Heat-Killed Bifidobacterium longum BBMN68 in Pasteurized Yogurt Alleviates Mugwort Pollen-Induced Allergic Airway Responses through Gut Microbiota Modulation in a Murine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Pasteurized Yogurt Containing Heat-Killed BBMN68
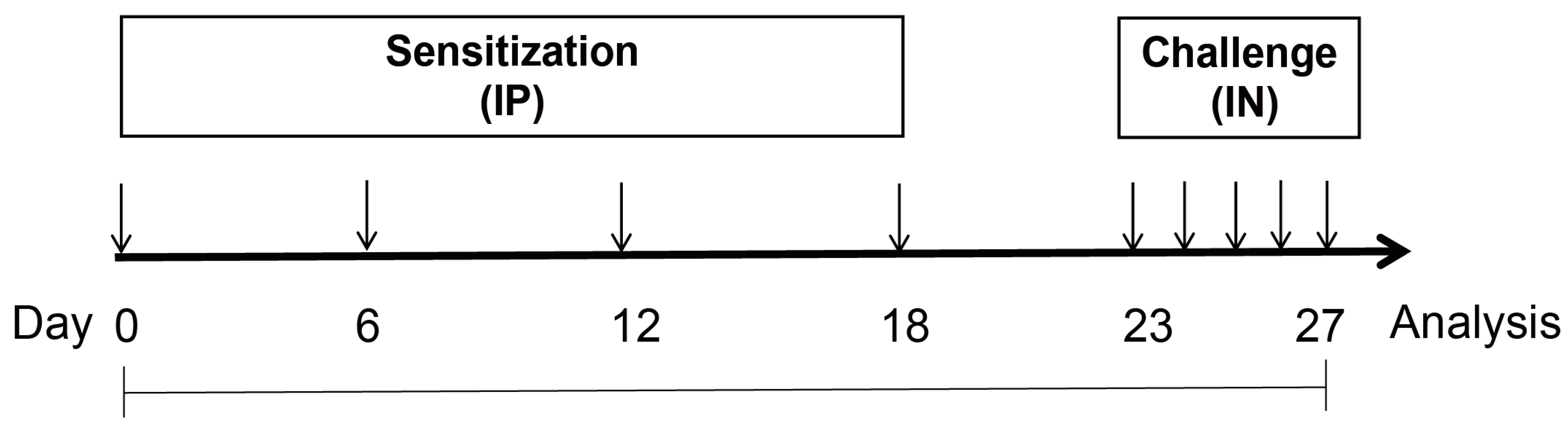
2.2. Animals and Experimental Design
2.3. Determination of Serum Immunoglobulins and Cytokines
2.4. The Differential Cell Counts in BALF
2.5. Lung Histology and Hematoxylin-Eosin Staining
2.6. Fecal Microbiota Analysis
2.7. Statistical Analysis
3. Results
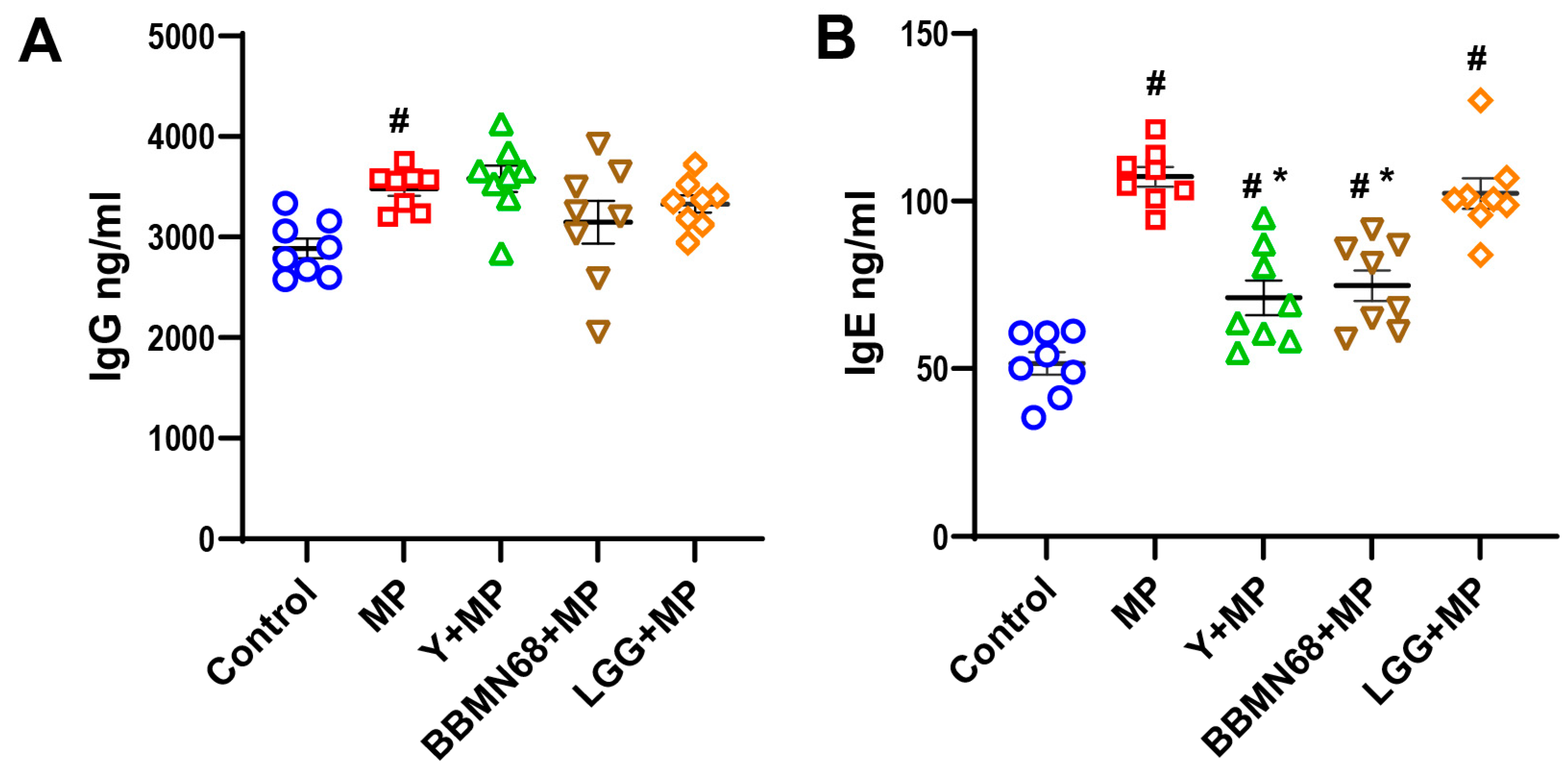
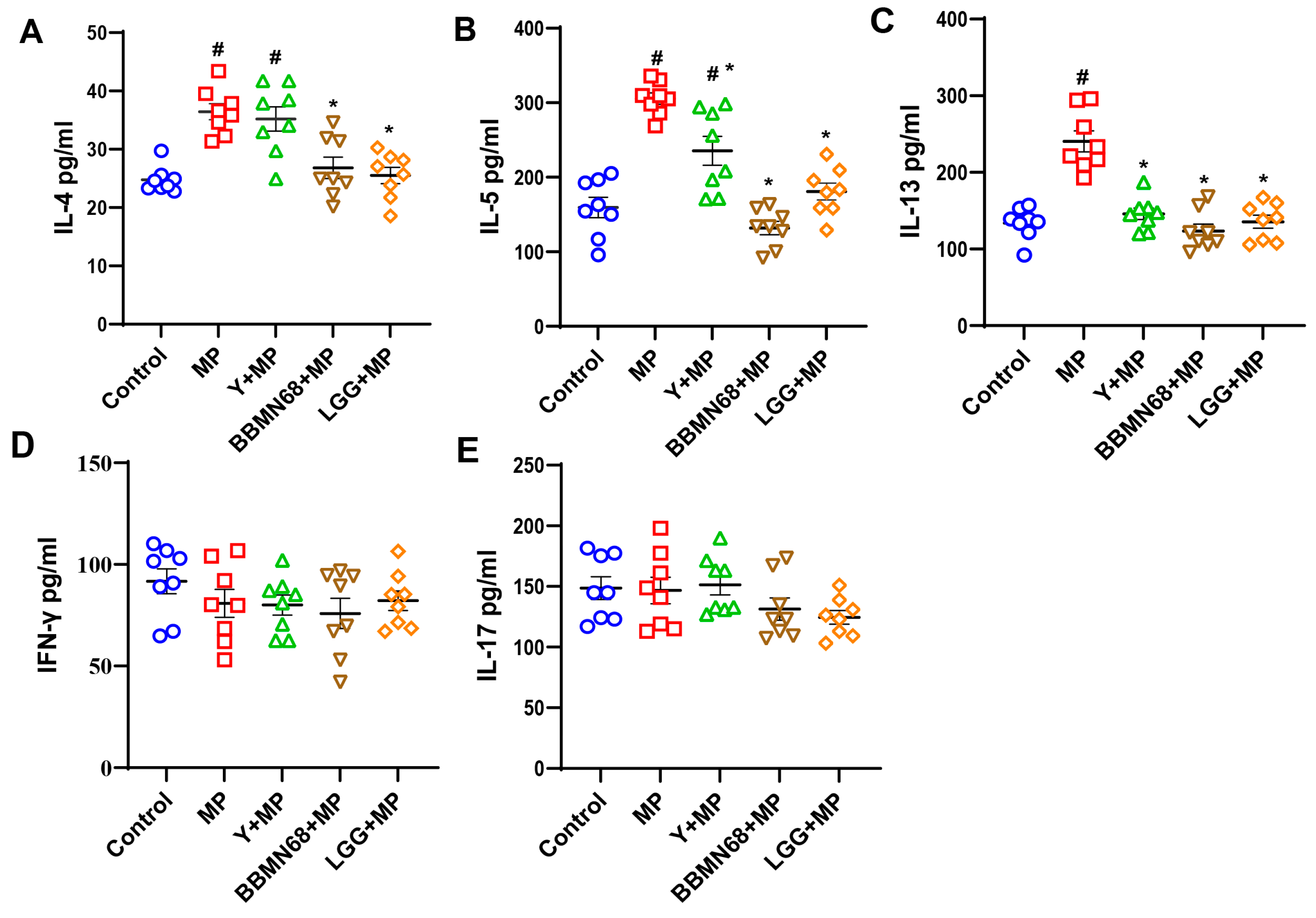
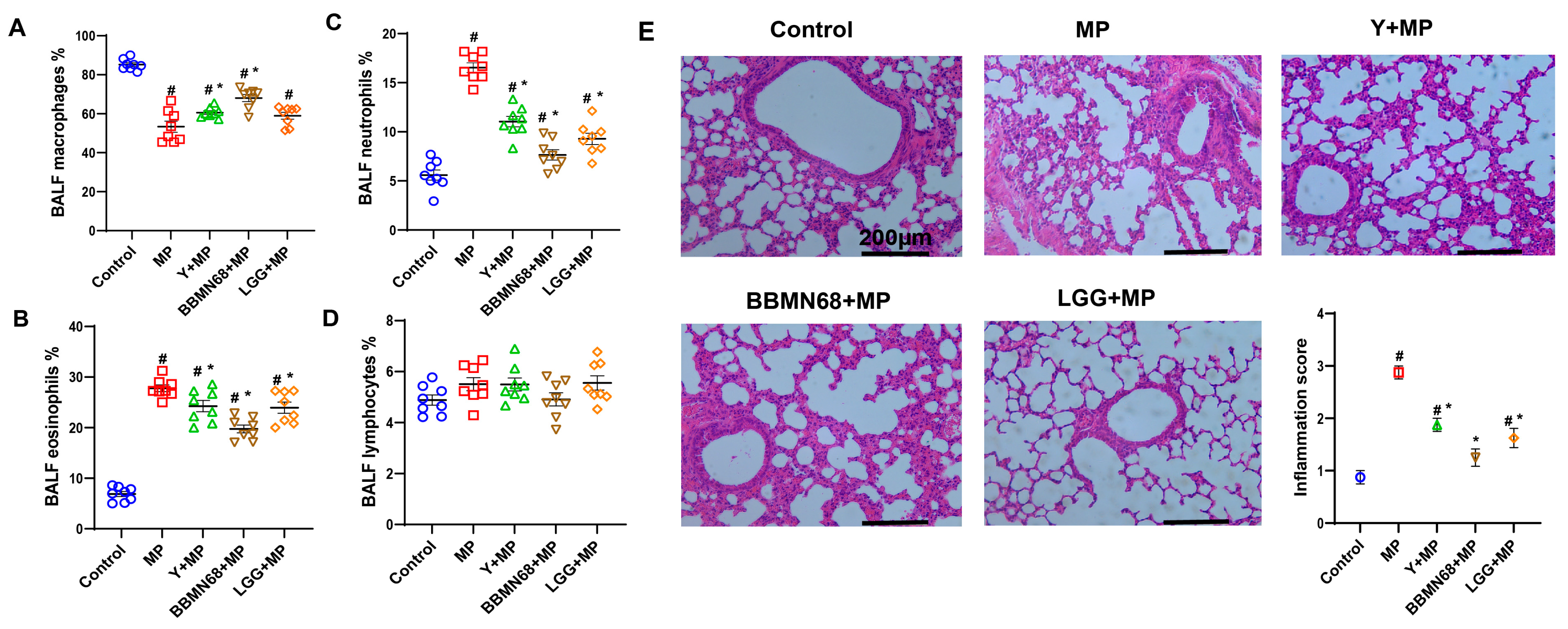
3.1. Protective Effects of Heat-Killed BBMN68 in Pasteurized Yogurt on Allergic Airway Responses
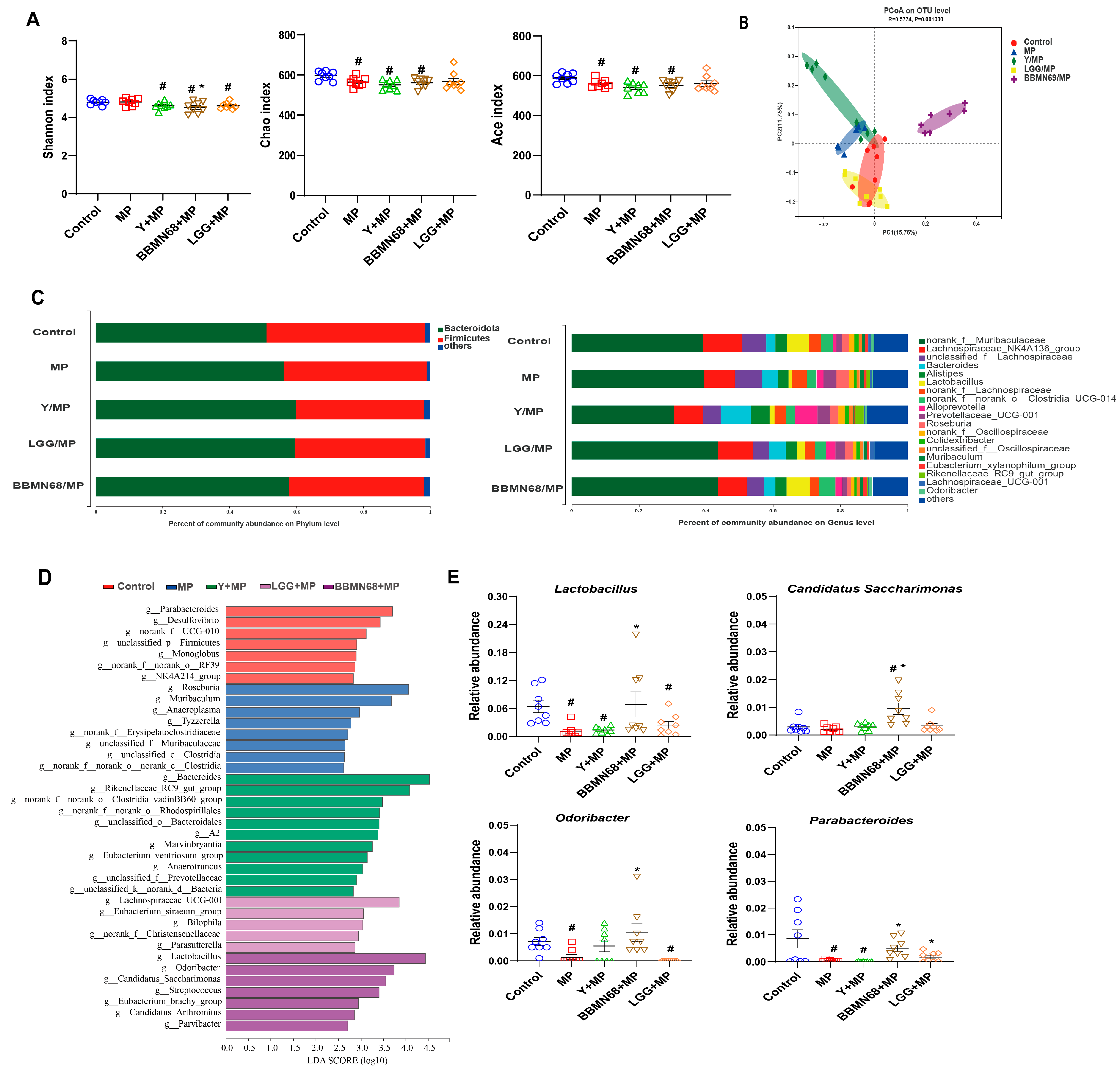
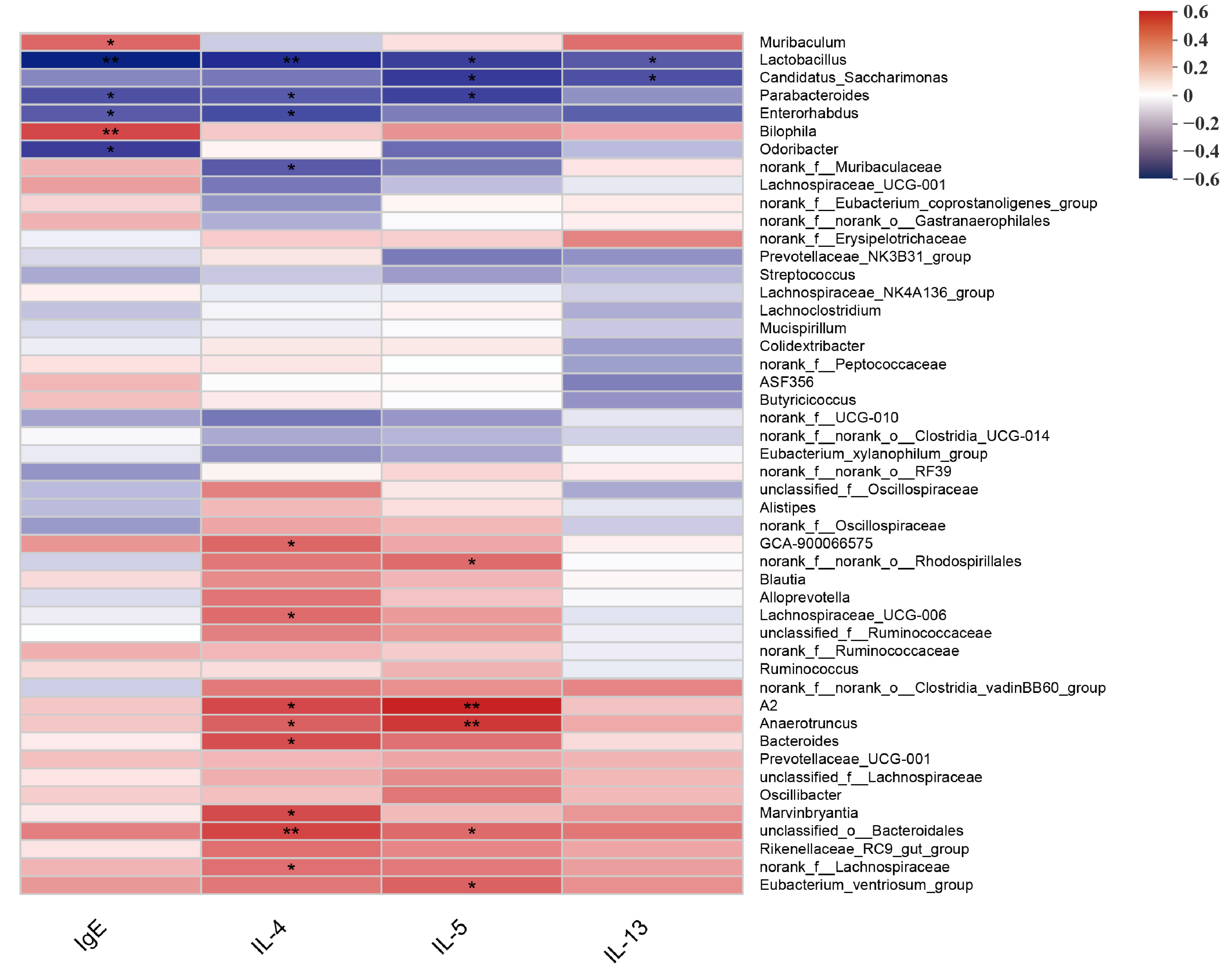
3.2. Intervention of Heat-Killed BBMN68 in Pasteurized Yogurt on Gut Microbiota Composition Systemically Correlates with Allergic Airway Response
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finkelman, F.D.; Hogan, S.P.; Hershey, G.K.K.; Rothenberg, M.E.; Wills-Karp, M. Importance of cytokines in murine allergic airway disease and human asthma. J. Immunol. 2010, 184, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.J.; Bao, M.J.; Zhu, J.P.; Yao, H.Y.; Xie, Y.C.; Guan, Y.; Li, F.F.; Dong, X.W.; Zheng, Y.M.; Xie, Q.M. Oral administration of allergen extracts from mugwort pollen desensitizes specific allergen-induced allergy in mice. Vaccine 2012, 30, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Meadows, A.; Kaambwa, B.; Novielli, N.; Huissoon, A.; Fry-Smith, A.; Meads, C.; Barton, P.; Dretzke, J. A systematic review and economic evaluation of subcutaneous and sublingual allergen immunotherapy in adults and children with seasonal allergic rhinitis. Health Technol. Assess 2013, 17, 1–322. [Google Scholar] [CrossRef]
- Spacova, I.; van Beeck, W.; Seys, S.; Devos, F.; Vanoirbeek, J.; Vanderleyden, J.; Ceuppens, J.; Petrova, M.; Lebeer, S. Lactobacillus rhamnosus probiotic prevents airway function deterioration and promotes gut microbiome resilience in a murine asthma model. Gut Microbes 2020, 11, 1729–1744. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The International Scientifc Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Antoine, J.M.; Herz, U.; Rijkers, G.T.; Wells, J.M.; Mercenier, A. Guidance for substantiating the evidence for benefcial efects of probiotics: Prevention and management of allergic diseases by probiotics. J. Nutr. 2010, 140, S713–S721. [Google Scholar] [CrossRef]
- Mennini, M.; Dahdah, L.; Artesani, M.C.; Fiocchi, A.; Martelli, A. Probiotics in asthma and allergy prevention. Front. Pediatr. 2017, 5, 165. [Google Scholar] [CrossRef]
- West, C.E.; Dzidic, M.; Prescott, S.L.; Jenmalm, M.C. Bugging allergy; role of pre-, pro- and synbiotics in allergy prevention. Allergol. Int. 2017, 66, 529–538. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Salminen, S.; Poussa, T.; Arvilommi, H.; Isolauri, E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 2003, 361, 1869–1871. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, D.; Guo, Y.; Sun, Y.; Zeng, X. Peptidoglycan diversity and anti-inflammatory capacity in Lactobacillus strains. Carbohydr. Polym. 2015, 128, 130–137. [Google Scholar] [CrossRef]
- Spacova, I.; Petrova, M.I.; Fremau, A.; Pollaris, L.; Vanoirbeek, J.; Ceuppens, J.L.; Seys, S.; Lebeer, S. Intranasal administration of probiotic Lactobacillus rhamnosus GG prevents birch pollen-induced allergic asthma in a murine model. Allergy 2019, 74, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, N.; Caicedo, R.; Neu, J. Alive and Dead Lactobacillus rhamnosus GG Decrease Tumor Necrosis Factor-α–Induced Interleukin-8 Production in Caco-2 Cells. J. Nutr. 2005, 135, 1752–1756. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Russell, W.M.; Douglas-Escobar, M.; Hauser, N.; Lopez, M.; Neu, J. Live and heat-killed Lactobacillus rhamnosus GG: Effects on proinflammatory and anti-inflammatory cytokines/chemokines in gastrostomy-fed infant rats. Pediatr. Res. 2009, 66, 203–207. [Google Scholar] [CrossRef]
- Li, L.; Sun, Q.; Xiao, H.; Zhang, Q.; Xu, S.; Lai, L.; Li, Z.; Li, C. Aerosol inhalation of heat-killed clostridium butyricum CGMCC0313-1 alleviates allergic airway inflammation in mice. J. Immunol. Res. 2022, 2022, 8447603. [Google Scholar] [CrossRef]
- Hong, H.J.; Kim, E.; Cho, D.; Kim, T.S. Differential suppression of heat-killed lactobacilli isolated from kimchi, a Korean traditional food, on airway hyper-responsiveness in mice. J. Clin. Immunol. 2010, 30, 449–458. [Google Scholar] [CrossRef]
- Hao, Y.; Huang, D.; Guo, H.; Xiao, M.; An, H.; Zhao, L.; Zuo, F.; Zhang, B.; Hu, S.; Song, S.; et al. Complete genome sequence of Bifidobacterium longum subsp. longum BBMN68, a new strain from a healthy chinese centenarian. J. Bacteriol. 2011, 193, 787–788. [Google Scholar] [CrossRef]
- Yang, H.Y.; Liu, S.L.; Ibrahim, S.A.; Zhao, L.; Jiang, J.L.; Sun, W.F.; Ren, F.Z. Oral administration of live Bifidobacterium substrains isolated from healthy centenarians enhanced immune function in BALB/c mice. Nutr. Res. 2009, 29, 281–289. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, L.; Guo, H.; Jiang, L.; Ren, F. Immunomodulatory effects of novel bifidobacterium and lactobacillus strains on murine macrophage cells. Afr. J. Microbiol. Res. 2011, 5, 8–15. [Google Scholar]
- Yang, J.; Zhang, H.; Jiang, L.; Guo, H.; Luo, X.; Ren, F. Bifidobacterium longum BBMN 68-specific modulated dendritic cells alleviate allergic responses to bovine β-lactoglobulin in mice. J. Appl. Microbiol. 2015, 119, 1127–1137. [Google Scholar] [CrossRef]
- Zhang, C.; Li, W.; Li, X.; Wan, D.; Mack, S.; Zhang, J.; Wagner, K.; Wang, C.; Tan, B.; Chen, J.; et al. Novel aerosol treatment of airway hyper-reactivity and inflammation in a murine model of asthma with a soluble epoxide hydrolase inhibitor. PLoS ONE 2022, 17, e0266608. [Google Scholar] [CrossRef] [PubMed]
- Kok, C.R.; Hutkins, R. Yogurt and other fermented foods as sources of health-promoting bacteria. Nutr. Rev. 2018, 76, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Daniel, N.; Nachbar, R.T.; Tran, T.T.T.; Ouellette, A.; Varin, T.V.; Cotillard, A.; Quinquis, L.; Gagné, A.; St-Pierre, P.; Trottier, J.; et al. Gut microbiota and fermentation-derived branched chain hydroxy acids mediate health benefits of yogurt consumption in obese mice. Nat. Commun. 2022, 13, 1343. [Google Scholar] [CrossRef]
- Meydani, S.N.; Ha, W.K. Immunologic effects of yogurt. Am. J. Clin. Nutr. 2000, 71, 861–872. [Google Scholar] [CrossRef]
- Ballesta, S.; Velasco, C.; Borobio, M.V.; Arguelles, F.; Perea, E.J. Fresh versus pasteurized yogurt: Comparative study of the effects on microbiological and immunological parameters, and gastrointestinal comfort. Enferm. Infecc. Microbiol. Clin. 2008, 26, 552–557. [Google Scholar] [CrossRef]
- Gasparri, C.; Perna, S.; Spadaccini, D.; Alalwan, T.; Girometta, C.; Infantino, V.; Rondanelli, M. Is vitamin D-fortified yogurt a value-added strategy for improving human health? A systematic review and meta-analysis of randomized trials. J. Dairy Sci. 2019, 102, 8587–8603. [Google Scholar] [CrossRef]
- Liu, Y.W.; Liao, T.W.; Chen, Y.H.; Chiang, Y.C.; Tsai, Y.C. Oral administration of heat-inactivated Lactobacillus plantarum K37 modulated airway hyperresponsiveness in ovalbumin-sensitized BALB/c mice. PLoS ONE 2014, 9, e100105. [Google Scholar] [CrossRef]
- Ou, C.C.; Lin, S.L.; Tsai, J.J.; Lin, M.Y. Heat-killed lactic acid bacteria enhance immunomodulatory potential by skewing the immune response toward Th1 polarization. J. Food Sci. 2011, 76, M260–M267. [Google Scholar] [CrossRef]
- Johnson-Henry, K.C.; Hagen, K.E.; Gordonpour, M.; Tompkins, T.A.; Sherman, P.M. Surface-layer protein extracts from Lactobacillus helveticus inhibit enterohaemorrhagic Escherichia coli O157:H7 adhesion to epithelial cells. Cell Microbiol. 2007, 9, 356–367. [Google Scholar] [CrossRef]
- Yan, F.; Cao, H.; Cover, T.L.; Whitehead, R.; Washington, M.K.; Polk, D.B. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 2007, 132, 562–575. [Google Scholar] [CrossRef]
- Wu, M.H.; Pan, T.M.; Wu, Y.J.; Chang, S.J.; Chang, M.S.; Hu, C.Y. Exopolysaccharide activities from probiotic bifidobacterium: Immunomodulatory effects (on J774A.1 macrophages) and antimicrobial properties. Int. J. Food Microbiol. 2010, 144, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Z.; Qiu, L.; Zhang, F.; Xu, X.; Wei, H.; Tao, X. Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04. J. Dairy Sci. 2017, 100, 6895–6905. [Google Scholar] [CrossRef] [PubMed]
- Martorell, P.; Alvarez, B.; Llopis, S.; Navarro, V.; Ortiz, P.; Gonzalez, N.; Balaguer, F.; Rojas, A.; Chenoll, E.; Ramón, D.; et al. Heat-treated Bifidobacterium longum CECT-7347: A whole-cell postbiotic with antioxidant, anti-Inflammatory, and gut-barrier protection properties. Antioxidants 2021, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Kiekens, S.; Vandenheuvel, D.; Broeckx, G.; Claes, I.; Allonsius, C.; De Boeck, I.; Thys, S.; Timmermans, J.P.; Kiekens, F.; Lebeer, S. Impact of spray-drying on the pili of Lactobacillus rhamnosus GG. Microb. Biotechnol. 2019, 12, 849–855. [Google Scholar] [CrossRef]
- George, L.; Brightling, C.E. Eosinophilic airway inflammation: Role in asthma and chronic obstructive pulmonary disease. Ther. Adv. Chronic Dis. 2016, 7, 34–51. [Google Scholar] [CrossRef]
- Pellaton, C.; Nutten, S.; Thierry, A.C.; Boudousquié, C.; Barbier, N.; Blanchard, C.; Corthésy, B.; Mercenier, A.; Spertini, F. Intragastric and intranasal administration of Lactobacillus paracasei NCC2461 modulates allergic airway inflammation in mice. Int. J. Inflam. 2012, 2012, 686739. [Google Scholar]
- Vatrella, A.; Maglio, A.; Pelaia, C.; Ciampo, L.; Pelaia, G.; Vitale, C. Eosinophilic inflammation: An appealing target for pharmacologic treatments in severe asthma. Biomedicines 2022, 10, 2181. [Google Scholar] [CrossRef]
- McLoughlin, R.; Berthon, B.S.; Rogers, G.B.; Baines, K.J.; Leong, L.E.X.; Gibson, P.G.; Williams, E.J.; Wood, L.G. Soluble fibre supplementation with and without a probiotic in adults with asthma: A 7-day randomised, double blind, three way cross-over trial. eBioMedicine 2019, 46, 473–485. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Li, Q.; Su, H.; Sun, X. Exploration of the effect of mixed probiotics on microbiota of allergic asthma mice. Cell Immunol. 2021, 367, 104399. [Google Scholar] [CrossRef]
- Barcik, W.; Boutin, R.C.T.; Sokolowska, M.; Finlay, B.B. The role of lung and gut microbiota in the pathology of asthma. Immunity 2020, 52, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Hufnagl, K.; Pali-Schöll, I.; Roth-Walter, F.; Jensen-Jarolim, E. Dysbiosis of the gut and lung microbiome has a role in asthma. Semin. Immunopathol. 2020, 42, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut–lung axis. Mucosal. Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Ganapathy, V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef]
- Desch, A.N.; Henson, P.M.; Jakubzick, C.V. Pulmonary dendritic cell development and antigen acquisition. Immunol. Res. 2013, 55, 178–186. [Google Scholar] [CrossRef]
- Kopf, M.; Schneider, C.; Nobs, S.P. The development and function of lung-resident macrophages and dendritic cells. Nat. Immunol. 2015, 16, 36–44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, X.; Yin, X.; Wu, X.; Zhang, Q.; Jiang, Y.; He, J.; Zhao, Y.; Zhang, C.; Ren, Y.; Lai, M.; et al. Heat-Killed Bifidobacterium longum BBMN68 in Pasteurized Yogurt Alleviates Mugwort Pollen-Induced Allergic Airway Responses through Gut Microbiota Modulation in a Murine Model. Foods 2023, 12, 2049. https://doi.org/10.3390/foods12102049
Niu X, Yin X, Wu X, Zhang Q, Jiang Y, He J, Zhao Y, Zhang C, Ren Y, Lai M, et al. Heat-Killed Bifidobacterium longum BBMN68 in Pasteurized Yogurt Alleviates Mugwort Pollen-Induced Allergic Airway Responses through Gut Microbiota Modulation in a Murine Model. Foods. 2023; 12(10):2049. https://doi.org/10.3390/foods12102049
Chicago/Turabian StyleNiu, Xiaokang, Xindi Yin, Xiuying Wu, Qi Zhang, Yunyun Jiang, Jingjing He, Yuyang Zhao, Chao Zhang, Yimei Ren, Mengxuan Lai, and et al. 2023. "Heat-Killed Bifidobacterium longum BBMN68 in Pasteurized Yogurt Alleviates Mugwort Pollen-Induced Allergic Airway Responses through Gut Microbiota Modulation in a Murine Model" Foods 12, no. 10: 2049. https://doi.org/10.3390/foods12102049
APA StyleNiu, X., Yin, X., Wu, X., Zhang, Q., Jiang, Y., He, J., Zhao, Y., Zhang, C., Ren, Y., Lai, M., Sang, Y., & Wang, R. (2023). Heat-Killed Bifidobacterium longum BBMN68 in Pasteurized Yogurt Alleviates Mugwort Pollen-Induced Allergic Airway Responses through Gut Microbiota Modulation in a Murine Model. Foods, 12(10), 2049. https://doi.org/10.3390/foods12102049






