Developing Technology for the Production of Innovative Coatings with Antioxidant Properties for Packaging Fish Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Herbs as Active Ingredients for Coatings and Choice of Extraction Method
2.1.1. Plant Material
2.1.2. Preparation of Ethanol and Water Extracts of Herbs for Assessing Antioxidant Activity
2.1.3. Antioxidant Analysis of Herbal Extracts
Ferric Reducing Antioxidant Power (FRAP)
DPPH Radical Scavenging
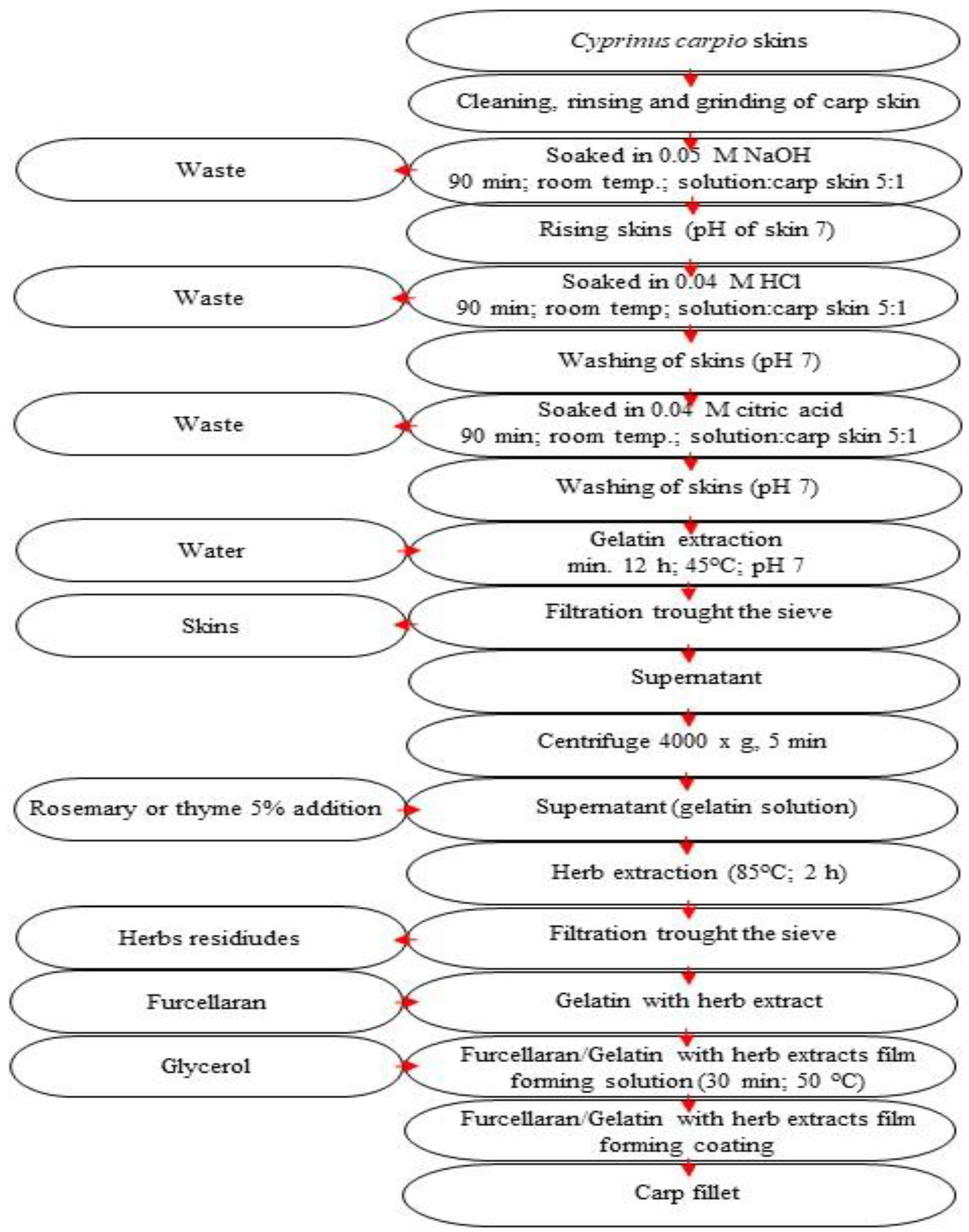
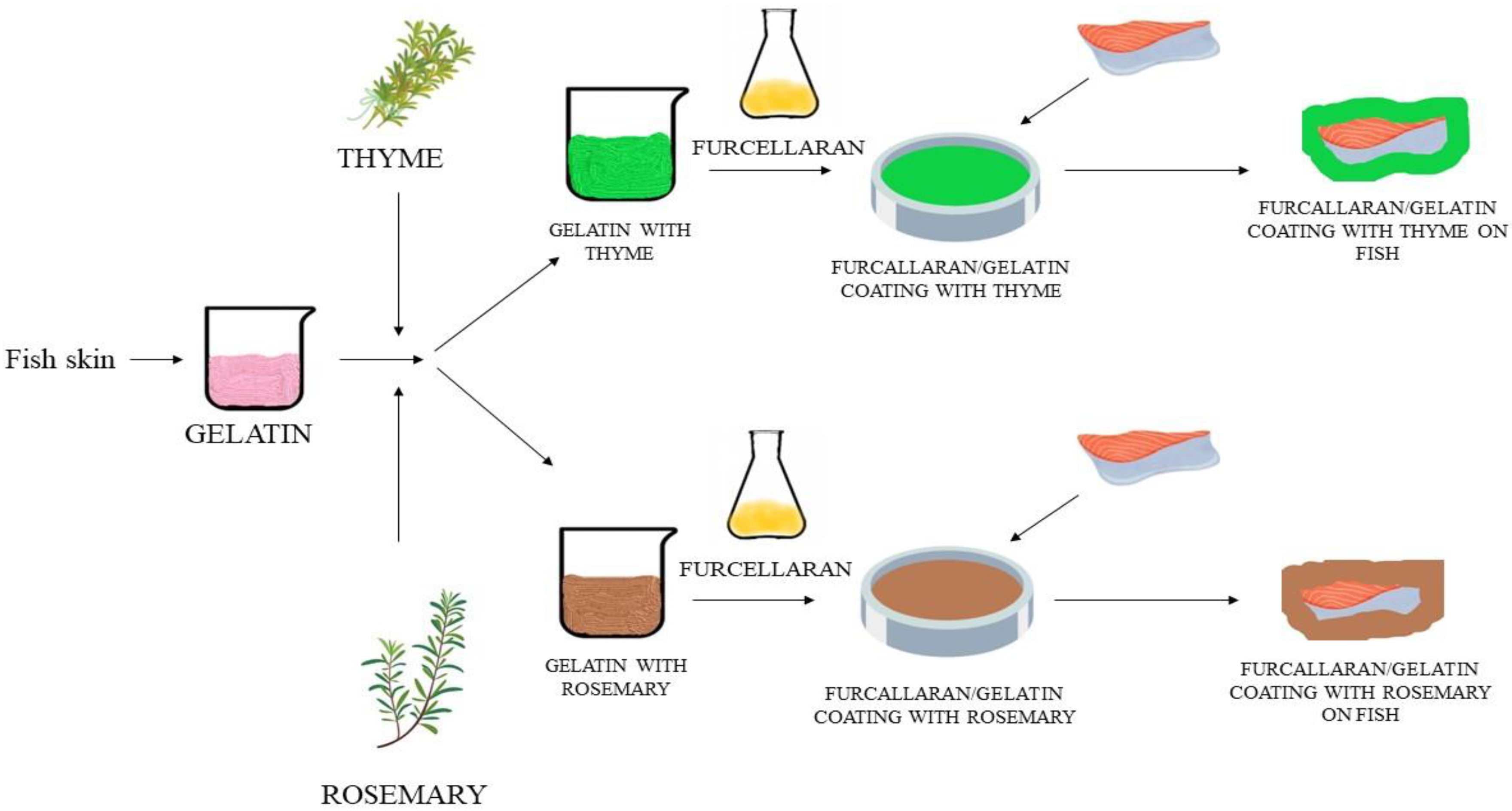
2.2. Preparation of Coatings
2.3. Properties of Antioxidant Coatings
2.3.1. Total Phenol Content (TPC) in Coatings
2.3.2. Antioxidant Activity of Coatings
2.3.3. Ultraviolet–Visible (UV-Vis) Spectroscopy Analysis
2.3.4. Colour Parameters of Coatings
2.4. Influence of Tested Coatings on Quality of Refrigerated Carp Fillets
2.4.1. Preparation of Samples
2.4.2. Texture, Colour and Water Activity of the Carp Samples
2.4.3. Oxidation Rate of Fish Lipids
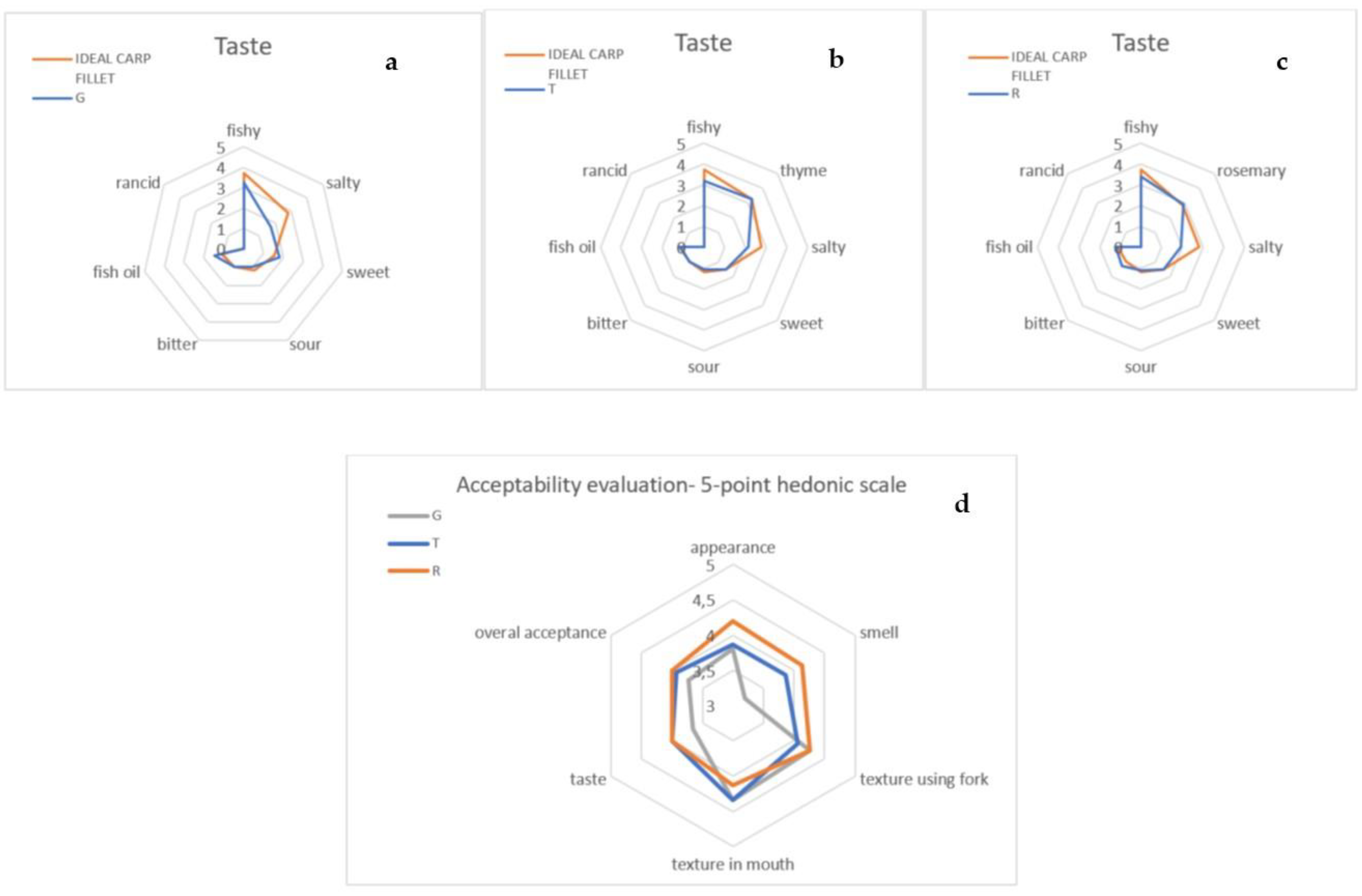
2.4.4. Sensory Analysis of Carp Fish Fillets Covered with Coatings
2.5. Statistical Analysis
3. Results and Discussion
3.1. Development of Active Coatings
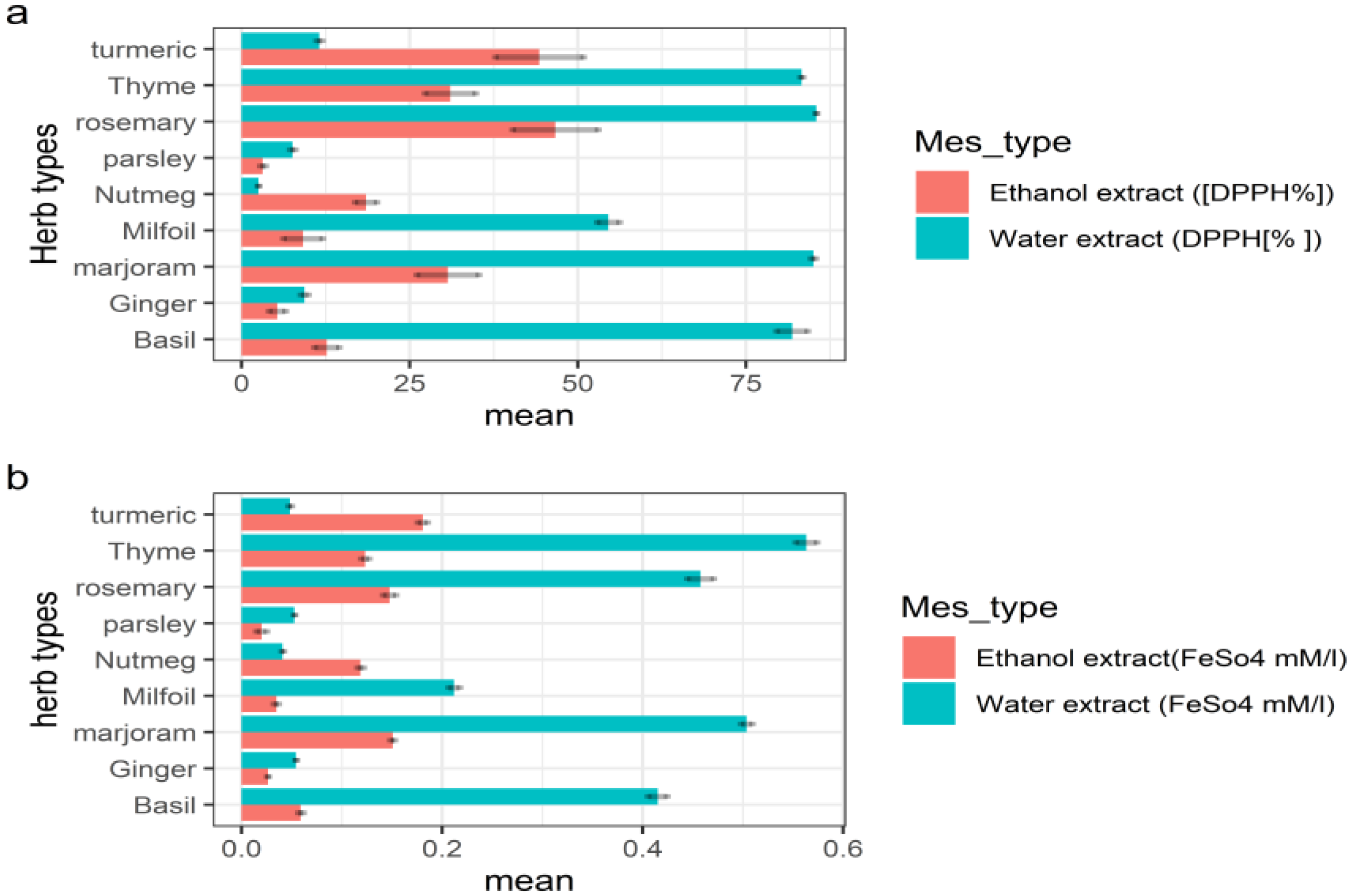
3.1.1. Antioxidant Activity of Herbs
3.1.2. Antioxidant Activity of Coatings
3.1.3. TPC in the Active Coating
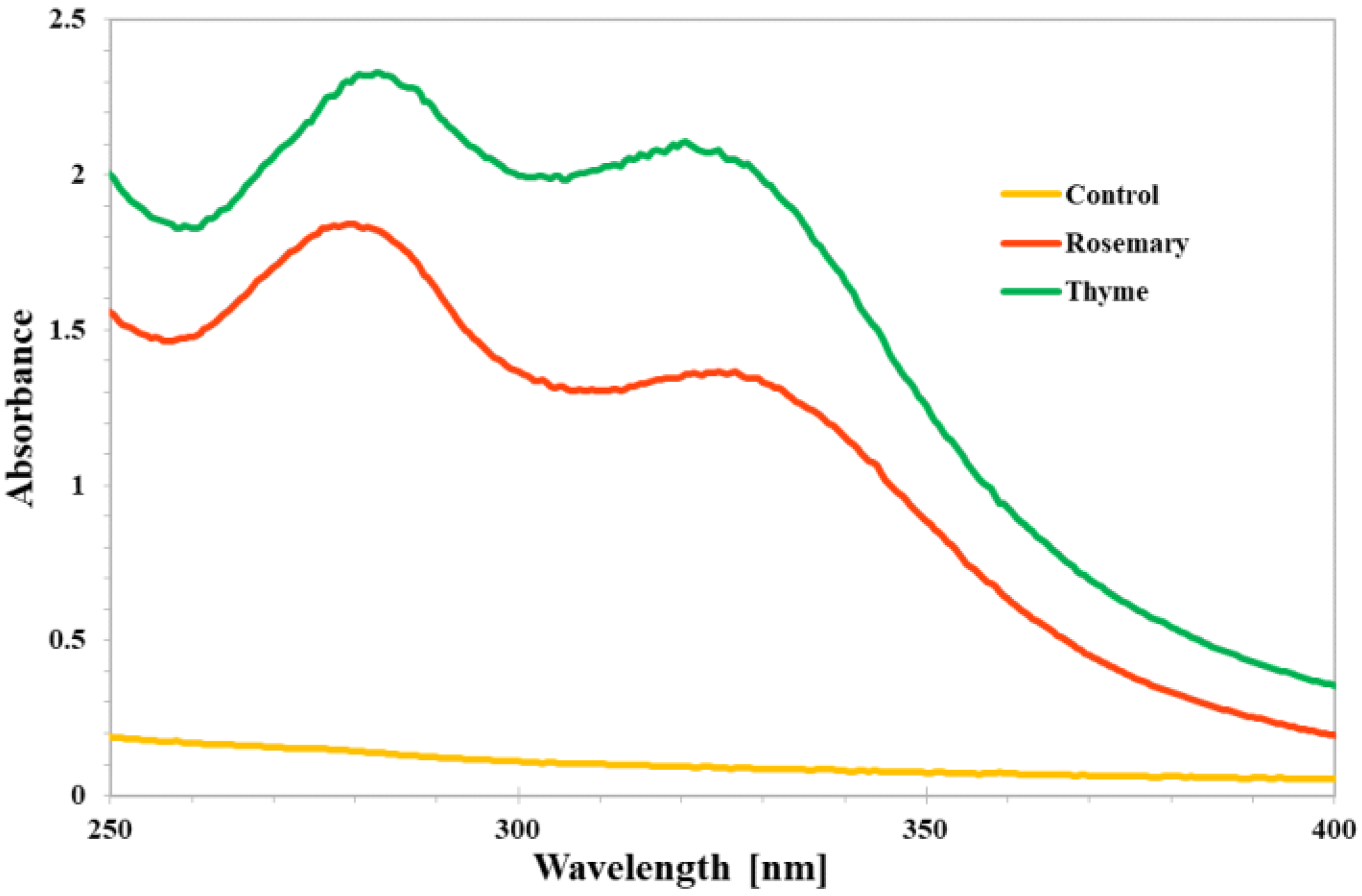
3.1.4. UV-Vis Analysis of the Coatings
3.1.5. Colour Parameters of the Active Coating
3.2. Influence of Tested Films on Functional Properties and Safety of Carp Fillets
3.2.1. Colour Parameters of Carp Fillet in Coatings during Storage
3.2.2. Texture Profile Analyses of Carp Fillets in Coatings during Storage
3.2.3. Water Activity of Carp Fillets in Coatings during Storage
3.2.4. Oxidative Stability of Carp Fillets in Active Coatings during Storage
3.3. Sensory Analysis of Carp Fish Fillets Covered with Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tosati, J.V.; Messias, V.C.; Carvalho, P.I.; Rodrigues Pollonio, M.A.; Meireles, M.A.A.; Monteiro, A.R. Antimicrobial effect of edible coating blend based on turmeric starch residue and gelatin applied onto fresh frankfurter sausage. Food Bioprocess Technol. 2017, 10, 2165–2175. [Google Scholar] [CrossRef]
- Kulawik, P.; Jamróz, E.; Zając, M.; Guzik, P.; Tkaczewska, J. The effect of furcellaran-gelatin edible coatings with green and pu-erh tea extracts on the microbiological, physicochemical and sensory changes of salmon sushi stored at 4 C. Food Control 2019, 100, 83–91. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.; Verma, A.K.; Barba, F.J.; Singh, V.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Menaga, D.; Rahman, P.K.; Rajakumar, S.; Ayyasamy, P. Antioxidant and cytotoxic activities of a novel isomeric molecule (PF5) obtained from methanolic extract of Pleurotus florida mushroom. J. Bioresour. Bioprod. 2021, 6, 338–349. [Google Scholar] [CrossRef]
- Yap, J.Y.; Hii, C.L.; Ong, S.P.; Lim, K.H.; Abas, F.; Pin, K.Y. Quantification of Carpaine and antioxidant properties of extracts from Carica Papaya plant leaves and stalks. J. Bioresour. Bioprod. 2021, 6, 350–358. [Google Scholar] [CrossRef]
- Sun, X.; Guo, X.; Ji, M.; Wu, J.; Zhu, W.; Wang, J.; Cheng, C.; Chen, L.; Zhang, Q. Preservative effects of fish gelatin coating enriched with CUR/βCD emulsion on grass carp (Ctenopharyngodon idellus) fillets during storage at 4 C. Food Chem. 2019, 272, 643–652. [Google Scholar] [CrossRef]
- Jimenez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and biodegradable starch films: A review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Poverenov, E.; Rutenberg, R.; Danino, S.; Horev, B.; Rodov, V. Gelatin-chitosan composite films and edible coatings to enhance the quality of food products: Layer-by-layer vs. blended formulations. Food Bioprocess Technol. 2014, 7, 3319–3327. [Google Scholar] [CrossRef]
- Jamróz, E.; Juszczak, L.; Kucharek, M. Development of starch-furcellaran-gelatin films containing tea tree essential oil. J. Appl. Polym. Sci. 2018, 135, 46754. [Google Scholar] [CrossRef]
- Lai, W.-F. Design of polymeric films for antioxidant active food packaging. Int. J. Mol. Sci. 2021, 23, 12. [Google Scholar] [CrossRef]
- Hellwig, M. Analysis of protein oxidation in food and feed products. J. Agric. Food Chem. 2020, 68, 12870–12885. [Google Scholar] [CrossRef]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef]
- Hashemi, M.; Daneshamooz, S.; Raeisi, M.; Jannat, B.; Taheri, S.; Noori, S. An overview on antioxidants activity of polysaccharide edible films and coatings contains essential oils and herb extracts in meat and meat products. Adv. Anim. Vet. Sci. 2020, 8, 198–207. [Google Scholar] [CrossRef]
- Ponce, A.G.; Roura, S.I.; del Valle, C.E.; Moreira, M.R. Antimicrobial and antioxidant activities of edible coatings enriched with natural plant extracts: In vitro and in vivo studies. Postharvest Biol. Technol. 2008, 49, 294–300. [Google Scholar] [CrossRef]
- Moreno, M.A.; Vallejo, A.M.; Ballester, A.-R.; Zampini, C.; Isla, M.I.; López-Rubio, A.; Fabra, M.J. Antifungal edible coatings containing Argentinian propolis extract and their application in raspberries. Food Hydrocoll. 2020, 107, 105973. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Langroodi, A.M. Chitosan coatings incorporated with propolis extract and Zataria multiflora Boiss oil for active packaging of chicken breast meat. Int. J. Biol. Macromol. 2019, 141, 401–409. [Google Scholar] [CrossRef]
- Sepahpour, S.; Selamat, J.; Abdul Manap, M.Y.; Khatib, A.; Abdull Razis, A.F. Comparative analysis of chemical composition, antioxidant activity and quantitative characterization of some phenolic compounds in selected herbs and spices in different solvent extraction systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef]
- Jamróz, E.; Tkaczewska, J.; Juszczak, L.; Zimowska, M.; Kawecka, A.; Krzyściak, P.; Skóra, M. The influence of lingonberry extract on the properties of novel, double-layered biopolymer films based on furcellaran, CMC and a gelatin hydrolysate. Food Hydrocoll. 2022, 124, 107334. [Google Scholar] [CrossRef]
- Oriani, V.B.; Molina, G.; Chiumarelli, M.; Pastore, G.M.; Hubinger, M.D. Properties of cassava starch-based edible coating containing essential oils. J. Food Sci. 2014, 79, E189–E194. [Google Scholar] [CrossRef] [PubMed]
- Khantaphant, S.; Benjakul, S. Comparative study on the proteases from fish pyloric caeca and the use for production of gelatin hydrolysate with antioxidative activity. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 151, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, E.; Kulawik, P.; Guzik, P.; Duda, I. The verification of intelligent properties of furcellaran films with plant extracts on the stored fresh Atlantic mackerel during storage at 2° C. Food Hydrocoll. 2019, 97, 105211. [Google Scholar] [CrossRef]
- Joly-Duhamel, C.; Hellio, D.; Ajdari, A.; Djabourov, M. All gelatin networks: 2. The master curve for elasticity. Langmuir 2002, 18, 7158–7166. [Google Scholar] [CrossRef]
- Zhou, P.; Regenstein, J.M. Effects of alkaline and acid pretreatments on Alaska pollock skin gelatin extraction. J. Food Sci. 2005, 70, c392–c396. [Google Scholar] [CrossRef]
- Eysturskarð, J.; Haug, I.J.; Elharfaoui, N.; Djabourov, M.; Draget, K.I. Structural and mechanical properties of fish gelatin as a function of extraction conditions. Food Hydrocoll. 2009, 23, 1702–1711. [Google Scholar] [CrossRef]
- Duan, R.; Zhang, J.; Xing, F.; Konno, K.; Xu, B. Study on the properties of gelatins from skin of carp (Cyprinus carpio) caught in winter and summer season. Food Hydrocoll. 2011, 25, 368–373. [Google Scholar] [CrossRef]
- Akagündüz, Y.; Mosquera, M.; Giménez, B.; Alemán, A.; Montero, P.; Gómez-Guillén, M.C. Sea bream bones and scales as a source of gelatin and ACE inhibitory peptides. LWT-Food Sci. Technol. 2014, 55, 579–585. [Google Scholar] [CrossRef]
- Jamróz, E.; Kopel, P.; Juszczak, L.; Kawecka, A.; Bytesnikova, Z.; Milosavljević, V.; Kucharek, M.; Makarewicz, M.; Adam, V. Development and characterisation of furcellaran-gelatin films containing SeNPs and AgNPs that have antimicrobial activity. Food Hydrocoll. 2018, 83, 9–16. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food Chem. 2006, 99, 835–841. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Przybylski, R. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. trees. Food Chem. 2007, 104, 1106–1114. [Google Scholar] [CrossRef]
- Kim, J.-S. Comparison of Antioxidant Properties of Water and Ethanol Extracts Obtained from Dried Boxthorn (Lycium chinensis) Fruit. Food Nutr. Sci. 2012, 3, 1307–1320. [Google Scholar]
- Gramza, A.; Khokhar, S.; Yoko, S.; Gliszczynska-Swiglo, A.; Hes, M.; Korczak, J. Antioxidant activity of tea extracts in lipids and correlation with polyphenol content. Eur. J. Lipid Sci. Technol. 2006, 108, 351–362. [Google Scholar] [CrossRef]
- Petlevski, R.; Flajs, D.; Kalođera, Z.; Končić, M.Z. Composition and antioxidant activity of aqueous and ethanolic Pelargonium radula extracts. S. Afr. J. Bot. 2013, 85, 17–22. [Google Scholar] [CrossRef]
- Ulbin-Figlewicz, N.; Zimoch, A.; Jarmoluk, A. Plant extracts as components of edible antimicrobial protective coatings. Czech J. Food Sci. 2013, 31, 596–600. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Alvarenga, J.F.R.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef]
- Andrade, M.A.; Ribeiro-Santos, R.; Bonito, M.C.C.; Saraiva, M.; Sanches-Silva, A. Characterization of rosemary and thyme extracts for incorporation into a whey protein based film. Lwt-Food Sci. Technol. 2018, 92, 497–508. [Google Scholar] [CrossRef]
- Škrovánková, S.; Mišurcová, L.; Machů, L. Antioxidant activity and protecting health effects of common medicinal plants. Adv. Food Nutr. Res. 2012, 67, 75–139. [Google Scholar]
- Jancikova, S.; Jamróz, E.; Kulawik, P.; Tkaczewska, J.; Dordevic, D. Furcellaran/gelatin hydrolysate/rosemary extract composite films as active and intelligent packaging materials. Int. J. Biol. Macromol. 2019, 131, 19–28. [Google Scholar] [CrossRef]
- Fiore, A.; Park, S.; Volpe, S.; Torrieri, E.; Masi, P. Active packaging based on PLA and chitosan-caseinate enriched rosemary essential oil coating for fresh minced chicken breast application. Food Packag. Shelf Life 2021, 29, 100708. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Vargas, M.; Chiralt, A.; González-Martínez, C. Release of polyphenols from starch-chitosan based films containing thyme extract. Carbohydr. Polym. 2017, 175, 122–130. [Google Scholar] [CrossRef]
- Ulewicz-Magulska, B.; Wesolowski, M. Total phenolic contents and antioxidant potential of herbs used for medical and culinary purposes. Plant Foods Hum. Nutr. 2019, 74, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Afshar, M.; Najafian, S.; Radi, M. Seasonal variation on the major bioactive compounds: Total phenolic and flavonoids contents, and antioxidant activity of rosemary from shiraz. Nat. Prod. Res. 2021, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gião, M.S.; González-Sanjosé, M.L.; Rivero-Pérez, M.D.; Pereira, C.I.; Pintado, M.E.; Malcata, F.X. Infusions of Portuguese medicinal plants: Dependence of final antioxidant capacity and phenol content on extraction features. J. Sci. Food Agric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- Zeković, Z.; Lepojeviíc, Ž.; Vujić, D. Supercritical extraction of thyme (Thymus vulgaris L.). Chromatographia 2000, 51, 175–179. [Google Scholar] [CrossRef]
- Gallego, M.G.; Gordon, M.H.; Segovia, F.; Almajano Pablos, M.P. Gelatine-based antioxidant packaging containing Caesalpinia decapetala and Tara as a coating for ground beef patties. Antioxidants 2016, 5, 10. [Google Scholar] [CrossRef]
- Du, H.; Liu, C.; Unsalan, O.; Altunayar-Unsalan, C.; Xiong, S.; Manyande, A.; Chen, H. Development and characterization of fish myofibrillar protein/chitosan/rosemary extract composite edible films and the improvement of lipid oxidation stability during the grass carp fillets storage. Int. J. Biol. Macromol. 2021, 184, 463–475. [Google Scholar] [CrossRef]
- Pérez-Santaescolástica, C.; Munekata, P.E.; Feng, X.; Liu, Y.; Bastianello Campagnol, P.C.; Lorenzo, J.M. Active edible coatings and films with Mediterranean herbs to improve food shelf-life. Crit. Rev. Food Sci. Nutr. 2022, 62, 2391–2403. [Google Scholar] [CrossRef]
- Liu, T.; Liu, L.; Gong, X.; Chi, F.; Ma, Z. Fabrication and comparison of active films from chitosan incorporating different spice extracts for shelf life extension of refrigerated pork. Lwt-Food Sci. Technol. 2021, 135, 110181. [Google Scholar] [CrossRef]
- Hosseini, H.; Hamgini, E.Y.; Jafari, S.M.; Bolourian, S. Improving the oxidative stability of sunflower seed kernels by edible biopolymeric coatings loaded with rosemary extract. J. Stored Prod. Res. 2020, 89, 101729. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Wang, B.-J.; Weng, Y.-M. Antioxidant and antimicrobial edible zein/chitosan composite films fabricated by incorporation of phenolic compounds and dicarboxylic acids. LWT-Food Sci. Technol. 2015, 63, 115–121. [Google Scholar] [CrossRef]
- Jouki, M.; Mortazavi, S.A.; Yazdi, F.T.; Koocheki, A.; Khazaei, N. Use of quince seed mucilage edible films containing natural preservatives to enhance physico-chemical quality of rainbow trout fillets during cold storage. Food Sci. Hum. Wellness 2014, 3, 65–72. [Google Scholar] [CrossRef]
- Jung, S.; Ghoul, M.; de Lamballerie-Anton, M. Influence of high pressure on the color and microbial quality of beef meat. LWT-Food Sci. Technol. 2003, 36, 625–631. [Google Scholar] [CrossRef]
- Zeng, L.; Ma, M.; Li, C.; Luo, L. Stability of tea polyphenols solution with different pH at different temperatures. Int. J. Food Prop. 2017, 20, 1–18. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Ihl, M.; Sobral, P.d.A.; Gómez-Guillén, M.; Bifani, V. Natural additives in bioactive edible films and coatings: Functionality and applications in foods. Food Eng. Rev. 2013, 5, 200–216. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Bravo, L.; Gómez-Guillén, M.; Alemán, A.; Montero, P. Antioxidant properties of tuna-skin and bovine-hide gelatin films induced by the addition of oregano and rosemary extracts. Food Chem. 2009, 112, 18–25. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Han, Z.; Zeng, X.A. Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 52–61. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Q.; Ma, T.; Bao, K.; Yu, X.; Duan, Z.; Shen, X.; Li, C. Effect of EGCG-gelatin biofilm on the quality and microbial composition of tilapia fillets during chilled storage. Food Chem. 2020, 305, 125454. [Google Scholar] [CrossRef]
- Feng, X.; Ng, V.K.; Mikš-Krajnik, M.; Yang, H. Effects of fish gelatin and tea polyphenol coating on the spoilage and degradation of myofibril in fish fillet during cold storage. Food Bioprocess Technol. 2017, 10, 89–102. [Google Scholar] [CrossRef]
- Saito, M.; Saito, K.; Kunisaki, N.; Kimura, S. Green tea polyphenols inhibit metalloproteinase activities in the skin, muscle, and blood of rainbow trout. J. Agric. Food Chem. 2002, 50, 7169–7174. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Jridi, M.; Nasri, R.; Mora, L.; Toldrá, F.; Nasri, M. Rheological and structural properties of Hemiramphus far skin gelatin: Potential use as an active fish coating agent. Food Hydrocoll. 2019, 87, 331–341. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Tkaczewska, J.; Guzik, P.; Zając, M.; Juszczak, L.; Krzyściak, P.; Turek, K. The effects of active double-layered furcellaran/gelatin hydrolysate film system with Ala-Tyr peptide on fresh Atlantic mackerel stored at −18 °C. Food Chem. 2020, 338, 127867. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Zając, M.; Jamróz, E.; Derbew, H. Utilising waste from soybean processing as raw materials for the production of preparations with antioxidant properties, serving as natural food preservatives-A pilot study. LWT-Food Sci. Technol. 2022, 160, 113282. [Google Scholar] [CrossRef]
- Ebadi, Z.; Khodanazary, A.; Hosseini, S.M.; Zanguee, N. The shelf life extension of refrigerated Nemipterus japonicus fillets by chitosan coating incorporated with propolis extract. Int. J. Biol. Macromol. 2019, 139, 94–102. [Google Scholar] [CrossRef]
- Makri, M. Effect of oregano and rosemary essential oils on lipid oxidation of stored frozen minced gilthead sea bream muscle. J. Für Verbrauch. Und Lebensm. 2013, 8, 67–70. [Google Scholar] [CrossRef]
- Choulitoudi, E.; Ganiari, S.; Tsironi, T.; Ntzimani, A.; Tsimogiannis, D.; Taoukis, P.; Oreopoulou, V. Edible coating enriched with rosemary extracts to enhance oxidative and microbial stability of smoked eel fillets. Food Packag. Shelf Life 2017, 12, 107–113. [Google Scholar] [CrossRef]
- Nawaz, T.; Fatima, M.; Shah, S.Z.H.; Afzal, M. Coating effect of rosemary extract combined with chitosan on storage quality of mori (Cirrhinus mrigala). J. Food Process. Preserv. 2020, 44, e14833. [Google Scholar] [CrossRef]
- Khalafalla, F.A.; Ali, F.H.; Hassan, A.-R.H. Quality improvement and shelf-life extension of refrigerated Nile tilapia (Oreochromis niloticus) fillets using natural herbs. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 33–40. [Google Scholar] [CrossRef]
- Tural, S.; Turhan, S. Effect of anchovy by-product protein coating incorporated with thyme essential oil on the shelf life of anchovy (Engraulis encrasicolus L.) fillets. Food Sci. Biotechnol. 2017, 26, 1291–1299. [Google Scholar] [CrossRef]
- Oguzhan Yildiz, P. The effects of chitosan coatings enriched with thyme oil on the quality of rainbow trout. J. Food Meas. Charact. 2017, 11, 1398–1405. [Google Scholar] [CrossRef]
- Lei, L.; Wan, K.; Zhang, L.; Cong, M.; Wang, Y.; Fu, Y.; Wang, L.; Ren, L. Practical application effect of bionic design replicating from dry lotus leaves surface based on carboxymethyl chitosan incorporated black carrot powder on fried shrimp. Mater. Today Commun. 2022, 33, 104673. [Google Scholar] [CrossRef]
- Opeña, A.M.; Baleta, F.N.; Bolaños, J.M. Sensory, organoleptic, and proximate composition of smoked surgeon fish Acanthurus sp. using selected herbs as flavor enhancer. Int. J. Food Sci. Nutr. 2017, 2, 174–181. [Google Scholar]
- Buchtova, H.; Dordević, Đ.; Kočárek, S.; Chomat, P. Analysis of chemical and sensory parameters in different kinds of escolar (Lepidocybium flavobrunneum) products. Czech J. Food Sci. 2015, 33, 346–353. [Google Scholar] [CrossRef]
| Antioxidant Properties of Coatings | Colour Parameters | ||||
|---|---|---|---|---|---|
| Type of Coating | FRAP Value [mM Fe2SO4/L] | TPC [mg gallic acid/L] | L* (D65) | a* (D65) | b* (D65) |
| Coating without herbs | 0.03 c ± 0.00 | 0.00 c ± 0.00 | 27.46 a ± 0.02 | −0.26 c ± 0.01 | −0.69 c ± 0.01 |
| Coating with rosemary | 0.93 b ± 0.00 | 276.21 b ± 3.31 | 24.88 b ± 0.02 | 0.34 a ± 0.01 | 1.20 a ± 0.02 |
| Coating with thyme | 0.95 a ± 0.00 | 290.41 a ± 5.81 | 23.66 c ± 0.01 | 0.11 b ± 0.01 | 0.11 b ± 0.01 |
| Group | Day | L* (D65) | a* (D65) | b* (D65) | |||
|---|---|---|---|---|---|---|---|
| K | 0 | 50.76 bcd | ±0.25 | −0.95 efg | ±0.17 | 4.53 f | ±0.34 |
| 6 | 48.00 de | ±0.59 | −0.06 cde | ±0.17 | 4.23 f | ±0.18 | |
| 9 | 52.60 ab | ±0.80 | −1.29 fg | ±0.10 | 4.44 f | ±0.30 | |
| 12 | 54.52 a | ±0.81 | −1.60 g | ±0.17 | 6.27 de | ±0.45 | |
| G | 6 | 50.61 bcd | ±0.81 | 1.19 bc | ±0.46 | 7.48 cde | ±0.61 |
| 9 | 48.15 de | ±0.78 | −0.26 | ±0.36 | 6.03 e | ±0.23 | |
| 12 | 51.95 abc | ±1.56 | −0.68 ef | ±0.26 | 6.99 cde | ±0.43 | |
| T | 6 | 40.74 h | ±0.77 | 2.63 a | ±0.22 | 10.43 ab | ±0.50 |
| 9 | 42.96 gh | ±0.61 | 2.06 ab | ±0.26 | 13.65 a | ±0.61 | |
| 12 | 40.53 h | ±0.67 | 3.22 a | ±0.28 | 13.53 a | ±0.58 | |
| R | 6 | 44.47 fg | ±0.82 | 0.62 bcd | ±0.19 | 8.96 bc | ±0.33 |
| 9 | 46.45 ef | ±0.75 | −0.01 cde | ±0.26 | 7.44 cde | ±0.56 | |
| 12 | 48.86 cdb | ±0.36 | −0.06 cde | ±0.14 | 8.23 bcd | ±0.23 | |
| Effect of group | *** | *** | *** | ||||
| Effect of storage time | *** | *** | *** | ||||
| Effect of group × time | *** | *** | *** | ||||
| Day of Storage | Group | Hardness [N] | Adhesiveness | Springiness | Cohesiveness | Chewiness |
|---|---|---|---|---|---|---|
| 0 | K | 16.73 a ± 4.29 | −6.99 abc ± 0.86 | 0.64 a ± 0.05 | 0.54 a ± 0.09 | 5.07 a ± 1.26 |
| 3 | 16.27 a ± 3.18 | −5.84 ab ± 1.42 | 0.55 a ± 0.04 | 0.43 a ± 0.04 | 4.55 ab ± 0.92 | |
| 6 | 21.65 a ± 3.01 | −6.20 ab ± 1.20 | 0.60 a ± 0.03 | 0.44 a ± 0.04 | 6.23 a ± 0.70 | |
| 9 | 20.77 a ± 0.90 | −6.20 ab ± 0.38 | 0.57 a ± 0.03 | 0.45 a ± 0.02 | 5.53 a ±1.24 | |
| 12 | 14.81 ab± 1.10 | −5.27 ab ± 1,21 | 0.58 a ± 0.02 | 0.46 a ± 0.03 | 4.03 ab ± 0.70 | |
| 3 | G | 16.73 ab ±4.29 | −7.67 abc ± 0.96 | 0.59 a ± 0.03 | 0.38 a ± 0.01 | 3.42 ab ± 0.11 |
| 6 | 17.20 a± 2.67 | −7.34 abc ± 1.24 | 0.52 a ± 0.03 | 0.41 a ± 0.02 | 3.97 ab ± 0.71 | |
| 9 | 11.06 ab ± 0.44 | −6.06 ab ± 0.62 | 0.56 a ± 0.02 | 0.44 a ± 0.01 | 2.76 ab ± 0.35 | |
| 12 | 17.86 a ± 0.22 a | −7.70 abc ± 0.90 | 0.58 a ± 0.03 | 0.41 a ± 0.02 | 4.20 ab ± 0.24 | |
| 3 | T | 15.75 ab ± 0.55 | −8.37 abc ± 0.84 | 0.53 a ± 0.03 | 0.42 a ± 0.04 | 3.99 ab ± 0.67 |
| 6 | 12.94 ab ± 0.55 | −8.54 abc ± 1.42 | 0.53 a ± 0.03 | 0.41 a ± 0.03 | 2.79 ab ± 1.52 | |
| 9 | 14.63 a ± 0.43 | −11.29 b ± 1.74 | 0.6 a ± 0.04 | 0.43 a ± 0.01 | 3.82 ab ± 0.33 | |
| 12 | 4.56 b ± 0.14 | −4.41 a ± 0.53 | 0.6 a ± 0.02 | 0.38 a ± 0.01 | 1.05 b ± 0.47 | |
| 3 | R | 13.79 ab ± 1.03 | −7.67 abc ± 0.96 | 0.59 a ± 0.03 | 0.38 a ± 0.02 | 3.98 ab ± 0.41 |
| 6 | 15.27 a ± 1.37 | −11.84 c ± 1.11 | 0.59 a ± 0.01 | 0.44 a ± 0.02 | 4.80 ab ± 0.46 | |
| 9 | 15.77 a ± 0.48 | −10.44 bdc ± 0.80 | 0.62 a ± 0.03 | 0.49 a ± 0.01 | 3.78 ab ± 0.60 | |
| 12 | 13.18 ab ± 0.68 | −9.72 bdc ± 1.20 | 0.63 a ± 0.04 | 0.46 a ± 0.03 | 3.78 ab ± 0.60 | |
| Effect of group | * | *** | Ns | Ns | *** | |
| Effect of storage time | Ns | Ns | Ns | Ns | ||
| Effect of group × time | Ns | *** | Ns | Ns | Ns | |
| Group | Day of Storage | Water Activity | TBARS [mg/kg] |
|---|---|---|---|
| K | 0 | 0.953 e ± 0.00 | 0.40 e ± 0.13 |
| 3 | 0.964 ab ± 0.00 | 1.31 b ± 0.20 | |
| 6 | 0.962 abc ± 0.00 | 1.30 b ± 0.12 | |
| 9 | 0.958 bcde ± 0.00 | 1.35 b ± 0.46 | |
| 12 | 0.959 bcde ± 0.00 | 1.70 a ± 0.26 | |
| G | 3 | 0.954 de ± 0.00 | 0.71 cd ± 0.17 |
| 6 | 0.959 abcde ± 0.00 | 0.86 c ± 0.38 | |
| 9 | 0.956 cde ± 0.00 | 0.69 cd ± 0.12 | |
| 12 | 0.959 bcde ± 0.00 | 1.18 b ± 0.20 | |
| T | 3 | 0.958 bcde ± 0.00 | 0.19 e ± 0.02 |
| 6 | 0.961 abcd ± 0.00 | 0.40 e ± 0.11 | |
| 9 | 0.967 a ± 0.00 | 0.24 e ± 0.09 | |
| 12 | 0.957 bcde ± 0.0 | 0.36 e ± 0.04 | |
| R | 3 | 0.961 dbg ± 0.00 | 0.23 e ± 0.07 |
| 6 | 0.957 bcde ± 0.00 | 0.40 e ± 0.09 | |
| 9 | 0.963 ebi ± 0.00 | 0.20 e ± 0.04 | |
| 12 | 0.958 bcde ± 0.00 | 0.44 de ± 0.05 | |
| Effect of group | *** | *** | |
| Effect of storage time | *** | *** | |
| Effect of group × time | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derbew Gedif, H.; Tkaczewska, J.; Jamróz, E.; Zając, M.; Kasprzak, M.; Pająk, P.; Grzebieniarz, W.; Nowak, N. Developing Technology for the Production of Innovative Coatings with Antioxidant Properties for Packaging Fish Products. Foods 2023, 12, 26. https://doi.org/10.3390/foods12010026
Derbew Gedif H, Tkaczewska J, Jamróz E, Zając M, Kasprzak M, Pająk P, Grzebieniarz W, Nowak N. Developing Technology for the Production of Innovative Coatings with Antioxidant Properties for Packaging Fish Products. Foods. 2023; 12(1):26. https://doi.org/10.3390/foods12010026
Chicago/Turabian StyleDerbew Gedif, Hana, Joanna Tkaczewska, Ewelina Jamróz, Marzena Zając, Mirosław Kasprzak, Paulina Pająk, Wiktoria Grzebieniarz, and Nikola Nowak. 2023. "Developing Technology for the Production of Innovative Coatings with Antioxidant Properties for Packaging Fish Products" Foods 12, no. 1: 26. https://doi.org/10.3390/foods12010026
APA StyleDerbew Gedif, H., Tkaczewska, J., Jamróz, E., Zając, M., Kasprzak, M., Pająk, P., Grzebieniarz, W., & Nowak, N. (2023). Developing Technology for the Production of Innovative Coatings with Antioxidant Properties for Packaging Fish Products. Foods, 12(1), 26. https://doi.org/10.3390/foods12010026








