Identification of Optimal Fermentation Temperature for Dry-Fermented Sausage Using Strains Isolated from Korean Fermented Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Preparation
2.2. Sausage Manufacturing and Sampling
2.3. Microbial Analysis
2.4. pH
2.5. Moisture Content
2.6. Color
2.7. TBARS
2.8. VBN
2.9. Electronic Nose (E-Nose)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Microbial Population
3.2. pH
3.3. Moisture Content
3.4. Color
3.5. TBARS
3.6. VBN
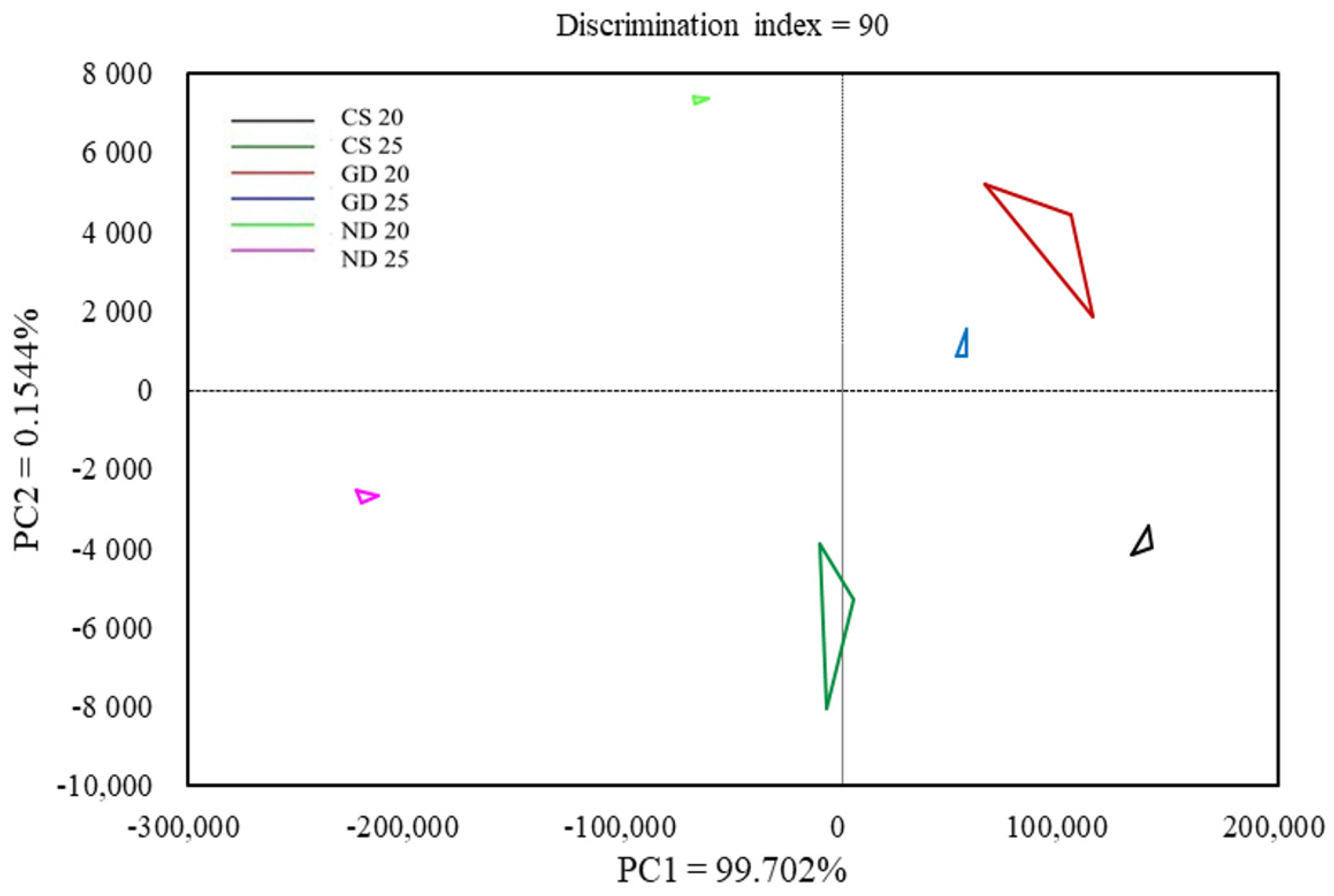
3.7. Electronic Nose (E-Nose)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montanari, C.; Barbieri, F.; Gardini, F.; Tabanelli, G. Competition between starter cultures and wild microbial population in sausage fermentation: A case study regarding a typical Italian Salami (Ventricina). Foods 2021, 10, 2138. [Google Scholar] [CrossRef] [PubMed]
- García-Cano, I.; Rocha-Mendoza, D.; Ortega-Anaya, J.; Wang, K.; Kosmerl, E.; Jiménez-Flores, R. Lactic acid bacteria isolated from dairy products as potential producers of lipolytic, proteolytic and antibacterial proteins. Appl. Microbiol. Biotechnol. 2019, 103, 5243–5257. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tian, Y.; Zhu, J.; Wen, R.; Chen, Q.; Kong, B. Technological characterization and flavor-producing potential of lactic acid bacteria isolated from traditional dry fermented sausages in northeast China. Food Microbiol. 2022, 106, 104059. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Kong, B.; Han, Q.; Liu, Q.; Xu, L. The role of bacterial fermentation in the hydrolysis and oxidation of sarcoplasmic and myofibrillar proteins in Harbin dry sausages. Meat Sci. 2016, 121, 196–206. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Wen, R.; Chen, Q.; Kong, B. Role of lactic acid bacteria in flavour development in traditional Chinese fermented foods: A review. Criti. Rev. Food Sci. Nutr. 2022, 62, 2741–2755. [Google Scholar] [CrossRef]
- Chen, Q.; Kong, B.; Han, Q.; Xia, X.; Xu, L. The role of bacterial fermentation in lipolysis and lipid oxidation in Harbin dry sausages and its flavour development. LWT Food Sci. Technol. 2017, 77, 389–396. [Google Scholar] [CrossRef]
- Shukla, S.; Lee, J.S.; Park, H.K.; Yoo, J.A.; Hong, S.Y.; Kim, J.K.; Kim, M. Effect of novel starter culture on reduction of biogenic amines, quality improvement, and sensory properties of Doenjang, a traditional Korean soybean fermented sauce variety. J. Food Sci. 2015, 80, M1794–M1803. [Google Scholar] [CrossRef]
- Yun, Y.R.; Lee, J.J.; Lee, H.J.; Choi, Y.J.; Lee, J.H.; Park, S.J.; Park, S.H.; Seo, H.Y.; Min, S.G. Comparison of quality characteristics of commercial kimchi manufactured in Korea, China, and the United States. Foods 2021, 10, 2488. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, Y.; Yoon, Y. Lactic acid bacteria in kimchi might be a cause for carcinogen production in intestine. Food Control 2021, 126, 108045. [Google Scholar] [CrossRef]
- Jeong, D.M.; Yoo, S.J.; Jeon, M.S.; Chun, B.H.; Han, D.M.; Jeon, C.O.; Eyun, S.I.; Seo, Y.J.; Kang, H.A. Genomic features, aroma profiles, and probiotic potential of the Debaryomyces hansenii species complex strains isolated from Korean soybean fermented food. Food Microbiol. 2022, 105, 104011. [Google Scholar] [CrossRef]
- Yeong, M.S.; Hee, M.S.; Choon, C.H. Characterization of high-ornithine-producing Weissella koreensis DB1 isolated from kimchi and its application in rice bran fermentation as a starter culture. Foods 2020, 9, 1545. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.W.; Lee, B.; Her, J.Y.; Lee, K.G.; Lee, J.H. Safety and technological characterization of coagulase-negative staphylococci isolates from traditional Korean fermented soybean foods for starter development. Int. J. Food Microbiol. 2016, 236, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mi, R.; Qi, B.; Xiong, S.; Li, J.; Qu, C.; Chen, W.; Wang, S. Effect of proteolytic starter culture isolated from Chinese Dong fermented pork (Nanx Wudl) on microbiological, biochemical and organoleptic attributes in dry fermented sausages. Food Sci. Hum. Wellness 2021, 10, 13–22. [Google Scholar] [CrossRef]
- Cebrián, E.; Núñez, F.; Álvarez, M.; Roncero, E.; Rodríguez, M. Biocontrol of ochratoxigenic Penicillium nordicum in dry-cured fermented sausages by Debaryomyces hansenii and Staphylococcus xylosus. Int. J. Food Microbiol. 2022, 375, 109744. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Liu, Q.; Wang, Y.; Chen, Q.; Kong, B. The potential correlation between bacterial diversity and the characteristic volatile flavour of traditional dry sausages from Northeast China. Food Microbiol. 2020, 91, 103505. [Google Scholar] [CrossRef]
- Wen, R.; Sun, F.; Li, X.; Chen, Q.; Kong, B. The potential correlations between the fungal communities and volatile compounds of traditional dry sausages from Northeast China. Food Microbiol. 2021, 98, 103787. [Google Scholar] [CrossRef]
- Yang, E.; Fan, L.; Yan, J.; Jiang, Y.; Doucette, C.; Fillmore, S.; Walker, B. Influence of culture media, pH and temperature on growth and bacteriocin production of bacteriocinogenic lactic acid bacteria. Amb Express 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Zarzecka, U.; Zadernowska, A.; Chajęcka-Wierzchowska, W. Starter cultures as a reservoir of antibiotic resistant microorganisms. LWT 2020, 127, 109424. [Google Scholar] [CrossRef]
- Jeong, C.H.; Lee, S.H.; Kim, H.Y. Microbiological composition and sensory characterization analysis of fermented sausage using strains isolated from Korean fermented foods. Food Sci Anim. Res. 2022, 42, 928–941. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Gaithersburg Association of Official Analytical Chemists International: Rockville, MD, USA, 2006. [Google Scholar]
- Jeong, C.H.; Lee, S.H.; Kim, H.Y. Analysis of food storage stability of biodegradable containers made of pork skin gelatin polymer with walnut shell powder. Polymers 2022, 14, 1940. [Google Scholar] [CrossRef]
- Conway, E.J.; O’malley, E. Microdiffusion methods. Ammonia and urea using buffered absorbents (revised methods for ranges greater than 10 μg. N). Biochem. J. 1942, 36, 655. [Google Scholar] [CrossRef] [PubMed]
- Go, H.Y.; Lee, S.H.; Kim, H.Y. The effect of hot-air dried Lentinula edodes on the quality and oranoleptic properties of rolled-dumplings. Food Sci. Anim. Resour. 2022, 42, 593. [Google Scholar] [CrossRef]
- Yu, D.; Feng, M.Q.; Sun, J. Influence of mixed starters on the degradation of proteins and the formation of peptides with antioxidant activities in dry fermented sausages. Food Control 2021, 123, 107743. [Google Scholar] [CrossRef]
- Bora, B. The role of lactic acid bacteria in the production of fermented meat products. Vigyan Varta 2022, 3, 13–15. [Google Scholar]
- Agüero, N.D.L.; Frizzo, L.S.; Ouwehand, A.C.; Aleu, G.; Rosmini, M.R. Technological characterization of probiotic lactic acid bacteria as starter cultures for dry fermented sausages. Foods 2020, 9, 596. [Google Scholar] [CrossRef] [PubMed]
- Kurt, Ş. The effects of grape seed flour on the quality of Turkish dry fermented sausage (Sucuk) during ripening and refrigerated storage. Korean J. Food Sci. Anim. Resour. 2016, 36, 300. [Google Scholar] [CrossRef]
- Wang, Y.; He, L.; Xing, Y.; Zhou, W.; Pian, R.; Yang, F.; Chen, X.; Zhang, Q. Bacterial diversity and fermentation quality of Moringa oleifera leaves silage prepared with lactic acid bacteria inoculants and stored at different temperatures. Bioresour. Technol. 2019, 284, 349–358. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, P.; Zhou, Y.; Ma, F.; Chen, C. Effect of inoculating Lactobacillus pentosus R3 on N-nitrosamines and bacterial communities in dry fermented sausages. Food Control 2018, 87, 126–134. [Google Scholar] [CrossRef]
- Hilbig, J.; Hildebrandt, L.; Herrmann, K.; Weiss, J.; Loeffler, M. Influence of homopolysaccharide-producing lactic acid bacteria on the spreadability of raw fermented sausages (onion mettwurst). J. Food Sci. 2020, 85, 289–297. [Google Scholar] [CrossRef]
- Wang, N.; Huang, D.; Shao, M.; Sun, R.; Xu, Q. Use of activated carbon to reduce ammonia emissions and accelerate humification in composting digestate from food waste. Bioresour. Technol. 2022, 347, 126701. [Google Scholar] [CrossRef]
- Canon, F.; Nidelet, T.; Guédon, E.; Thierry, A.; Gagnaire, V. Understanding the mechanisms of positive microbial interactions that benefit lactic acid bacteria co-cultures. Front. Microbiol. 2020, 11, 2088. [Google Scholar] [CrossRef]
- Taoukis, P.S.; Richardson, M. Principles of Intermediate-Moisture Foods and Related Technology. In Water Activity in Foods: Fundamentals and Applications; BarbosaCanovas, G.V., Fontana, A.J., Jr., Schmidt, S.J., Labuza, T.P., Eds.; Blackwell Publishing Professional: Ames, IA, USA, 2007; pp. 273–312. [Google Scholar]
- Ministry of Food and Drug Safety. Available online: https://www.foodsafetykorea.go.kr/foodcode/03_02.jsp?idx=37 (accessed on 23 September 2022).
- Hughes, J.M.; Clarke, F.M.; Purslow, P.P.; Warner, R.D. Meat color is determined not only by chromatic heme pigments but also by the physical structure and achromatic light scattering properties of the muscle. Compr. Rev. Food Sci. Food Saf. 2020, 19, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Eisinaitė, V.; Tamkutė, L.; Vinauskienė, R.; Leskauskaitė, D. Freeze-dried celery as an indirect source of nitrate in cold-smoked sausages: Effect on safety and color formation. LWT 2020, 129, 109586. [Google Scholar] [CrossRef]
- Liu, J.; Mesfin, F.M.; Hunter, C.E.; Olson, K.R.; Shelley, W.C.; Markel, T.A. Recent development of the molecular and cellular mechanisms of hydrogen sulfide gasotransmitter. Antioxidants 2022, 11, 1788. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.I.; Han, M.; Suh, J.; Yoo, S.J.; Lee, H.; Jo, C. Effect of dielectric barrier discharge plasma on the discoloration of myoglobin. In Proceedings of the 62nd International Congress of Meat Science and Technology, Bangkok, Thailand, 14–19 August 2016; pp. 60–63. [Google Scholar]
- Van Ba, H.; Seo, H.W.; Kim, J.H.; Cho, S.H.; Kim, Y.S.; Ham, J.S.; Park, B.Y.; Kim, H.Y.; Kim, T.B.; Seong, P.N. The effects of starter culture types on the technological quality, lipid oxidation and biogenic amines in fermented sausages. LWT 2016, 74, 191–198. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Reitznerová, A.; Šuleková, M.; Nagy, J.; Marcinčák, S.; Semjon, B.; Čertík, M.; Klempová, T. Lipid peroxidation process in meat and meat products: A comparison study of malondialdehyde determination between modified 2-thiobarbituric acid spectrophotometric method and reverse-phase high-performance liquid chromatography. Molecules 2017, 22, 1988. [Google Scholar] [CrossRef]
- Zhao, B.; Zhou, H.; Zhang, S.; Pan, X.; Li, S.; Zhu, N.; Wu, Q.; Wang, S.; Qiao, X.; Chen, W. Changes of protein oxidation, lipid oxidation and lipolysis in Chinese dry sausage with different sodium chloride curing salt content. Food Sci. Hum. Wellness 2020, 9, 328–337. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Domínguez, R.; Purriños, L.; Pérez-Santaescolástica, C.; Pateiro, M.; Barba, F.J.; Tomasevic, I.; Campagnol, P.C.B.; Lorenzo, J.M. Characterization of volatile compounds of dry-cured meat products using HS-SPME-GC/MS technique. Food Anal. Methods 2019, 12, 1263–1284. [Google Scholar] [CrossRef]
- Jin, S.K.; Jeong, H.J.; Choi, J.S. Effect of surimi on quality characteristics of dry and semi-dry (non-fermented) pork sausages during cold storage. J. Food Meas. Charact. 2017, 11, 1370–1377. [Google Scholar] [CrossRef]
- Seleshe, S.; Kang, S.N. Effect of different Pediococcus pentosaceus and Lactobacillus plantarum strains on quality characteristics of dry fermented sausage after completion of ripening period. Food Sci. Anim. Resour. 2021, 41, 636. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Y.; Li, X.A.; Zhang, H.; Chen, Q.; Kong, B. Application of lactic acid bacteria for improving the quality of reduced-salt dry fermented sausage: Texture, color, and flavor profiles. LWT 2022, 154, 112723. [Google Scholar] [CrossRef]
- Wen, R.; Yin, X.; Hu, Y.; Chen, Q.; Kong, B. Technological properties and flavour formation potential of yeast strains isolated from traditional dry fermented sausages in Northeast China. LWT 2022, 154, 112853. [Google Scholar] [CrossRef]
- Sidira, M.; Kandylis, P.; Kanellaki, M.; Kourkoutas, Y. Effect of immobilized Lactobacillus casei on volatile compounds of heat treated probiotic dry-fermented sausages. Food Chem. 2015, 178, 201–207. [Google Scholar] [CrossRef]
- Ferrocino, I.; Bellio, A.; Giordano, M.; Macori, G.; Romano, A.; Rantsiou, K.; Decastelli, L.; Cocolin, L. Shotgun metagenomics and volatilome profile of the microbiota of fermented sausages. Appl. Environ. Microbiol. 2018, 84, e02120-17. [Google Scholar] [CrossRef]
| Trait | Time (Days) | CS 5 | GD 6 | ND 7 | SEM 8 | |||
|---|---|---|---|---|---|---|---|---|
| 20 °C | 25 °C | 20 °C | 25 °C | 20 °C | 25 °C | |||
| AC 1 | 0 | 4.33 Cb | 4.33 Cb | 4.86 Ca | 4.86 Ca | 5.02 Ca | 5.02 Da | 0.10 |
| 3 | 7.91 Bc | 9.07 Aa | 7.70 Bd | 7.84 Ac | 7.91 Ac | 8.31 Ab | 0.10 | |
| 10 | 7.97 Bc | 8.80 Aa | 8.07 Ac | 7.96 Ac | 7.97 Ac | 8.42 Ab | 0.21 | |
| 17 | 8.38 Aa | 8.29 Ba | 7.84 ABb | 7.77 Ab | 7.78 ABb | 8.14 Ba | 0.19 | |
| 24 | 8.10 ABa | 8.11 Ba | 7.71 Bb | 7.49 Bb | 7.67 Bb | 8.04 Ba | 0.16 | |
| 31 | 7.89 Ba | 8.10 Ba | 7.56 Bb | 7.49 Bb | 7.58 Bb | 7.86 Ba | 0.16 | |
| SEM | 0.20 | 0.11 | 0.19 | 0.15 | 0.09 | 0.17 | ||
| LABC 2 | 0 | 3.96 Db | 3.96 Bb | 4.70 Ca | 4.70 Da | 4.56 Ea | 4.56 Da | 0.15 |
| 3 | 8.47 ABb | 9.21 Aa | 7.57 Bc | 8.89 Aa | 6.83 Dd | 8.34 Cb | 0.23 | |
| 10 | 8.66 Abc | 9.13 Aa | 8.00 Ad | 8.86 ABb | 8.48 Ac | 8.78 Ab | 0.15 | |
| 17 | 8.67 Ab | 9.15 Aa | 7.96 Ac | 8.74 ABCb | 7.98 ABc | 8.70 ABb | 0.15 | |
| 24 | 8.19 Bc | 9.18 Aa | 7.97 Ad | 8.71 BCb | 7.54 BCe | 8.66 ABb | 0.13 | |
| 31 | 7.85 Cc | 9.14 Aa | 7.52 Bd | 8.69 Cb | 7.45 Cd | 8.53 Bb | 0.16 | |
| SEM | 0.16 | 0.26 | 0.22 | 0.10 | 0.10 | 0.13 | ||
| STPC 3 | 0 | 3.44 Ca | 3.44 Ca | 3.60 Da | 3.60 Da | 3.61 Ca | 3.61 Ca | 0.09 |
| 3 | 3.59 Cd | 3.70 Cd | 5.36 Cc | 6.02 Cb | 5.55 Bc | 6.43 Ba | 0.19 | |
| 10 | 3.71 BCd | 4.47 Bc | 5.94 Bb | 6.21 Cb | 5.88 ABb | 7.24 Aa | 0.33 | |
| 17 | 3.84 BCe | 4.47 Bd | 6.03 Bc | 6.82 Bb | 6.10 Ac | 7.24 Aa | 0.19 | |
| 24 | 4.25 Bc | 4.49 Bc | 6.09 Bb | 7.05 ABa | 6.28 Ab | 7.25 Aa | 0.27 | |
| 31 | 4.94 Ac | 4.95 Ac | 6.42 Ab | 7.21 Aa | 6.37 Ab | 7.51 Aa | 0.23 | |
| SEM | 0.16 | 0.30 | 0.26 | 0.18 | 0.18 | 0.23 | ||
| YMC 4 | 0 | 4.76 Fb | 4.76 Cb | 5.27 Da | 5.27 Da | 5.09 Ca | 5.09 Da | 0.11 |
| 3 | 5.21 Ed | 5.64 Bc | 5.69 CDc | 6.13 Cb | 6.57 Ba | 6.08 Cb | 0.11 | |
| 10 | 5.91 Db | 6.05 ABb | 6.15 Cb | 6.33 Cab | 6.63 Ba | 6.17 Cb | 0.21 | |
| 17 | 6.42 Cab | 6.06 ABb | 7.21 Ba | 6.36 Cab | 6.72 Bab | 6.61 Bab | 0.33 | |
| 24 | 6.74 Bb | 6.03 ABc | 7.47 Ba | 6.91 Bb | 6.85 Bb | 6.63 Bb | 0.24 | |
| 31 | 7.17 Ab | 6.51 Ac | 8.05 Aa | 7.52 Aab | 7.52 Aab | 7.58 Aab | 0.27 | |
| SEM | 0.31 | 0.25 | 0.10 | 0.20 | 0.10 | 0.32 | ||
| Time (Days) | CS 1 | GD 2 | ND 3 | SEM 4 | |||
|---|---|---|---|---|---|---|---|
| 20 °C | 25 °C | 20 °C | 25 °C | 20 °C | 25 °C | ||
| 0 | 5.93 Aa | 5.93 Aa | 5.96 Aa | 5.96 Aa | 5.94 Aa | 5.94 Aa | 0.02 |
| 3 | 5.67 Bc | 4.89 Be | 5.83 Ba | 5.29 Bd | 5.78 Cab | 5.73 Bbc | 0.06 |
| 10 | 4.79 De | 4.64 Cf | 5.38 Eb | 5.03 Cd | 5.78 Ca | 5.12 Dc | 0.04 |
| 17 | 4.75 De | 4.66 Cf | 5.33 Eb | 4.94 Cd | 5.70 Da | 5.09 Dc | 0.05 |
| 24 | 4.79 De | 4.69 Cf | 5.61 Db | 5.07 Cd | 5.82 Ba | 5.19 Dc | 0.06 |
| 31 | 4.99 Ce | 4.67 Cf | 5.76 Cb | 5.03 Cd | 5.96 Aa | 5.53 Cc | 0.05 |
| SEM | 0.05 | 0.05 | 0.05 | 0.07 | 0.02 | 0.05 | |
| Time (Days) | CS 1 | GD 2 | ND 3 | SEM 4 | |||
|---|---|---|---|---|---|---|---|
| 20 °C | 25 °C | 20 °C | 25 °C | 20 °C | 25 °C | ||
| 0 | 64.71 Aa | 64.71 Aa | 64.71 Aa | 64.71 Aa | 64.71 Aa | 64.71 Aa | 2.06 |
| 3 | 57.75 Ba | 54.87 Bbc | 57.67 Ba | 53.01 Bc | 56.97 Bab | 54.45 Bc | 1.41 |
| 10 | 48.24 Ca | 41.23 Cc | 43.74 Cb | 39.70 Cd | 44.74 Cb | 43.97 Cb | 0.84 |
| 17 | 42.29 Da | 36.53 Db | 42.09 Ca | 36.47 Db | 41.43 Da | 35.33 Db | 1.04 |
| 24 | 37.84 Ea | 25.81 Ec | 38.14 Da | 31.35 Eb | 39.62 Da | 23.58 Ec | 1.42 |
| 31 | 35.82 Ea | 23.39 Ed | 32.49 Eb | 28.86 Fc | 34.53 Ea | 23.23 Ed | 1.14 |
| SEM | 1.16 | 1.63 | 1.36 | 1.09 | 1.34 | 1.33 | |
| Trait | Time (Days) | CS 1 | GD 2 | ND 3 | SEM 4 | |||
|---|---|---|---|---|---|---|---|---|
| 20 °C | 25 °C | 20 °C | 25 °C | 20 °C | 25 °C | |||
| CIE L* | 0 | 61.10 Aa | 61.10 Aa | 61.10 Aa | 61.10 Aa | 61.10 Aa | 61.10 Aa | 0.36 |
| 3 | 58.20 Ba | 52.92 Bd | 56.04 Bab | 52.27 Bd | 54.85 Bbc | 53.30 Bcd | 1.30 | |
| 10 | 58.57 Ba | 53.82 Bb | 55.37 Bb | 50.38 BCc | 54.47 Bb | 48.27 Cc | 1.44 | |
| 17 | 56.33 Ca | 47.82 Cb | 54.92 Ba | 48.76 Cb | 55.35 Ba | 44.7 Dc | 1.67 | |
| 24 | 55.13 Ca | 47.38 Cb | 54.32 Ba | 48.25 Cb | 53.85 Ba | 45.33 Dc | 1.55 | |
| 31 | 51.73 Da | 47.65 Cb | 51.70 Ca | 45.82 Dbc | 50.70 Ca | 45.28 Dc | 1.50 | |
| SEM | 1.20 | 1.75 | 1.12 | 1.18 | 1.60 | 0.96 | ||
| CIE a* | 0 | 7.00 Aa | 7.00 Aa | 7.00 Aa | 7.00 Aa | 7.00 Aa | 7.00 Aa | 0.20 |
| 3 | 6.97 Aa | 4.60 Bc | 6.30 ABab | 6.27 ABab | 6.13 ABab | 5.60 Bbc | 0.62 | |
| 10 | 5.75 Aa | 4.78 Ba | 5.80 BCa | 5.82 Ba | 5.50 BCa | 4.88 Ca | 0.83 | |
| 17 | 6.37 Aa | 4.60 Bb | 4.97 CDb | 4.28 Cb | 5.08 Cb | 4.70 Cb | 0.62 | |
| 24 | 6.28 Aa | 3.72 Bcd | 4.58 CDbc | 3.67 Cd | 4.60 CDb | 4.58 Cbc | 0.60 | |
| 31 | 4.00 Ba | 3.58 Ba | 4.23 Da | 3.80 Ca | 3.70 Da | 4.25 Ca | 0.95 | |
| SEM | 0.73 | 0.78 | 0.64 | 0.72 | 0.61 | 0.34 | ||
| CIE b* | 0 | 6.97 Ea | 6.97 Ea | 6.97 Ba | 6.97 Ca | 6.97 Ca | 6.97 Ba | 0.15 |
| 3 | 8.00 Db | 9.45 Da | 8.30 Ab | 9.35 Ba | 8.38 Bb | 9.47 Aa | 0.39 | |
| 10 | 8.14 CDb | 9.72 CDa | 8.53 Ab | 9.40 Ba | 8.40 ABb | 9.80 Aa | 0.52 | |
| 17 | 8.46 BCc | 10.40 BCa | 8.43 Ac | 9.40 Bb | 8.48 ABc | 9.95 Aa | 0.32 | |
| 24 | 8.85 ABc | 10.33 ABa | 8.43 Ac | 9.47 Bb | 8.53 ABc | 9.93 Aa | 0.36 | |
| 31 | 9.25 Ac | 11.33 Aa | 8.68 Ad | 10.08 Ab | 8.73 Ad | 10.00 Ab | 0.31 | |
| SEM | 0.34 | 0.42 | 0.38 | 0.15 | 0.31 | 0.26 | ||
| Time (Days) | CS 1 | GD 2 | ND 3 | SEM 4 | |||
|---|---|---|---|---|---|---|---|
| 20 °C | 25 °C | 20 °C | 25 °C | 20 °C | 25 °C | ||
| 0 | 0.61 Da | 0.61 Ea | 0.61 Da | 0.61 Da | 0.61 Ca | 0.61 Ea | 0.04 |
| 3 | 0.58 Dc | 1.44 Da | 0.66 Dbc | 1.41 Da | 0.67 Cbc | 0.97 Db | 0.12 |
| 10 | 0.87 Dc | 4.90 Ca | 0.72 Dc | 3.37 Db | 0.76 Cc | 1.09 Dc | 0.23 |
| 17 | 2.70 Cd | 5.06 Ca | 1.47 Ce | 4.49 Cb | 1.13 Be | 3.27 Cc | 0.22 |
| 24 | 3.21 Bd | 7.06 Ba | 1.63 Be | 4.64 Bb | 1.30 Be | 3.98 Bc | 0.23 |
| 31 | 4.35 Ad | 7.58 Aa | 2.12 Ae | 5.23 Ac | 1.68 Af | 6.71 Ab | 0.18 |
| SEM | 0.18 | 0.11 | 0.07 | 0.37 | 0.08 | 0.21 | |
| Time (Days) | CS 1 | GD 2 | ND 3 | SEM 4 | |||
|---|---|---|---|---|---|---|---|
| 20 °C | 25 °C | 20 °C | 25 °C | 20 °C | 25 °C | ||
| 0 | 2.18 Da | 2.18 Fa | 2.18 Da | 2.18 Ea | 2.18 Da | 2.18 Fa | 0.21 |
| 3 | 4.20 Cc | 4.82 Ec | 4.63 Cc | 6.33 Da | 5.67 Cb | 6.72 Ea | 0.29 |
| 10 | 5.60 Bd | 6.65 Dc | 7.50 Bb | 7.95 Cab | 7.06 Bc | 8.36 Da | 0.35 |
| 17 | 5.94 ABe | 7.22 Cd | 7.84 ABc | 8.66 Bb | 7.09 Bd | 9.18 Ca | 0.18 |
| 24 | 6.16 Ae | 7.99 Bc | 7.84 ABcd | 9.13 ABb | 7.39 Bd | 10.42 Ba | 0.31 |
| 31 | 6.44 Ad | 9.24 Ab | 8.36 Ac | 9.56 Ab | 8.46 Ac | 11.57 Aa | 0.40 |
| SEM | 0.30 | 0.23 | 0.30 | 0.36 | 0.24 | 0.32 | |
| Expected Volatile Compounds | CS 1 | GD 2 | ND 3 | SEM 4 | |||
|---|---|---|---|---|---|---|---|
| 20 °C | 25 °C | 20 °C | 25 °C | 20 °C | 25 °C | ||
| Acetaldehyde | 4774.68 | 860.51 | 4193.42 | 5824.64 | 1693.92 | 2312.24 | 24.31 |
| Ethanol | 258,474.64 | 166,689.25 | 230,930.89 | 205,370.92 | 126,849.58 | 27,718.30 | 820.27 |
| Propan-2-one | 16,124.12 | 13,101.51 | 9816.87 | 9347.94 | 5343.18 | 10,098.84 | 88.85 |
| Butanal | 282.15 | 1959.60 | 499.20 | 2293.30 | 824.75 | 691.50 | 13.31 |
| Butan-2-one | 2223.46 | 1205.25 | 1084.56 | 1172.71 | 1237.28 | 828.16 | 20.77 |
| Ethyl acetate | 6869.33 | 5603.10 | 3333.27 | 4883.84 | 2849.78 | 488.18 | 22.17 |
| Acetoin | 139.09 | 3028.29 | 440.63 | 4098.40 | 1052.01 | 885.65 | 24.51 |
| Hexanal | 3045.24 | 2207.68 | 1033.54 | 869.48 | 358.95 | 1005.81 | 14.67 |
| Hexanoic acid | 709.14 | 1856.97 | 594.75 | 1766.51 | 535.34 | 1687.16 | 7.19 |
| p-Cymene | 597.16 | 1848.63 | 508.64 | 1899.63 | 453.26 | 1822.97 | 6,28 |
| Limonene | 697.44 | 1725.32 | 576.28 | 1683.38 | 507.83 | 1717.92 | 5.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, C.-H.; Lee, S.-H.; Yoon, Y.; Choi, H.-Y.; Kim, H.-Y. Identification of Optimal Fermentation Temperature for Dry-Fermented Sausage Using Strains Isolated from Korean Fermented Foods. Foods 2023, 12, 137. https://doi.org/10.3390/foods12010137
Jeong C-H, Lee S-H, Yoon Y, Choi H-Y, Kim H-Y. Identification of Optimal Fermentation Temperature for Dry-Fermented Sausage Using Strains Isolated from Korean Fermented Foods. Foods. 2023; 12(1):137. https://doi.org/10.3390/foods12010137
Chicago/Turabian StyleJeong, Chang-Hwan, Sol-Hee Lee, Yohan Yoon, Hyung-Youn Choi, and Hack-Youn Kim. 2023. "Identification of Optimal Fermentation Temperature for Dry-Fermented Sausage Using Strains Isolated from Korean Fermented Foods" Foods 12, no. 1: 137. https://doi.org/10.3390/foods12010137
APA StyleJeong, C.-H., Lee, S.-H., Yoon, Y., Choi, H.-Y., & Kim, H.-Y. (2023). Identification of Optimal Fermentation Temperature for Dry-Fermented Sausage Using Strains Isolated from Korean Fermented Foods. Foods, 12(1), 137. https://doi.org/10.3390/foods12010137






