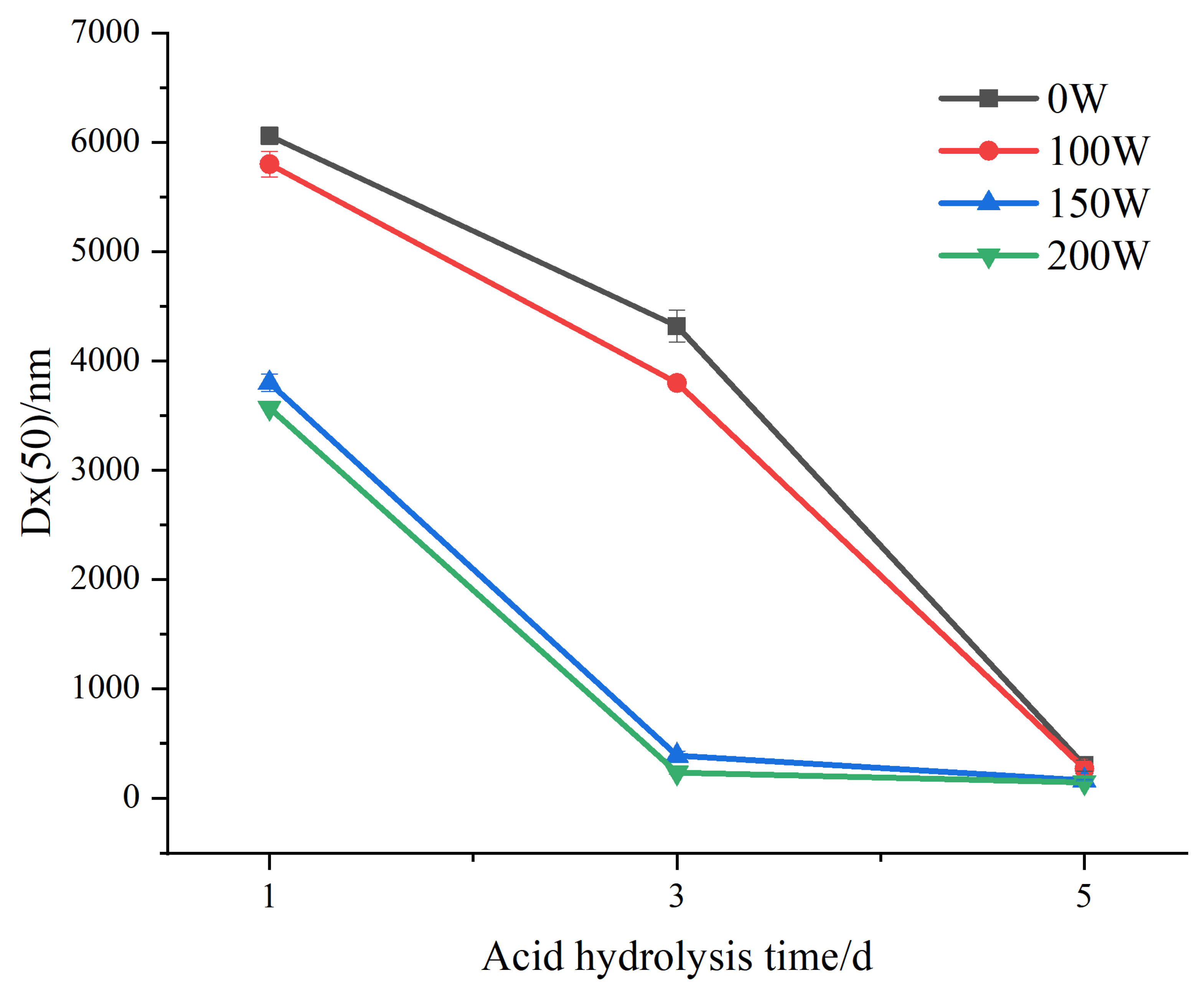

| Acid Hydrolysis Days/d | Ultrasonic Power/W | D [3,2]/nm | D [4,3]/nm | Dx (10)/nm | Dx (50)/nm | Dx (90)/nm |
|---|---|---|---|---|---|---|
| 1 | 0 | 5370 ± 60.3 a | 6280 ± 86.2 a | 3420 ± 55.1 a | 6060 ± 80.8 a | 9480 ± 128.6 b |
| 100 | 5040 ± 100.0 b | 6330 ± 125.0 a | 2920 ± 60.3 b | 5800 ± 115.0 b | 10,600 ± 200.0 a | |
| 150 | 1510 ± 63.5 c | 3940 ± 92.4 b | 470 ± 16.7 c | 3800 ± 80.8 c | 7310 ± 138.6 c | |
| 200 | 170 ± 7.6 d | 3560 ± 1.7 c | 49.7 ± 1.5 d | 3570 ± 0.6 d | 7480 ± 1.0 c | |
| 3 | 0 | 3870 ± 105.4 a | 4580 ± 141.9 a | 2370 ± 50.3 a | 4320 ± 145.7 a | 7250 ± 218.3 a |
| 100 | 1510 ± 63.5 b | 3940 ± 92.4 b | 470 ± 16.7 b | 3800 ± 0.8 b | 7310 ± 138.6 a | |
| 150 | 109 ± 4.7 c | 1310 ± 55.1 c | 35.6 ± 1.3 c | 392 ± 38.7 c | 3850 ± 79.4 b | |
| 200 | 88.7 ± 1.3 c | 1050 ± 69.3 d | 29.6 ± 0.3 c | 237 ± 13.0 d | 6030 ± 133.2 c | |
| 5 | 0 | 107 ± 4.9 a | 1280 ± 167 bc | 36.4 ± 1.2 a | 302 ± 49.9 a | 3700 ± 443.8 b |
| 100 | 94.3 ± 6.6 b | 1070 ± 115.8 c | 31.9 ± 1.6 b | 272 ± 53.5 a | 3390 ± 245.0 b | |
| 150 | 79 ± 1.3 c | 1660 ± 40.0 a | 27.5 ± 0.3 c | 166 ± 7.6 b | 5390 ± 37.9 a | |
| 200 | 75.3 ± 0.2 c | 1500 ± 173.2 ab | 26.7 ± 0.1 c | 147 ± 4.6 b | 5120 ± 10.0 a |
| Acid Hydrolysis Days/d | Ultrasonic Power/W | Mw (×104 Da) | Mn (×104 Da) | Mw/Mn |
|---|---|---|---|---|
| 1 | 0 | 4.3830 | 2.5387 | 1.7265 |
| 100 | 4.1468 | 1.9667 | 2.1085 | |
| 150 | 4.1307 | 1.9490 | 2.1194 | |
| 200 | 4.0334 | 1.8858 | 2.1388 | |
| 3 | 0 | 3.8200 | 2.0957 | 1.8228 |
| 100 | 3.7195 | 1.7706 | 2.1007 | |
| 150 | 3.6718 | 1.7470 | 2.1018 | |
| 200 | 3.5768 | 1.6661 | 2.1468 | |
| 5 | 0 | 3.5936 | 1.9048 | 1.8866 |
| 100 | 3.5684 | 1.7653 | 2.0214 | |
| 150 | 3.5002 | 1.7083 | 2.0489 | |
| 200 | 3.4179 | 1.5933 | 2.1452 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, R.; Huang, M.; Zeng, M.; Liu, S.; Chen, W.; Guo, Z. Structural Properties of Lotus Seed Starch Nanocrystals Prepared Using Ultrasonic-Assisted Acid Hydrolysis. Foods 2023, 12, 2050. https://doi.org/10.3390/foods12102050
Jia R, Huang M, Zeng M, Liu S, Chen W, Guo Z. Structural Properties of Lotus Seed Starch Nanocrystals Prepared Using Ultrasonic-Assisted Acid Hydrolysis. Foods. 2023; 12(10):2050. https://doi.org/10.3390/foods12102050
Chicago/Turabian StyleJia, Ru, Minli Huang, Muhua Zeng, Sidi Liu, Wenjing Chen, and Zebin Guo. 2023. "Structural Properties of Lotus Seed Starch Nanocrystals Prepared Using Ultrasonic-Assisted Acid Hydrolysis" Foods 12, no. 10: 2050. https://doi.org/10.3390/foods12102050
APA StyleJia, R., Huang, M., Zeng, M., Liu, S., Chen, W., & Guo, Z. (2023). Structural Properties of Lotus Seed Starch Nanocrystals Prepared Using Ultrasonic-Assisted Acid Hydrolysis. Foods, 12(10), 2050. https://doi.org/10.3390/foods12102050






