Isolation of a Novel Anti-Diabetic α-Glucosidase Oligo-Peptide Inhibitor from Fermented Rice Bran
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Seed and Fermentation Broth
2.3. Fermentation Product Extraction
2.4. Determination of α-Glucosidase Inhibitory Activity
2.5. Separation and Purification
2.5.1. Ultrafiltration
2.5.2. DEAE Sepharose Fast Flow Ion Column Chromatography
2.5.3. Sephadex G-25 Column Chromatography
2.6. Identification of Rice Bran Peptides
2.7. Molecular Docking Studies
2.8. α-Glucosidase Inhibition by Synthetic Peptides In Vitro
2.9. Mechanism of α-Glucosidase Inhibition
2.10. In Vitro Simulated Gastrointestinal (GI) Digestion System
2.11. Statistical Analysis
3. Results
3.1. Isolation, Purification, and Identification of Rice Bran Peptides
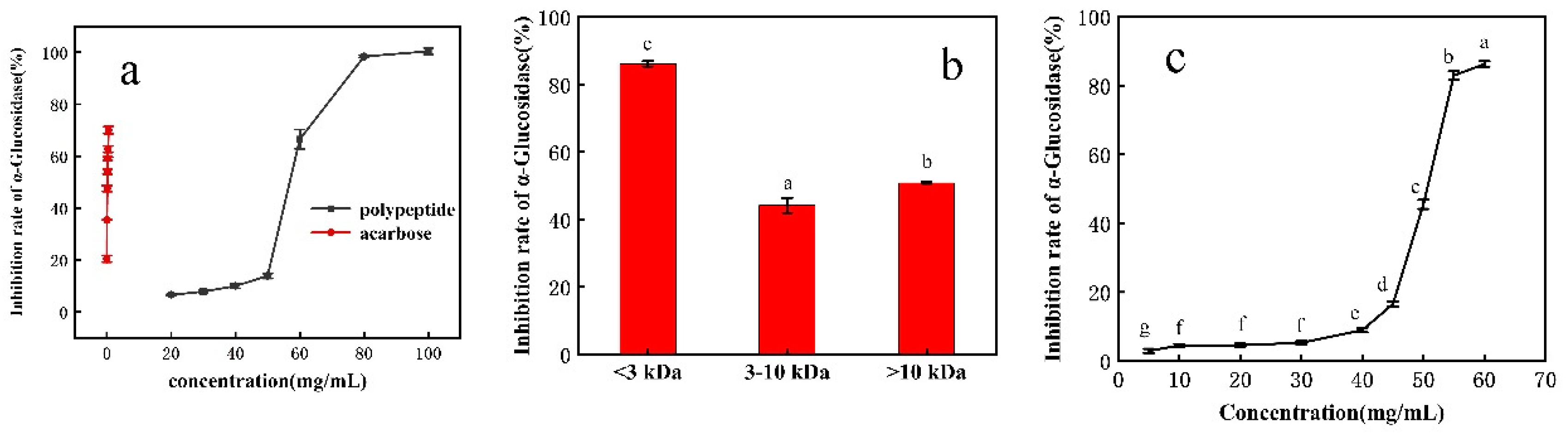
3.1.1. Inhibition Rate of Fermentation Products against α-Glucosidase
3.1.2. α-Glucosidase Inhibitory Activity of Ultrafiltered Rice Bran Peptides
3.1.3. Purification by DEAE Sepharose Fast Flow Ion Column
3.1.4. Purification by Sephadex G-25 Column and Identification
3.2. Identification and Molecular Docking of Rice Bran Peptides
3.3. In Vitro α-Glucosidase Inhibition Activity
3.4. Characteristics of Oligopeptide GLLGY
3.5. α-Glucosidase Inhibitory Mechanism of Oligopeptide GLLGY
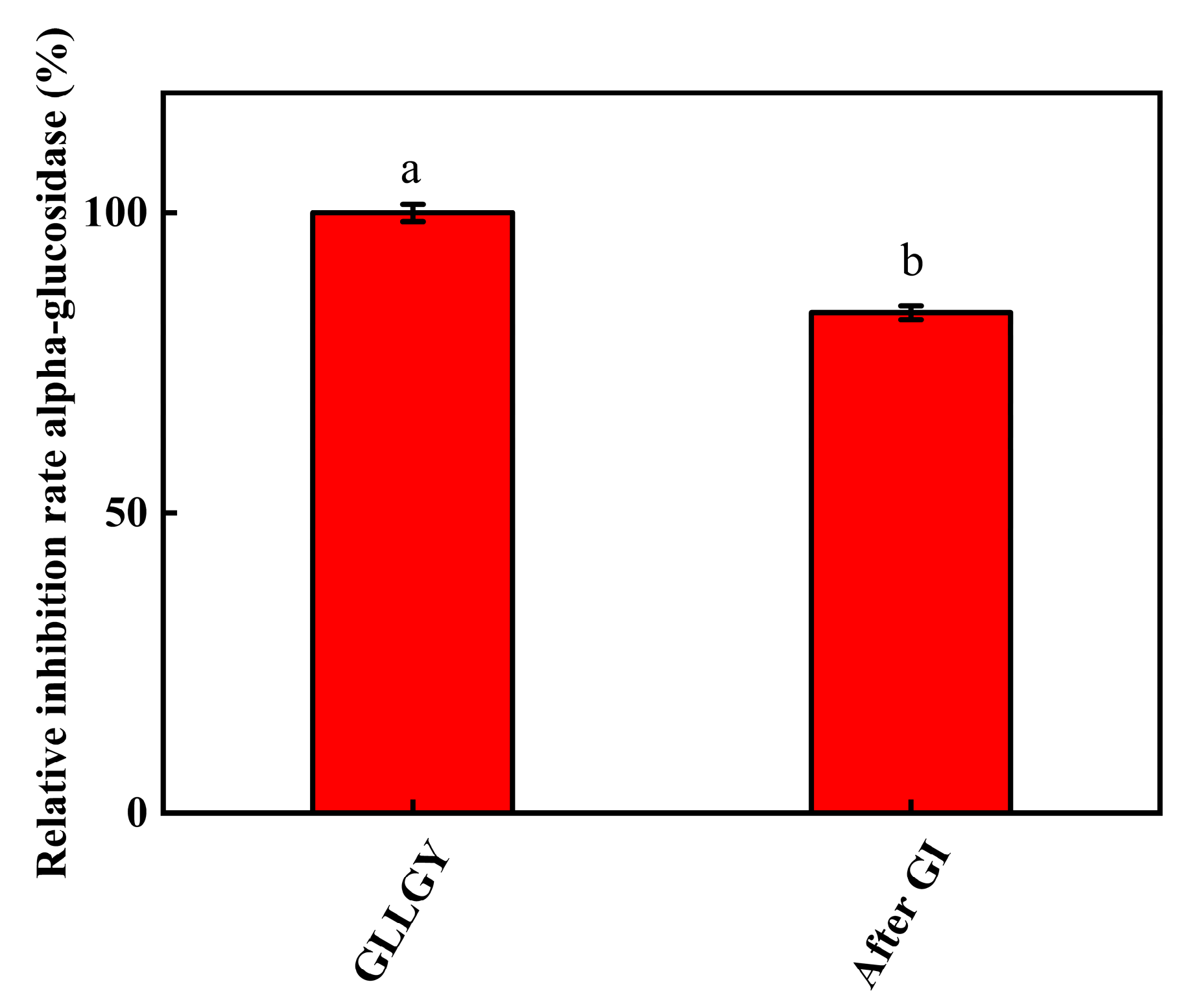
3.6. Effect of Simulated Gastrointestinal (GI) Digestion on the α-Glucosidase Activity of GLLGY
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aamir, A.H.; Ul-Haq, Z.; Mahar, S.A.; Qureshi, F.M.; Ahmad, I.; Jawa, A.; Sheikh, A.; Raza, A.; Fazid, S.; Jadoon, Z.; et al. Diabetes Prevalence Survey of Pakistan (DPS-PAK): Prevalence of type 2 diabetes mellitus and prediabetes using HbA1c: A population-based survey from Pakistan. BMJ Open 2019, 9, e025300. [Google Scholar] [CrossRef] [PubMed]
- Støy, J.; De Franco, E.; Ye, H.; Park, S.Y.; Bell, G.I.; Hattersley, A.T. In celebration of a century with insulin—Update of insulin gene mutations in diabetes. Mol. Metab. 2021, 52, 101280. [Google Scholar] [CrossRef] [PubMed]
- Helfer, G.; Wu, Q.F. Chemerin: A multifaceted adipokine involved in metabolic disorders. J. Endocrinol. 2018, 238, R79–R94. [Google Scholar] [CrossRef]
- Xie, X.; Chen, C.; Fu, X. Screening α-glucosidase inhibitors from four edible brown seaweed extracts by ultra-filtration and molecular docking. LWT 2021, 138, 110654. [Google Scholar] [CrossRef]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef]
- Di Stefano, E.; Oliviero, T.; Udenigwe, C.C. Functional significance and structure–activity relationship of food-derived α-glucosidase inhibitors. Curr. Opin. Food Sci. 2018, 20, 7–12. [Google Scholar] [CrossRef]
- Hu, S.; Fan, X.; Qi, P.; Zhang, X. Identification of anti-diabetes peptides from Spirulina platensis. J. Funct. Foods 2019, 56, 333–341. [Google Scholar] [CrossRef]
- Ramírez Fuentes, L.; Richard, C.; Chen, L. Sequential alcalase and flavourzyme treatment for preparation of α-amylase, α-glucosidase, and dipeptidyl peptidase (DPP)-IV inhibitory peptides from oat protein. J. Funct. Foods 2021, 87, 104829. [Google Scholar] [CrossRef]
- Urbizo-Reyes, U.; Liceaga, A.M.; Reddivari, L.; Kim, K.-H.; Anderson, J.M. Enzyme kinetics, molecular docking, and in silico characterization of canary seed (Phalaris canariensis L.) peptides with ACE and pancreatic lipase inhibitory activity. J. Funct. Foods 2022, 88, 104892. [Google Scholar] [CrossRef]
- Abbasi, S.; Moslehishad, M.; Salami, M. Antioxidant and alpha-glucosidase enzyme inhibitory properties of hydrolyzed protein and bioactive peptides of quinoa. Int. J. Biol. Macromol. 2022, 213, 602–609. [Google Scholar] [CrossRef]
- Elam, E.; Feng, J.; Lv, Y.-M.; Ni, Z.-J.; Sun, P.; Thakur, K.; Zhang, J.-G.; Ma, Y.-L.; Wei, Z.-J. Recent advances on bioactive food derived anti-diabetic hydrolysates and peptides from natural resources. J. Funct. Foods 2021, 86, 104674. [Google Scholar] [CrossRef]
- Park, H.Y.; Lee, K.W.; Choi, H.D. Rice bran constituents: Immunomodulatory and therapeutic activities. Food Funct. 2017, 8, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kumar, G.; Kumar, V.; Singh, D. Enhanced Phytase Production by Bacillus subtilis subsp. subtilis in Solid State Fermentation and its Utility in Improving Food Nutrition. Protein Pept. Lett. 2021, 28, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Anu; Kumar, S.; Kumar, A.; Kumar, V.; Singh, B. Optimization of cellulase production by Bacillus subtilis subsp. subtilis JJBS300 and biocatalytic potential in saccharification of alkaline-pretreated rice straw. Prep. Biochem. Biotechnol. 2021, 51, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Shobako, N.; Ohinata, K. Anti-Hypertensive Effects of Peptides Derived from Rice Bran Protein. Nutrients 2020, 12, 3060. [Google Scholar] [CrossRef] [PubMed]
- Uraipong, C.; Zhao, J. Rice bran protein hydrolysates exhibit strong in vitro α-amylase, β-glucosidase and ACE-inhibition activities. J. Sci. Food Agric. 2016, 96, 1101–1110. [Google Scholar] [CrossRef]
- Manzoor, M.; Singh, J.; Gani, A. Exploration of bioactive peptides from various origin as promising nutraceutical treasures: In vitro, in silico and in vivo studies. Food Chem. 2022, 373, 131395. [Google Scholar] [CrossRef]
- Kumar, S.; Bhardwaj, V.K.; Singh, R.; Das, P.; Purohit, R. Identification of acridinedione scaffolds as potential inhibitor of DENV-2 C protein: An in silico strategy to combat dengue. J. Cell Biochem. 2022, 123, 935–946. [Google Scholar] [CrossRef]
- Kumar Bhardwaj, V.; Purohit, R.; Kumar, S. Himalayan bioactive molecules as potential entry inhibitors for the human immunodeficiency virus. Food Chem. 2021, 347, 128932. [Google Scholar] [CrossRef]
- Singh, R.; Bhardwaj, V.K.; Sharma, J.; Purohit, R.; Kumar, S. In-silico evaluation of bioactive compounds from tea as potential SARS-CoV-2 nonstructural protein 16 inhibitors. J. Tradit. Complement. Med. 2022, 12, 35–43. [Google Scholar] [CrossRef]
- Rajendran, V.; Sethumadhavan, R. Drug resistance mechanism of PncA in Mycobacterium tuberculosis. J. Biomol. Struct. Dyn. 2014, 32, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, V. Structural analysis of oncogenic mutation of isocitrate dehydrogenase 1. Mol. Biosyst. 2016, 12, 2276–2287. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, V.; Gopalakrishnan, C.; Sethumadhavan, R. Pathological role of a point mutation (T315I) in BCR-ABL1 protein-A computational insight. J. Cell. Biochem. 2018, 119, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, Y.; Zhao, A.; Wu, Y.; Zhang, Y.; Yang, X. Quantitative analyses for several nutrients and volatile components during fermentation of soybean by Bacillus subtilis natto. Food Chem. 2022, 374, 131725. [Google Scholar] [CrossRef]
- Su, W.; Jiang, Z.; Wang, C.; Xu, B.; Lu, Z.; Wang, F.; Zong, X.; Jin, M.; Wang, Y. Dynamics of defatted rice bran in physicochemical characteristics, microbiota and metabolic functions during two-stage co-fermentation. Int. J. Food Microbiol. 2022, 362, 109489. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Guo, Y.; Xu, W.; Wang, W.; Wu, S.; Chen, W.; Huang, Y. Label-free quantification proteomics reveals the active peptides from protein degradation during anaerobic fermentation of tea. LWT 2021, 150, 111950. [Google Scholar] [CrossRef]
- Cecile Urbain Marie, G.; Perreault, V.; Henaux, L.; Carnovale, V.; Aluko, R.E.; Marette, A.; Doyen, A.; Bazinet, L. Impact of a high hydrostatic pressure pretreatment on the separation of bioactive peptides from flaxseed protein hydrolysates by electrodialysis with ultrafiltration membranes. Sep. Purif. Technol. 2019, 211, 242–251. [Google Scholar] [CrossRef]
- Zhao, Q.; Wei, G.; Li, K.; Duan, S.; Ye, R.; Huang, A. Identification and molecular docking of novel α-glucosidase inhibitory peptides from hydrolysates of Binglangjiang buffalo casein. LWT 2022, 156, 113062. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.; Li, X. Novel peptides with α-glucosidase inhibitory activity from Changii Radix hydrolysates. Process Biochem. 2021, 111, 200–206. [Google Scholar] [CrossRef]
- Jiang, M.; Hui, Y.; He, R.; Ma, Y. Purification and a molecular docking study of α-glucosidase-inhibitory peptides from a soybean protein hydrolysate with ultrasonic pretreatment. Eur. Food Res. Technol. 2018, 244, 1995–2005. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Bougatef, A.; Nedjar-Arroume, N.; Manni, L.; Ravallec, R.; Barkia, A.; Guillochon, D.; Nasri, M. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 2010, 118, 559–565. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Bester, M.J.; Neitz, A.W.H.; Gaspar, A.R.M. Structural properties of bioactive peptides with α-glucosidase inhibitory activity. Chem. Biol. Drug Des. 2018, 91, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Deng, Z.; Zhao, C.; Xiao, T.; Weng, C.; Li, J.; Zheng, L. Nutritional composition and proteomic analysis of soft-shelled turtle (Pelodiscus sinensis) egg and identification of oligopeptides with alpha-glucosidase inhibitory activity. Food Res. Int. 2021, 145, 110414. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Bester, M.J.; Neitz, A.W.; Gaspar, A.R.M. Rational in silico design of novel α-glucosidase inhibitory peptides and in vitro evaluation of promising candidates. Biomed Pharm. 2018, 107, 234–242. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Qiao, S. Advances in research on solid-state fermented feed and its utilization: The pioneer of private customization for intestinal microorganisms. Anim. Nutr. 2021, 7, 905–916. [Google Scholar] [CrossRef]
- Rocchetti, G.; Miragoli, F.; Zacconi, C.; Lucini, L.; Rebecchi, A. Impact of cooking and fermentation by lactic acid bacteria on phenolic profile of quinoa and buckwheat seeds. Food Res. Int. 2019, 119, 886–894. [Google Scholar] [CrossRef]
- Wei, G.; Zhao, Q.; Wang, D.; Fan, Y.; Shi, Y.; Huang, A. Novel ACE inhibitory, antioxidant and α-glucosidase inhibitory peptides identified from fermented rubing cheese through peptidomic and molecular docking. LWT 2022, 159, 113196. [Google Scholar] [CrossRef]
- Li, M.; Bao, X.; Zhang, X.; Ren, H.; Cai, S.; Hu, X.; Yi, J. Exploring the phytochemicals and inhibitory effects against α-glucosidase and dipeptidyl peptidase-IV in Chinese pickled chili pepper: Insights into mechanisms by molecular docking analysis. LWT 2022, 162, 113467. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oboh, G.; Boligon, A.A.; Athayde, M.L. Effect of fermented soybean condiment supplemented diet on α-amylase and α-glucosidase activities in Streptozotocin-induced diabetic rats. J. Funct. Foods 2014, 9, 1–9. [Google Scholar] [CrossRef]
- Gu, X.; Gao, T.; Hou, Y.; Li, D.; Fu, L. Identification and characterization of two novel α-glucosidase inhibitory peptides from almond (Armeniaca sibirica) oil manufacture residue. LWT-Food Sci. Technol. 2020, 134, 110215. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. Changes in the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates during a simulated gastrointestinal digestion. Food Chem. 2010, 120, 810–816. [Google Scholar] [CrossRef]
- Ahmed, T.; Sun, X.; Udenigwe, C.C. Role of structural properties of bioactive peptides in their stability during simulated gastrointestinal digestion: A systematic review. Trends Food Sci. Technol. 2022, 120, 265–273. [Google Scholar] [CrossRef]
- Ju, H.; Chen, H.; Xiang, A.; Wang, Y.; Yue, T.; Yuan, Y. Identification and characterization of Lactobacillus paracasei strain MRS-4 antibacterial activity against Alicyclobacillus acidoterrestris. LWT 2021, 150, 111991. [Google Scholar] [CrossRef]
- Ren, Y.; Liang, K.; Jin, Y.; Zhang, M.; Chen, Y.; Wu, H.; Lai, F. Identification and characterization of two novel α-glucosidase inhibitory oligopeptides from hemp (Cannabis sativa L.) seed protein. J. Funct. Foods 2016, 26, 439–450. [Google Scholar] [CrossRef]
- Ur Rehman, N.; Rafiq, K.; Khan, A.; Ahsan Halim, S.; Ali, L.; Al-Saady, N.; Hilal Al-Balushi, A.; Al-Busaidi, H.K.; Al-Harrasi, A. α-Glucosidase Inhibition and Molecular Docking Studies of Natural Brominated Metabolites from Marine Macro Brown Alga Dictyopteris hoytii. Mar. Drugs 2019, 17, 666. [Google Scholar] [CrossRef]
- Roig-Zamboni, V.; Cobucci-Ponzano, B.; Iacono, R.; Ferrara, M.C.; Germany, S.; Bourne, Y.; Parenti, G.; Moracci, M.; Sulzenbacher, G. Structure of human lysosomal acid α-glucosidase-a guide for the treatment of Pompe disease. Nat. Commun. 2017, 8, 1111. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yin, Y.; Zhao, W.; Yu, Y.; Liu, B.; Liu, J.; Chen, F. Novel peptides derived from egg white protein inhibiting alpha-glucosidase. Food Chem. 2011, 129, 1376–1382. [Google Scholar] [CrossRef]




| Peptides | Scan Time (min) | Accurate Mass (Da) | Protein Family | Score | Intensity | Binding Energy (kcal/mol) | Binding Residues of α-Glucosidase | Hydrogen Bond Distance (Å) | Number of Hydrogen Bonds |
|---|---|---|---|---|---|---|---|---|---|
| Acarbose | −7.4 | Asp282 Asp281 Asp404 Arg600 Asp616 His674 | 2.3/1.9 3.4/2.9 2.8 3.4/3.0 2.0/1.7 3.2/3.4 | 11 | |||||
| LFSGF | 60.4865 | 570.292 | ORYSJ Cellulose synthase-like protein | 79.7 | 0 | −8.0 | Asp282 Ala284 Asp616 | 3.5 3.0 2.1/2.4/2.6/3.2 | 6 |
| HWP | 54.567 | 439.209 | ORYSJ Mixed-linked glucan synthase | 193.6 | 193.6 | −7.8 | Arg600 Asp616 His674 | 3.1 1.9/2.1/3.2 3.2 | 5 |
| QSFF | 55.3714 | 528.248 | ORYSJ protein | 286.6 | 336,170 | −7.7 | Asp282 Ser523 Asn524 Asp616 | 2.3/3.0/3.5 3.0 2.3/3.5 2.6 | 7 |
| FPF | 61.4677 | 410.207 | BACSU PTS system oligo-beta-mannoside-specific EIIC component | 85.3 | 0 | −7.6 | Asp616 | 1.9/3.4 | 2 |
| FSGF | 51.4358 | 457.209 | ORYSJ Cellulose synthase-like protein | 249.9 | 534,630 | −7.6 | Asp282 Asp284 Ser523 Asn524 Ala555 | 2.9 3.5 2.9/2.4/3.1 3.3 3.3 | 7 |
| VYGF | 50.7739 | 485.241 | ORYSJ Cellulose synthase-like protein | 219.3 | 982,140 | −7.6 | Asp282 Asp518 Asp616 | 2.4/3.5 2.5 2.3 | 4 |
| GLIGY | 50.3365 | 522.292 | BACIU Phage tail length tape-measure protein | 171.7 | 594,040 | −7.2 | Asp282 Asp404 Ser523 Ala555 Asp616 His674 | 2.0/2.9 2.1 1.8/3.3 2.5 1.9/3.3 2.9 | 9 |
| GLLGY | 50.3365 | 522.292 | ORYSJ Phospholipase A1-II | 171.7 | 594,040 | −7.1 | Asp282 Ser523 Asp616 His674 | 3.1 2.3 2.2/3.2 2.9 | 5 |
| KGDPY | 51.7567 | 579.293 | BACSU Aspartokinase 1 | 88.8 | 0 | −6.4 | Asp282 Asp404 Asp616 His674 | 2.1/2.2 2.0 2.1/3.4 2.9 | 6 |
| QSFLQRYYFLFRILPL | 55.3714 | 2104.154 | ORYSJ Protein kinase domain-containing protein | 142.1 | 336,170 | −6.4 | Arg281 Asp282 Ala284 Pro285 Phe525 Arg527 Trp618 Glu622 Gln623 | 3.4/3.0/3.1 2.3/2.4 3.5 3.2 3.4 3.4 3.4 2.3 3.0 | 12 |
| PSR | 59.1375 | 359.218 | ORYSA DNA-directed RNA polymerase subunit α | 8.0 | 2,1243,000 | −6.3 | Asp282 Ser523 Arg600 Asp616 | 2.4/3.1/3.4/3.6 2.1/2.2/2.3 3.2 3.3/3.4 | 10 |
| ISIFLSFFFGLIAGT | 60.6946 | 1632.906 | BACIU Amino acid ABC transporter permease | 82.8 | 2,322,000 | −6.2 | Arg281 Asp282 Asp518 Asn524 Arg600 | 2.8/3.3 1.9 2.4/3.2 3.3/3.3 3.2 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Lai, X.; Wu, X.; Wang, H.; Weng, N.; Lu, J.; Lyu, M.; Wang, S. Isolation of a Novel Anti-Diabetic α-Glucosidase Oligo-Peptide Inhibitor from Fermented Rice Bran. Foods 2023, 12, 183. https://doi.org/10.3390/foods12010183
Hu J, Lai X, Wu X, Wang H, Weng N, Lu J, Lyu M, Wang S. Isolation of a Novel Anti-Diabetic α-Glucosidase Oligo-Peptide Inhibitor from Fermented Rice Bran. Foods. 2023; 12(1):183. https://doi.org/10.3390/foods12010183
Chicago/Turabian StyleHu, Jingfei, Xiaohua Lai, Xudong Wu, Huanyu Wang, Nanhai Weng, Jing Lu, Mingsheng Lyu, and Shujun Wang. 2023. "Isolation of a Novel Anti-Diabetic α-Glucosidase Oligo-Peptide Inhibitor from Fermented Rice Bran" Foods 12, no. 1: 183. https://doi.org/10.3390/foods12010183
APA StyleHu, J., Lai, X., Wu, X., Wang, H., Weng, N., Lu, J., Lyu, M., & Wang, S. (2023). Isolation of a Novel Anti-Diabetic α-Glucosidase Oligo-Peptide Inhibitor from Fermented Rice Bran. Foods, 12(1), 183. https://doi.org/10.3390/foods12010183








