Natural Deep Eutectic Solvents (NADESs) Combined with Sustainable Extraction Techniques: A Review of the Green Chemistry Approach in Food Analysis
Abstract
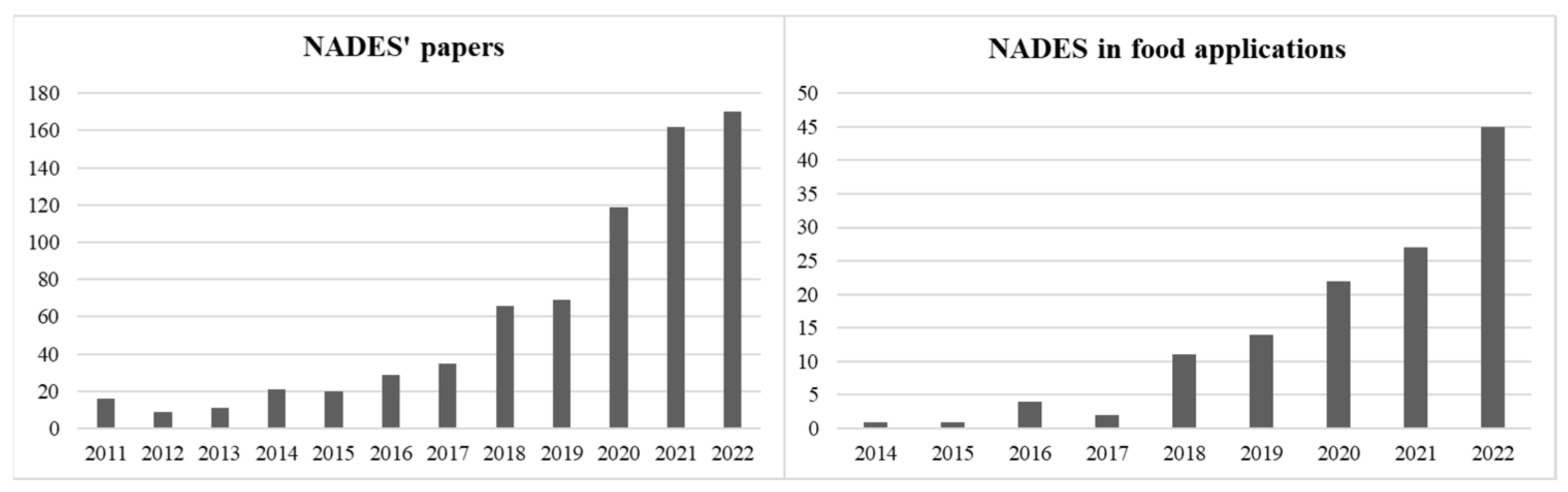
1. Introduction
2. Physicochemical Properties of NADES for the Extraction Process
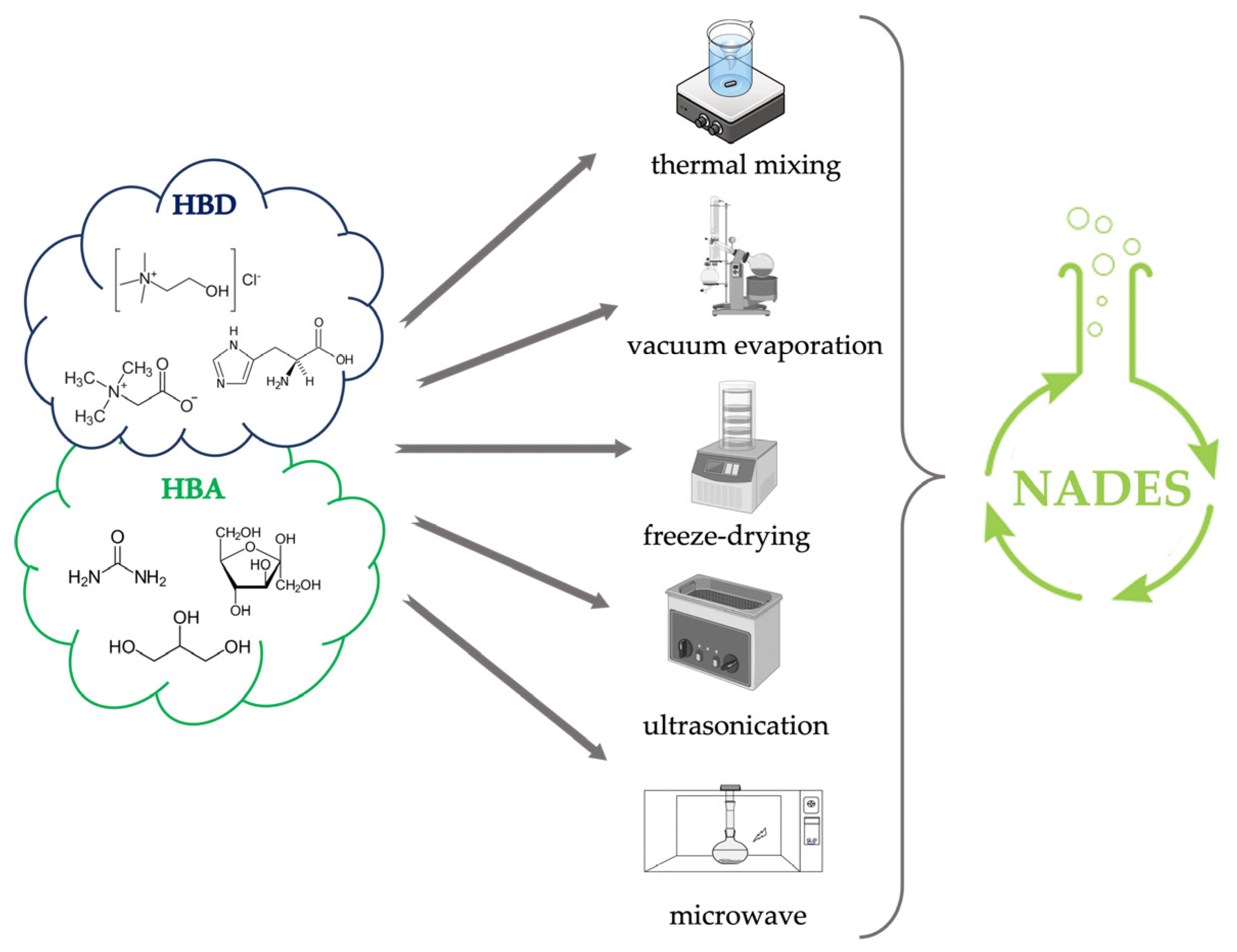
3. Preparation of NADES
- Microwave-assisted synthesis exploits the production of microwaves that upon interaction with precursors generate collisions between molecules and between the hydrogen bond donor and hydrogen bond acceptor components due to dipole rotation resulting in dielectric heating that speeds up the synthesis time [36,37]. According to Popovic et al., 2022, microwaves could be one of the fastest methods for the preparation of some NADES, taking even less than a minute [33]. However, because of the possible overheating caused by the technique, it is advisable to divide the process into several cycles of a few seconds interspersed with cooling pauses [38]. The entire preparation is carried out in closed systems with controlled pressure and temperature.
- Ultrasound-assisted is a little-explored but effective way of preparing NADESs. The cavitation process promotes, through the release of heat and pressure exerted because of bubbles implosion, the interaction between the hydrogen bond acceptor (HBA) and the hydrogen bond donor (HBD) [34]. According to when described by Santana et al., 2019, the preparation of NADES by ultrasound can also be performed by heating the mixture around 50 °C [34]. This approach requires several minutes with intermediate times between microwaves and the remaining techniques described above.
4. Use of NADES as Green Solvent in the Extraction Techniques
5. Toxicity and Sustainability
6. Recent Application in Food Analysis
6.1. Food Analytics
6.2. Extraction of Bioactive Compounds from Natural Sources
| Target Compound | Natural Matrix | NADE System | Molar Ratio * | Water/ NADES * | NADES Preparations | Extraction Technique | Reference |
|---|---|---|---|---|---|---|---|
| Anthocyanins | Grape skin | Citric acid/ D-(+)-maltose | 4:1 | 3:7 | Freeze-drying method | USAE | [57] |
| Anthocyanins | Mulberry | ChCl /citric acid/glucose | 1:1:1 | - | Heating and stirring | HSH and CBE | [66] |
| Bioactive compounds | Cytinus hypocistis | Proline/xylitol | 5:1 | - | Heating | USAE | [67] |
| Bioactive compounds | Annona muricata | ChCl/ xylitol | 1:1 | - | USAE | [69] | |
| Essential oils | Angelica sinensis radix | ChCl/citric acid | 1:1 | - | Heating and stirring | Hydrodistillation microwaves | [41] |
| Pectins | Mango peel | Betaine/citric acid choline chloride/malic acid | 1:1 1:1 | - - | Heating and stirring | USAE | [70] |
| Phenolic acids | Orange peel | ChCl/D-(+)-glucose/H2O | 5:2:5 | Mixing | USAE | [64] | |
| Phenolic compounds | Bitter melon | ChCl/acetic acid | 1:4.35 | 1:5 | Heating | USAE | [65] |
| Phenolic compounds | Moringa oleifera | Proline/glycine | 1:1 | 1:3 | Heating and stirring | USAE | [76] |
| Phenolic compounds | Olea europaea | H2O/ChCl/fructose | 5:5:2 | Mixing | USAE | [77] | |
| Polyphenols | Sambucus nigra | Glycine/lactic acid | 1:1 | 15% | Heating | USAE | [78] |
| Flavonoids | Radix scutellariae | Proline/glycine | 1:1 | 1:3 | Stirring | USAE | [79] |
| Tannins | Osmansthus fragrans | Lactic acid/glucose | 1:1 | 4:1 | Heating and stirring | MAE | [80] |
| Caffeine | Chinese dark tea | ChCl/lactic acid | 1:1 | 1:3 | Heating | SLE | [81] |
| (+)-catechin | Grape skin | ChCl/Oxalic acid | 1:1 | 1:4 | Heating and stirring | USAE | [82] |
| Curcumin | Standard solubility tests | ChCl/glycine | 1:1 | - | Heating and stirring | USAE | [72] |
| Isoflavones | Soy beans | ChC/citric acid | 1:1 | 30% | Stirring and heating | USAE | [39] |
| Lycopene | Tomato fruit | Thymol/lauric acid | 2:1 | - | Heating and stirring | Stirring | [73] |
| Solenesol | Tobacco leaves | ChCl/urea | 1:2 | 5% | Heating and stirring | USAE | [74] |
| Tryptanthrin, indirubin, and indigo | Baphicacanthus cusia | Lactic acid/L-menthol | 1:1 | - | USAE | USAE | [83] |
| Ursolic acid | Cynomorium songaricum Rupr. | ChCl/ D-(+)-glucose | 1:1 | - | Heating | USAE-ATPS | [84] |
| Rosmarinic acid, carnosol, carnosic acid | Rosmarinus officinalis | Lactic acid-glucose/menthol-lauric acid (biphasic system) | 5:1/2:1 | - | Heating and stirring | USAE | [68] |
6.3. Extraction of Bioactive Compounds from Agricultural Food by-Products
| Target Compound | Natural Matrix | NADE System | Molar Ratio * | Water/DES * | Extraction Technique | Reference |
|---|---|---|---|---|---|---|
| Phenolic compounds | Olive pomace | ChCl /citric acid | 1:2 | 20% | Homogenization | [87] |
| Phenolic compounds | Olive pomace | Lactic acid/glucose/water | 5:1:9.3 | USAE | [88] | |
| Phenolic compounds | Hazelnut skin | ChCl /lactic acid | 1:2 | 35% | USAE | [91] |
| Phenolic compounds | Cocoa beans | Betaine/glucose | 5:2 | USAE | [92] | |
| Phenolic compounds | Waste mango peel | lactic acid/glucose | 5:1 | 20% | USAE | [90] |
| Anthocyanins | Grape pomace | ChCl /citric acid ChCl /proline/ malic acid | 2:1 1:1:1 | 30% 25% | USAE/MAE | [63] |
| Anthocyanins | Sour cherry pomace | ChCl/malic acid | 1:1 | 20% | MAE | [33] |
| Anthocyanins | Blueberry peel | ChCl/malic acid ChCl/citric acid | 1.5:1 2:1 | 22% | MAE | [93] |
| Hydroxytyrosol | Olive leaves | Citric acid/glycine/water | 2:1:1 | USAE | [89] |
6.4. Food Safety
| Target Compound | Matrix | NADE System | Molar Ratio * | Extraction Technique | Ref. |
|---|---|---|---|---|---|
| 2-chlorophenol, O-cresol | Wastewater | Menthol/octanoic acid Menthol/decanoic acid | 1:1 | Liquid–liquid extraction | [94] |
| Alkylphenols, bisphenols, and alkylphenol ethoxylates | Functional beverage Bottled water | Menthol/octanoic acid Menthol/decanoic acid Thymol/octanoic acid Thymol/decanoic acid Menthol/octanoic acid Menthol/octanoic acid | 1:1 1:1 1:1 1:1 1:2 2:1 | Vortex-assisted dispersive liquid–liquid microextraction | [95] |
| Pesticides | Green and black teas | Chloride/polyethylene glycol ChCl/urea | 1:4 - | NADES functionalized 3D-graphene aerogel (3DG-Fe3O4) aerogel | [96] |
| Pesticides | Citrus and olive byproducts | Betaine/1,2-propylene glycol | 1:4 | USAE | [97] |
| Pesticides | Water | Thymol/myristyl alcohol Alanine/kojic acid/water | 2:1 1:2:5 | Dispersive liquid–liquid microextraction | [98] |
| Cadmium | Water and food samples | Salicylic acid/l-menthol | 1:4 | Ultrasound-vortex-assisted dispersive liquid–liquid microextraction | [101] |
| Zearalenone | Cereals | Menthol/1-hexanol | 2:1 | Dispersive liquid–liquid microextraction | [103] |
| 5-hydroxymethylfurfural | Glucose, starch, and food wastes | Chcl/glucose/water | 2:1:1 | Heating and stirring | [104] |
7. Conclusions and Future Prospective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ignat, I.; Volf, I.; Popa, V.I. A Critical Review of Methods for Characterisation of Polyphenolic Compounds in Fruits and Vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Rashid, R.; Mohd Wani, S.; Manzoor, S.; Masoodi, F.A.; Masarat Dar, M. Green Extraction of Bioactive Compounds from Apple Pomace by Ultrasound Assisted Natural Deep Eutectic Solvent Extraction: Optimisation, Comparison and Bioactivity. Food Chem. 2023, 398, 133871. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Issaoui, N.; Medimagh, M.; Fetisova, O.Y.; Berezhnaya, Y.D.; Elsuf’ev, E.V.; Al-Dossary, O.M.; Wojcik, M.J.; Xiang, Z.; Bousiakou, L.G. Experimental and Theoretical Study of the Sulfamic Acid-Urea Deep Eutectic Solvent. J. Mol. Liq. 2022, 363, 119859. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New Horizons in the Extraction of Bioactive Compounds Using Deep Eutectic Solvents: A Review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Understanding the Basics and Properties of Deep Eutectic Solvents. In Deep Eutectic Solvents for Medicine, Gas Solubilization and Extraction of Natural Substances; Springer: Berlin/Heidelberg, Germany, 2021; Volume 56, pp. 1–40. [Google Scholar] [CrossRef]
- Dai, Y.; Varypataki, E.M.; Golovina, E.A.; Jiskoot, W.; Witkamp, G.J.; Choi, Y.H.; Verpoorte, R. Natural Deep Eutectic Solvents in Plants and Plant Cells: In Vitro Evidence for Their Possible Functions. Adv. Bot. Res. 2021, 97, 159–184. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Durand, E.; Villeneuve, P.; Bourlieu-lacanal, C.; Carrière, F. Natural Deep Eutectic Solvents: Hypothesis for Their Possible Roles in Cellular Functions and Interaction with Membranes and Other Organized Biological Systems. Adv. Bot. Res. 2021, 97, 133–158. [Google Scholar] [CrossRef]
- Aroso, I.M.; Paiva, A.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents from Choline Chloride and Betaine—Physicochemical Properties. J. Mol. Liq. 2017, 241, 654–661. [Google Scholar] [CrossRef]
- Tang, B.; Bi, W.; Zhang, H.; Row, K.H. Deep Eutectic Solvent-Based HS-SME Coupled with GC for the Analysis of Bioactive Terpenoids in Chamaecyparis Obtusa Leaves. Chromatographia 2014, 77, 373–377. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of Deep Eutectic Solvents in Biotechnology and Bioengineering—Promises and Challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Namieśnik, J. Ionic Liquids and Deep Eutectic Mixtures: Sustainable Solvents for Extraction Processes. ChemSusChem 2014, 7, 1784–1800. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chen, L.; Li, M.; Cao, F.; Zhao, L.; Su, E. Two-Phase Systems Developed with Hydrophilic and Hydrophobic Deep Eutectic Solvents for Simultaneously Extracting Various Bioactive Compounds with Different Polarities. Green Chem. 2018, 20, 1879–1886. [Google Scholar] [CrossRef]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced Extraction of Bioactive Natural Products Using Tailor-Made Deep Eutectic Solvents: Application to Flavonoid Extraction from Flos Sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Rente, D.; Paiva, A.; Duarte, A.R. The Role of Hydrogen Bond Donor on the Extraction of Phenolic Compounds from Natural Matrices Using Deep Eutectic Systems. Molecules 2021, 26, 2336. [Google Scholar] [CrossRef]
- Zhekenov, T.; Toksanbayev, N.; Kazakbayeva, Z.; Shah, D.; Mjalli, F.S. Formation of Type III Deep Eutectic Solvents and Effect of Water on Their Intermolecular Interactions. Fluid Phase Equilib. 2017, 441, 43–48. [Google Scholar] [CrossRef]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and Thermal Behavior of Natural Deep Eutectic Solvents. J. Mol. Liq. 2016, 215, 534–540. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.G. Green and Efficient Extraction of Rutin from Tartary Buckwheat Hull by Using Natural Deep Eutectic Solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Tuntarawongsa, S.; Phaechamud, T. Menthol, Borneol, Camphor and WS-3 Eutectic Mixture. Adv. Mater. Res. 2012, 506, 355–358. [Google Scholar] [CrossRef]
- Van Osch, D.J.G.P.; Zubeir, L.F.; Van Den Bruinhorst, A.; Rocha, M.A.A.; Kroon, M.C. Hydrophobic Deep Eutectic Solvents as Water-Immiscible Extractants. Green Chem. 2015, 17, 4518–4521. [Google Scholar] [CrossRef]
- Van Osch, D.J.G.P.; Dietz, C.H.J.T.; Van Spronsen, J.; Kroon, M.C.; Gallucci, F.; Van Sint Annaland, M.; Tuinier, R. A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Xu, K.; Huang, Y.; Wen, Q.; Ding, X. Development of Green Betaine-Based Deep Eutectic Solvent Aqueous Two-Phase System for the Extraction of Protein. Talanta 2016, 152, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, Y.; Huang, Y.; Li, N.; Wen, Q. A Green Deep Eutectic Solvent-Based Aqueous Two-Phase System for Protein Extracting. Anal. Chim. Acta 2015, 864, 9–20. [Google Scholar] [CrossRef]
- Pang, J.; Sha, X.; Chao, Y.; Chen, G.; Han, C.; Zhu, W.; Li, H.; Zhang, Q. Green Aqueous Biphasic Systems Containing Deep Eutectic Solvents and Sodium Salts for the Extraction of Protein. RSC Adv. 2017, 7, 49361–49367. [Google Scholar] [CrossRef]
- Deng, W.W.; Zong, Y.; Xiao, Y.X. Hexafluoroisopropanol-Based Deep Eutectic Solvent/Salt Aqueous Two-Phase Systems for Extraction of Anthraquinones from Rhei Radix et Rhizoma Samples. ACS Sustain. Chem. Eng. 2017, 5, 4267–4275. [Google Scholar] [CrossRef]
- Yang, Z. Natural Deep Eutectic Solvents and Their Applications in Biotechnology. Adv. Biochem. Eng. Biotechnol. 2019, 168, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Popovic, B.M.; Micic, N.; Potkonjak, A.; Blagojevic, B.; Pavlovic, K.; Milanov, D.; Juric, T. Novel Extraction of Polyphenols from Sour Cherry Pomace Using Natural Deep Eutectic Solvents—Ultrafast Microwave-Assisted NADES Preparation and Extraction. Food Chem. 2022, 366, 130562. [Google Scholar] [CrossRef] [PubMed]
- Santana, A.P.R.; Mora-Vargas, J.A.; Guimarães, T.G.S.; Amaral, C.D.B.; Oliveira, A.; Gonzalez, M.H. Sustainable Synthesis of Natural Deep Eutectic Solvents (NADES) by Different Methods. J. Mol. Liq. 2019, 293, 111452. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Ferrer, M.L.; Mateo, C.R.; Monte, F. Del Freeze-Drying of Aqueous Solutions of Deep Eutectic Solvents: A Suitable Approach to Deep Eutectic Suspensions of Self-Assembled Structures. Langmuir 2009, 25, 5509–5515. [Google Scholar] [CrossRef]
- Gomez, F.J.V.; Espino, M.; Fernández, M.A.; Silva, M.F. A Greener Approach to Prepare Natural Deep Eutectic Solvents. ChemistrySelect 2018, 3, 6122–6125. [Google Scholar] [CrossRef]
- Zhu, X.H.; Hang, Q.M. Microscopical and Physical Characterization of Microwave and Microwave-Hydrothermal Synthesis Products. Micron 2013, 44, 21–44. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Development of a Method Based on Natural Deep Eutectic Solvents for Extraction of Flavonoids from Food Samples. Food Anal. Methods 2018, 11, 1330–1344. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Evaluation of New Natural Deep Eutectic Solvents for the Extraction of Isoflavones from Soy Products. Talanta 2017, 168, 329–335. [Google Scholar] [CrossRef]
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alañón, M.E. Modifiers Based on Natural Deep Eutectic Mixtures to Enhance Anthocyanins Isolation from Grape Pomace by Pressurized Hot Water Extraction. LWT 2021, 149, 111889. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Q. An Efficient Extraction Method for Essential Oil from Angelica Sinensis Radix by Natural Deep Eutectic Solvents-Assisted Microwave Hydrodistillation. Sustain. Chem. Pharm. 2022, 29, 100792. [Google Scholar] [CrossRef]
- Morrison, H.G.; Sun, C.C.; Neervannan, S. Characterization of Thermal Behavior of Deep Eutectic Solvents and Their Potential as Drug Solubilization Vehicles. Int. J. Pharm. 2009, 378, 136–139. [Google Scholar] [CrossRef] [PubMed]
- De Morais, P.; Gonçalves, F.; Coutinho, J.A.P.; Ventura, S.P.M. Ecotoxicity of Cholinium-Based Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2015, 3, 3398–3404. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Gaurina Srček, V.; Cvjetko Bubalo, M.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Natural Deep Eutectic Solvents as Beneficial Extractants for Enhancement of Plant Extracts Bioactivity. LWT 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.S.; Saheed, O.K. Are Deep Eutectic Solvents Benign or Toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef]
- Radošević, K.; Cvjetko Bubalo, M.; Gaurina Srček, V.; Grgas, D.; Landeka Dragičević, T.; Redovniković, R.I. Evaluation of Toxicity and Biodegradability of Choline Chloride Based Deep Eutectic Solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Brito, T.A.; Santana, A.P.R.; Guimarães, T.G.S.; Lamarca, R.S.; Ferreira, K.C.; Gomes, P.C.F.L.; Oliveira, A.; Amaral, C.D.B.; Gonzalez, M.H. Greenness of Procedures Using NADES in the Preparation of Vegetal Samples: Comparison of Five Green Metrics. Talanta Open 2022, 6, 100131. [Google Scholar] [CrossRef]
- Putnik, P.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S.; Jambrak, A.R.; Granato, D.; Montesano, D.; Kovačević, D.B. Novel Food Processing and Extraction Technologies of High-Added Value Compounds from Plant Materials. Foods 2018, 7, 106. [Google Scholar] [CrossRef]
- Palos-Hernández, A.; Gutiérrez Fernández, M.Y.; Escuadra Burrieza, J.; Pérez-Iglesias, J.L.; González-Paramás, A.M. Obtaining Green Extracts Rich in Phenolic Compounds from Underex-ploited Food By-Products Using Natural Deep Eutectic Solvents. Opportunities and Challenges. Sustain. Chem. Pharm. 2022, 29, 100773. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namieśnik, J. Greener Organic Solvents in Analytical Chemistry. Curr. Opin. Green Sustain. Chem. 2017, 5, 1–4. [Google Scholar] [CrossRef]
- Li, X.; Row, K.H. Exploration of Mesoporous Stationary Phases Prepared Using Deep Eutectic Solvents Combining Choline Chloride with 1,2-Butanediol or Glycerol for Use in Size-Exclusion Chromatography. Chromatographia 2015, 78, 1321–1325. [Google Scholar] [CrossRef]
- Li, X.; Row, K.H. Development of Deep Eutectic Solvents Applied in Extraction and Separation. J. Sep. Sci. 2016, 39, 3505–3520. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Zhang, M.; Wan, Y.; Qiu, H. Utilization of Deep Eutectic Solvents as Novel Mobile Phase Additives for Improving the Separation of Bioactive Quaternary Alkaloids. Talanta 2016, 149, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green Solvents for Green Technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. New Perspective in Extraction of Plant Biologically Active Compounds by Green Solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Funari, C.S.; Sutton, A.T.; Carneiro, R.L.; Fraige, K.; Cavalheiro, A.J.; da Silva Bolzani, V.; Hilder, E.F.; Arrua, R.D. Natural Deep Eutectic Solvents and Aqueous Solutions as an Alternative Extraction Media for Propolis. Food Res. Int. 2019, 125, 108559. [Google Scholar] [CrossRef]
- Jeong, K.M.; Zhao, J.; Jin, Y.; Heo, S.R.; Han, S.Y.; Yoo, D.E.; Lee, J. Highly Efficient Extraction of Anthocyanins from Grape Skin Using Deep Eutectic Solvents as Green and Tunable Media. Arch. Pharm. Res. 2015, 38, 2143–2152. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Liu, Y.; Garzon, J.; Friesen, J.B.; Zhang, Y.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Countercurrent Assisted Quantitative Recovery of Metabolites from Plant-Associated Natural Deep Eutectic Solvents. Fitoterapia 2016, 112, 30–37. [Google Scholar] [CrossRef]
- Wang, X.H.; Wang, J.P. Effective Extraction with Deep Eutectic Solvents and Enrichment by Macroporous Adsorption Resin of Flavonoids from Carthamus Tinctorius L. J. Pharm. Biomed. Anal. 2019, 176, 112804. [Google Scholar] [CrossRef]
- Zhuang, B.; Dou, L.L.; Li, P.; Liu, E.H. Deep Eutectic Solvents as Green Media for Extraction of Flavonoid Glycosides and Aglycones from Platycladi Cacumen. J. Pharm. Biomed. Anal. 2017, 134, 214–219. [Google Scholar] [CrossRef]
- Bi, Y.; Chi, X.; Zhang, R.; Lu, Y.; Wang, Z.; Dong, Q.; Ding, C.; Yang, R.; Jiang, L. Highly Efficient Extraction of Mulberry Anthocyanins in Deep Eutectic Solvents: Insights of Degradation Kinetics and Stability Evaluation. Innov. Food Sci. Emerg. Technol. 2020, 66, 102512. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Cravotto, G.; Radojčić Redovniković, I. Enabling Technologies for the Extraction of Grape-Pomace Anthocyanins Using Natural Deep Eutectic Solvents in up-to-Half-Litre Batches Extraction of Grape-Pomace Anthocyanins Using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, A.H.; Pölzler, M.; Gollas, B.; Miller, M.A.; Wainright, J.S.; Savinell, R.F.; Lin, L. Phenolic Acid Extraction from Orange Peel with Natural Deep Eutectic Solvents. J. Phys. Conf. Ser. 2022, 2321, 012020. [Google Scholar] [CrossRef]
- Zannou, O.; Pashazadeh, H.; Ghellam, M.; Ali Redha, A.; Koca, I. Enhanced Ultrasonically Assisted Extraction of Bitter Melon (Momordica Charantia) Leaf Phenolic Compounds Using Choline Chloride-Acetic Acid–Based Natural Deep Eutectic Solvent: An Optimization Approach and in Vitro Digestion. Biomass Convers. Biorefin. 2022, 1, 1–13. [Google Scholar] [CrossRef]
- Guo, N.; Ping-Kou; Jiang, Y.W.; Wang, L.T.; Niu, L.J.; Liu, Z.M.; Fu, Y.J. Natural Deep Eutectic Solvents Couple with Integrative Extraction Technique as an Effective Approach for Mulberry Anthocyanin Extraction. Food Chem. 2019, 296, 78–85. [Google Scholar] [CrossRef]
- Zengin, G.; Cádiz-Gurrea, M.d.l.L.; Fernández-Ochoa, Á.; Leyva-Jiménez, F.J.; Carretero, A.S.; Momotko, M.; Yildiztugay, E.; Karatas, R.; Jugreet, S.; Mahomoodally, M.F.; et al. Selectivity Tuning by Natural Deep Eutectic Solvents (NADESs) for Extraction of Bioactive Compounds from Cytinus Hypocistis—Studies of Antioxidative, Enzyme-Inhibitive Properties and LC-MS Profiles. Molecules 2022, 27, 5788. [Google Scholar] [CrossRef]
- Vieira, C.; Rebocho, S.; Craveiro, R.; Paiva, A.; Duarte, A.R.C. Selective Extraction and Stabilization of Bioactive Compounds from Rosemary Leaves Using a Biphasic NADES. Front. Chem. 2022, 10, 954835. [Google Scholar] [CrossRef]
- Leal, F.C.; Farias, F.O.; do Amaral, W.; Toci, A.T.; Mafra, M.R.; Igarashi-Mafra, L. Green Solvents to Value Annona Muricata L. Leaves as Antioxidants Source: Process Optimization and Potential as a Natural Food Additive. Waste Biomass Valorization 2022, 13, 1233–1241. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, L.; Li, S.; Meng, T.; Wang, L.; Zhang, W. The Effect of Sonication-Synergistic Natural Deep Eutectic Solvents on Extraction Yield, Structural and Physicochemical Properties of Pectins Extracted from Mango Peels. Ultrason. Sonochem. 2022, 86, 106045. [Google Scholar] [CrossRef]
- Liang, X.; Yan, J.; Guo, S.; McClements, D.J.; Ma, C.; Liu, X.; Liu, F. Enhancing Lycopene Stability and Bioaccessibility in Homogenized Tomato Pulp Using Emulsion Design Principles. Innov. Food Sci. Emerg. Technol. 2021, 67, 102525. [Google Scholar] [CrossRef]
- Alioui, O.; Sobhi, W.; Tiecco, M.; Alnashef, I.M.; Attoui, A.; Boudechicha, A.; Kumar Yadav, K.; Fallatah, A.M.; Elboughdiri, N.; Jeon, B.H.; et al. Theoretical and Experimental Evidence for the Use of Natural Deep Eutectic Solvents to Increase the Solubility and Extractability of Curcumin. J. Mol. Liq. 2022, 359, 119149. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Tsiouras, A.; Mourtzinos, I. Extraction of Lycopene from Tomato Using Hydrophobic Natural Deep Eutectic Solvents Based on Terpenes and Fatty Acids. Foods 2022, 11, 2645. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Deng, M.; Zhao, L. Natural Deep Eutectic Solvent Combined with Ultrasonic Enhancement: A Green Extraction Strategy for Solanesol in Tobacco Leaves. Ind. Crops Prod. 2022, 187, 115355. [Google Scholar] [CrossRef]
- Min, L.; Chen, S.W.; Wei, J.L.; Wang, R.; Yu, L.L.; Wen, J.W.; Xiao, J.M. The Effects of Angelica Essential Oil in Social Interaction and Hole-Board Tests. Pharmacol. Biochem. Behav. 2005, 81, 838–842. [Google Scholar] [CrossRef]
- Ivanović, M.; Alañón, M.E.; Arráez-Román, D.; Segura-Carretero, A. Enhanced and Green Extraction of Bioactive Compounds from Lippia Citriodora by Tailor-Made Natural Deep Eutectic Solvents. Food Res. Int. 2018, 111, 67–76. [Google Scholar] [CrossRef]
- Ünlü, A.E. Green and Non-conventional Extraction of Bioactive Compounds from Olive Leaves: Screening of Novel Natural Deep Eutectic Solvents and Investigation of Process Parameters. Waste Biomass Valorization 2021, 12, 5329–5346. [Google Scholar] [CrossRef]
- Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A Green Extraction Process for Polyphenols from Elderberry (Sambucus Nigra) Flowers Using Deep Eutectic Solvent and Ultrasound-Assisted Pretreatment. Molecules 2020, 25, 921. [Google Scholar] [CrossRef]
- Shafie, M.H.; Yusof, R.; Gan, C.Y. Deep Eutectic Solvents (DES) Mediated Extraction of Pectin from Averrhoa Bilimbi: Optimization and Characterization Studies. Carbohydr. Polym. 2019, 216, 303–311. [Google Scholar] [CrossRef]
- Pan, C.; Zhao, L.; Zhao, D. Microwave-Assisted Green Extraction of Antioxidant Components from Osmanthus Fragrans (Lour) Flower Using Natural Deep Eutectic Solvents. J. Appl. Res. Med. Aromat. Plants 2021, 20, 100285. [Google Scholar] [CrossRef]
- Cai, C.; Li, F.; Liu, L.; Tan, Z. Deep Eutectic Solvents Used as the Green Media for the Efficient Extraction of Caffeine from Chinese Dark Tea. Sep. Purif. Technol. 2019, 227, 115723. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K.; Radojcic Redovnikovic, I. Green Extraction of Grape Skin Phenolics by Using Deep Eutectic Solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Cai, Y.; Ma, Q.; Zhao, Z.; Yang, D.; Xu, X. Optimization of Extraction of Bioactive Compounds from Baphicacanthus Cusia Leaves by Hydrophobic Deep Eutectic Solvents. Molecules 2021, 26, 1729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Teng, G.; Zhang, J. Deep Eutectic Solvents Aqueous Two-Phase System Based Ultrasonically Assisted Extraction of Ursolic Acid (UA) from Cynomorium Songaricum Rupr. Chem. Eng. Commun. 2018, 206, 419–431. [Google Scholar] [CrossRef]
- Matharu, A.S.; de Melo, E.M.; Houghton, J.A. Opportunity for High Value-Added Chemicals from Food Supply Chain Wastes. Bioresour. Technol. 2016, 215, 123–130. [Google Scholar] [CrossRef]
- Pourbayramian, R.; Abdi-Benemar, H.; Seifdavati, J.; Greiner, R.; Elghandour, M.M.M.Y.; Salem, A.Z.M. Bioconversion of Potato Waste by Rumen Fluid from Slaughterhouses to Produce a Potential Feed Additive Rich in Volatile Fatty Acids for Farm Animals. J. Clean. Prod. 2021, 280, 124411. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Extraction of Phenolic Compounds from Olive Pomace by Using Natural Deep Eutectic Solvents and Innovative Extraction Techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Morgana, N.M.; Magdalena, E.; Fernandez, M.d.l.A.; Fernanda, S.M. NADES for Food Industry Innovation: Novel Bioadditives Based on Olive Oil Byproducts. Food Bioprod. Process. 2022, 134, 193–201. [Google Scholar] [CrossRef]
- Zurob, E.; Cabezas, R.; Villarroel, E.; Rosas, N.; Merlet, G.; Quijada-Maldonado, E.; Romero, J.; Plaza, A. Design of Natural Deep Eutectic Solvents for the Ultrasound-Assisted Extraction of Hydroxytyrosol from Olive Leaves Supported by COSMO-RS. Sep. Purif. Technol. 2020, 248, 117054. [Google Scholar] [CrossRef]
- Lanjekar, K.J.; Gokhale, S.; Rathod, V.K. Utilization of Waste Mango Peels for Extraction of Polyphenolic Antioxidants by Ultrasound-Assisted Natural Deep Eutectic Solvent. Bioresour. Technol. Rep. 2022, 18, 101074. [Google Scholar] [CrossRef]
- Fanali, C.; Gallo, V.; Della Posta, S.; Dugo, L.; Mazzeo, L.; Cocchi, M.; Piemonte, V.; De Gara, L. Choline Chloride–Lactic Acid-Based NADES as an Extraction Medium in a Response Surface Methodology-Optimized Method for the Extraction of Phenolic Compounds from Hazelnut Skin. Molecules 2021, 26, 2652. [Google Scholar] [CrossRef]
- Manuela, P.; Drakula, S.; Cravotto, G.; Verpoorte, R.; Hruškar, M.; Radojčić Redovniković, I.; Radošević, K. Biological Activity and Sensory Evaluation of Cocoa By-Products NADES Extracts Used in Food Fortification. Innov. Food Sci. Emerg. Technol. 2020, 66, 102514. [Google Scholar] [CrossRef]
- Grillo, G.; Gunjević, V.; Radošević, K.; Redovniković, I.R.; Cravotto, G. Deep Eutectic Solvents and Nonconventional Technologies for Blueberry-Peel Extraction: Kinetics, Anthocyanin Stability, and Antiproliferative Activity. Antioxidants 2020, 9, 1069. [Google Scholar] [CrossRef] [PubMed]
- Sas, O.G.; Castro, M.; Domínguez, Á.; González, B. Removing Phenolic Pollutants Using Deep Eutectic Solvents. Sep. Purif. Technol. 2019, 227, 115703. [Google Scholar] [CrossRef]
- Baute-Pérez, D.; Santana-Mayor, Á.; Herrera-Herrera, A.V.; Socas-Rodríguez, B.; Rodríguez-Delgado, M.Á. Analysis of Alkylphenols, Bisphenols and Alkylphenol Ethoxylates in Microbial-Fermented Functional Beverages and Bottled Water: Optimization of a Dispersive Liquid-Liquid Microextraction Protocol Based on Natural Hydrophobic Deep Eutectic Solvents. Food Chem. 2022, 377, 131921. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Sereshti, H. A Green Alternative QuEChERS Developed Based on Green Deep Eutectic Solvents Coupled with Gas Chromatography-Mass Spectrometry for the Analysis of Pesticides in Tea Samples. Food Chem. 2022, 380, 132181. [Google Scholar] [CrossRef] [PubMed]
- Socas-Rodríguez, B.; Mendiola, J.A.; Rodríguez-Delgado, M.Á.; Ibáñez, E.; Cifuentes, A. Safety Assessment of Citrus and Olive By-Products Using a Sustainable Methodology Based on Natural Deep Eutectic Solvents. J. Chromatogr. A 2022, 1669, 462922. [Google Scholar] [CrossRef]
- Sereshti, H.; Seraj, M.; Soltani, S.; Rashidi Nodeh, H.; Hossein Shojaee AliAbadi, M.; Taghizadeh, M. Development of a Sustainable Dispersive Liquid–Liquid Microextraction Based on Novel Hydrophobic and Hydrophilic Natural Deep Eutectic Solvents for the Analysis of Multiclass Pesticides in Water. Microchem. J. 2022, 175, 107226. [Google Scholar] [CrossRef]
- Shirani, M.; Habibollahi, S.; Akbari, A. Centrifuge-Less Deep Eutectic Solvent Based Magnetic Nanofluid-Linked Air-Agitated Liquid–Liquid Microextraction Coupled with Electro-thermal Atomic Absorption Spectrometry for Simultaneous Determination of Cadmium, Lead, Copper, and Arsenic in Food Sample. Food Chem. 2019, 281, 304–311. [Google Scholar] [CrossRef]
- Waalkes, M.P. Cadmium Carcinogenesis in Review. J. Inorg. Biochem. 2000, 79, 241–244. [Google Scholar] [CrossRef]
- Shamsipur, M.; Mafakheri, N.; Babajani, N. A Natural Deep Eutectic Solvent–Based Ultrasound-Vortex-Assisted Dispersive Liquid–Liquid Microextraction Method for Ligand-Less Pre-Concentration and Determination of Traces of Cadmium Ions in Water and Some Food Samples. Food Anal. Methods 2022, 15, 1203–1213. [Google Scholar] [CrossRef]
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical Methods for Determination of Mycotoxins: A Review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Pochivalov, A.; Pavlova, K.; Garmonov, S.; Bulatov, A. Behaviour of Deep Eutectic Solvent Based on Terpenoid and Long-Chain Alcohol during Dispersive Liquid-Liquid Microextraction: Determination of Zearalenone in Cereal Samples. J. Mol. Liq. 2022, 366, 120231. [Google Scholar] [CrossRef]
- Zuo, M.; Wang, X.; Wang, Q.; Zeng, X.; Lin, L. Aqueous-Natural Deep Eutectic Sol-vent-Enhanced 5-Hydroxymethylfurfural Production from Glucose, Starch, and Food Wastes. ChemSusChem 2022, 15, e202101889. [Google Scholar] [CrossRef] [PubMed]
- Lores, H.; Romero, V.; Costas, I.; Bendicho, C.; Lavilla, I. Natural Deep Eutectic Solvents in Combination with Ultrasonic Energy as a Green Approach for Solubilisation of Proteins: Application to Gluten Determination by Immunoassay. Talanta 2017, 162, 453–459. [Google Scholar] [CrossRef]
| Extraction Technique | Type of NADES | Compounds | Extraction Yield | Ref. |
|---|---|---|---|---|
| Heating and stirring | ChCl:MalA | Phenols | 3.2 mg/g a | [33] |
| ChCl:Ur | Phenols | 2.7 mg/g a | ||
| ChCl:Fru | Phenols | 1.80 mg/g a | ||
| USAE | ChCl:MalA | Phenols | 2.5 mg/g a | |
| USAE | ChCl:glycerol | Phenols | 5.6 mg/g b | [2] |
| MAE | ChCl:MalA | Phenols | 3.0 mg/g a | [33] |
| PHWE | ChCl:Ox | Anthocyanins | 170.0 ± 6.5 mg/g c | [40] |
| ChCl:La | Anthocyanins | 146.1 ± 63.9 mg/g c | ||
| ChCl:Fru | Anthocyanins | 78.5 ± 6.5 mg/g c | ||
| ChCl:EtOH | Anthocyanins | 93.7 ± 8.24 mg/g c | ||
| ChCl:Pro | Anthocyanins | 145.5 ± 4.9 mg/g c | ||
| ChCl:Ur | Anthocyanins | 101.4 ± 27.9 mg/g c | ||
| CaMa | Anthocyanins | 39.2 ± 9.8 mg/g c | ||
| CaFru | Anthocyanins | 47.4 ± 1.6 mg/g c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannavacciuolo, C.; Pagliari, S.; Frigerio, J.; Giustra, C.M.; Labra, M.; Campone, L. Natural Deep Eutectic Solvents (NADESs) Combined with Sustainable Extraction Techniques: A Review of the Green Chemistry Approach in Food Analysis. Foods 2023, 12, 56. https://doi.org/10.3390/foods12010056
Cannavacciuolo C, Pagliari S, Frigerio J, Giustra CM, Labra M, Campone L. Natural Deep Eutectic Solvents (NADESs) Combined with Sustainable Extraction Techniques: A Review of the Green Chemistry Approach in Food Analysis. Foods. 2023; 12(1):56. https://doi.org/10.3390/foods12010056
Chicago/Turabian StyleCannavacciuolo, Ciro, Stefania Pagliari, Jessica Frigerio, Chiara Maria Giustra, Massimo Labra, and Luca Campone. 2023. "Natural Deep Eutectic Solvents (NADESs) Combined with Sustainable Extraction Techniques: A Review of the Green Chemistry Approach in Food Analysis" Foods 12, no. 1: 56. https://doi.org/10.3390/foods12010056
APA StyleCannavacciuolo, C., Pagliari, S., Frigerio, J., Giustra, C. M., Labra, M., & Campone, L. (2023). Natural Deep Eutectic Solvents (NADESs) Combined with Sustainable Extraction Techniques: A Review of the Green Chemistry Approach in Food Analysis. Foods, 12(1), 56. https://doi.org/10.3390/foods12010056







