Content Variations in Oleocanthalic Acid and Other Phenolic Compounds in Extra-Virgin Olive Oil during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solvents and Analytical Standards
2.2. Instruments
2.3. EVOO Samples, Storage Conditions, and Phytoextracts Preparation
2.4. Analysis of Phenolic Compounds Content
2.5. Determination of Total Phenolic Content
3. Results and Discussion
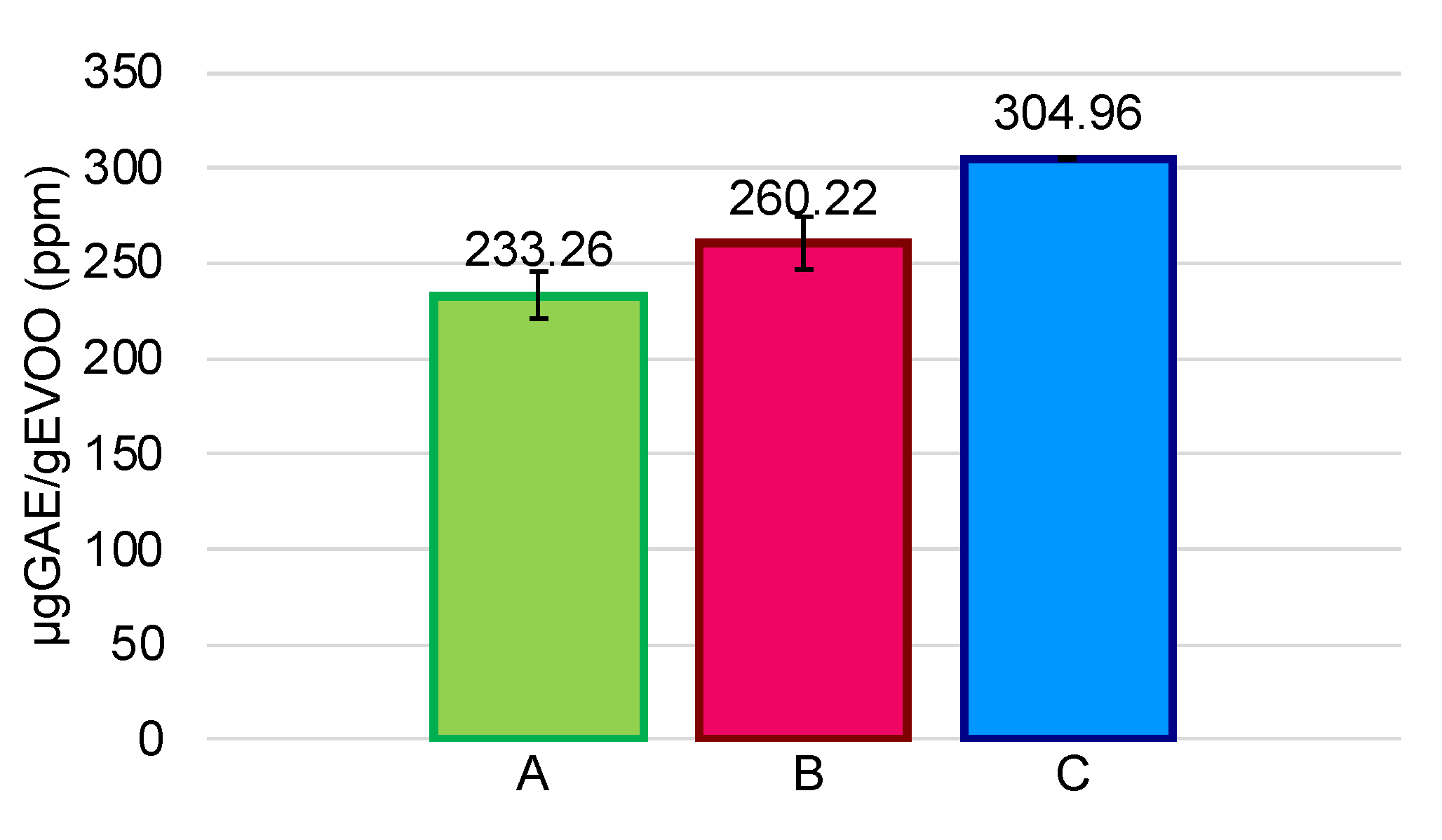
3.1. Initial Phenolic Content in EVOOs
3.2. Variations in the Phenolic Content in EVOOs during Storage
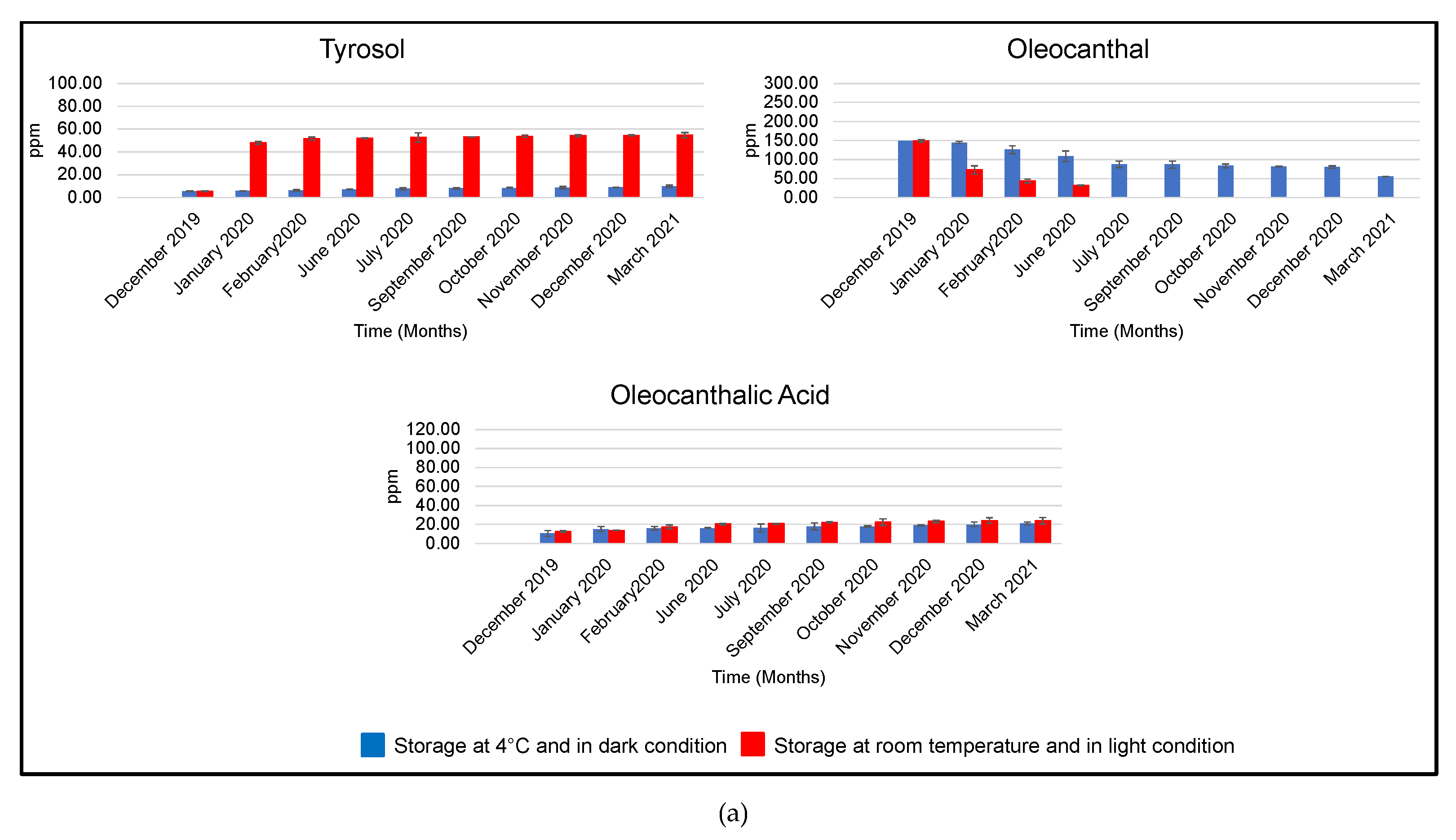
3.2.1. EVOOs Stored at 25 °C and Exposed to Light
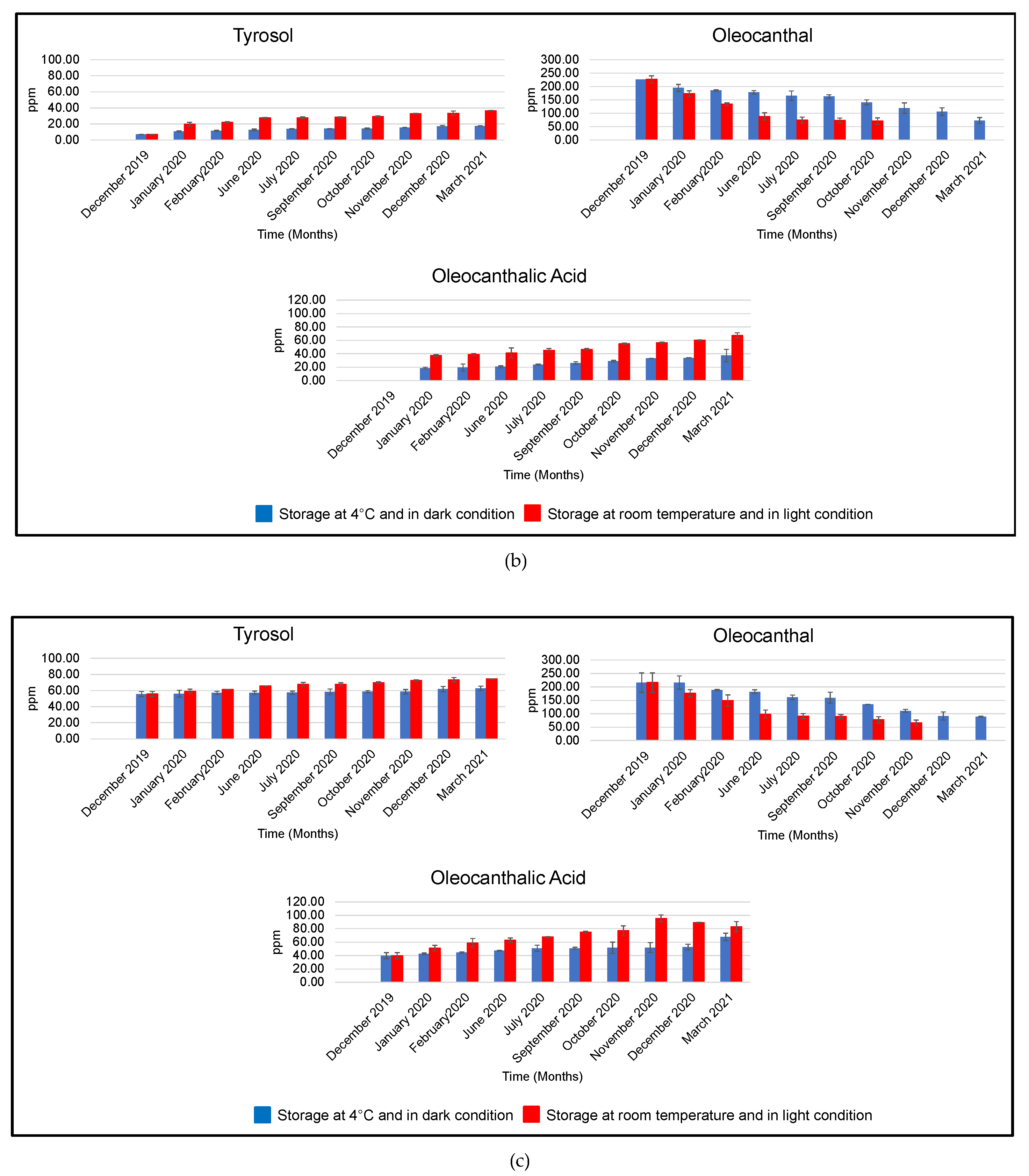
3.2.2. EVOOs Stored at 4 °C and in Darkness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parkinson, L.; Cicerale, S. The Health Benefiting Mechanisms of Virgin Olive Oil Phenolic Compounds. Molecules 2016, 21, 1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, P.A.L.; Marcadenti, A.; Portal, V.L. Effects of Olive Oil Phenolic Compounds on Inflammation in the Prevention and Treatment of Coronary Artery Disease. Nutrients 2017, 9, 1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reboredo-Rodríguez, P.; Varela-López, A.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Zhang, J.; Manna, P.P.; Bompadre, S.; Quiles, J.L.; et al. Phenolic Compounds Isolated from Olive Oil as Nutraceutical Tools for the Prevention and Management of Cancer and Cardiovascular Diseases. Int. J. Mol. Sci. 2018, 19, 2305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Morató, J.; Xicota, L.; Fitó, M.; Farré, M.; Dierssen, M.; de la Torre, R. Potential Role of Olive Oil Phenolic Compounds in the Prevention of Neurodegenerative Diseases. Molecules 2015, 20, 4655–4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scientific Opinion on the Substantiation of Health Claims Related to Polyphenols in Olive and Protection of LDL Particles from Oxidative Damage (ID 1333, 1638, 1639, 1696, 2865), Maintenance of Normal Blood HDL Cholesterol Concentrations (ID 1639), Maintenance of Normal Blood Pressure (ID 3781), …. Available online: https://www.efsa.europa.eu/it/efsajournal/pub/2033 (accessed on 15 February 2022).
- Kotsiou, K.; Tasioula-Margari, M. Monitoring the Phenolic Compounds of Greek Extra-Virgin Olive Oils during Storage. Food Chem. 2016, 200, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Palla, M.; Digiacomo, M.; Cristani, C.; Bertini, S.; Giovannetti, M.; Macchia, M.; Manera, C.; Agnolucci, M. Composition of Health-Promoting Phenolic Compounds in Two Extra Virgin Olive Oils and Diversity of Associated Yeasts. J. Food Compos. Anal. 2018, 74, 27–33. [Google Scholar] [CrossRef]
- Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Veneziani, G.; Di Maio, I.; Sordini, B.; Servili, M. Effect of Light Exposure on the Quality of Extra Virgin Olive Oils According to Their Chemical Composition. Food Chem. 2017, 229, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Mariotti, R.; Stanzione, V.; Pandolfi, S.; Mastio, V.; Baldoni, L.; Cultrera, N.G.M. Evolution of Extra Virgin Olive Oil Quality under Different Storage Conditions. Foods 2021, 10, 1945. [Google Scholar] [CrossRef] [PubMed]
- Tsolakou, A.; Diamantakos, P.; Kalaboki, I.; Mena-Bravo, A.; Priego-Capote, F.; Abdallah, I.M.; Kaddoumi, A.; Melliou, E.; Magiatis, P. Oleocanthalic Acid, a Chemical Marker of Olive Oil Aging and Exposure to a High Storage Temperature with Potential Neuroprotective Activity. J. Agric. Food Chem. 2018, 66, 7337–7346. [Google Scholar] [CrossRef] [PubMed]
- Esposito Salsano, J.; Pinto, D.; Rodrigues, F.; Saba, A.; Manera, C.; Digiacomo, M.; Macchia, M. Oleocanthalic Acid from Extra-Virgin Olive Oil: Analysis, Preparative Isolation and Radical Scavenging Activity. J. Food Compos. Anal. 2022, 105, 104160. [Google Scholar] [CrossRef]
- Gabbia, D.; Carpi, S.; Sarcognato, S.; Cannella, L.; Colognesi, M.; Scaffidi, M.; Polini, B.; Digiacomo, M.; Esposito Salsano, J.; Manera, C.; et al. The Extra Virgin Olive Oil Polyphenol Oleocanthal Exerts Antifibrotic Effects in the Liver. Front. Nutr. 2021, 8, 715183. [Google Scholar] [CrossRef] [PubMed]
- Carpi, S.; Polini, B.; Manera, C.; Digiacomo, M.; Salsano, J.E.; Macchia, M.; Scoditti, E.; Nieri, P. MiRNA Modulation and Antitumor Activity by the Extra-Virgin Olive Oil Polyphenol Oleacein in Human Melanoma Cells. Front. Pharmacol. 2020, 11, 574317. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Arena, C.; Carpi, S.; Polini, B.; Bertini, S.; Digiacomo, M.; Gado, F.; Saba, A.; Saccomanni, G.; Breschi, M.C.; et al. Cytotoxic Activity of Oleocanthal Isolated from Virgin Olive Oil on Human Melanoma Cells. Nutr. Cancer 2016, 68, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, S.; Ieri, F.; Romani, A. Minor Polar Compounds in Extra Virgin Olive Oil: Correlation between HPLC-DAD-MS and the Folin-Ciocalteu Spectrophotometric Method. J. Agric. Food Chem. 2014, 62, 826–835. [Google Scholar] [CrossRef] [PubMed]


| Total Time (min) | A% | B% |
|---|---|---|
| 0.0 | 95 | 5 |
| 45.0 | 70 | 30 |
| 65.0 | 30 | 70 |
| 70.0 | 20 | 80 |
| 85.0 | 20 | 80 |
| 90.0 | - | 100 |
| 95.0 | 95 | 5 |
| 105.0 | 95 | 5 |
| Compound | Calibration Curve | r2 |
|---|---|---|
| Gallic Acid | y = 0.0098 + 0.098 | 0.995 |
| Month | EVOO | Hydroxytyrosol | Tyrosol | Oleacein | Oleocanthal | Oleocanthalic Acid |
|---|---|---|---|---|---|---|
| December 2019 | A | 4.72 ± 0.11 | 5.47 ± 0.25 | 116.09 ± 18.39 | 148.93 ± 3.04 | 10.61 ± 2.96 |
| B | 5.67 ± 0.01 | 7.28 ± 0.01 | 110.69 ± 9.80 | 226.36 ± 13.30 | - | |
| C | 34.11 ± 0.57 | 55.62 ± 3.10 | 94.49 ± 3.75 | 216.12 ± 36.44 | 39.72 ± 4.59 | |
| January 2020 | A | 5.51 ± 0.12 | 5.86 ± 0.14 | 103.40 ± 8.20 | 144.54 ± 10.46 | 15.20 ± 2.64 |
| B | 7.58 ± 0.29 | 10.84 ± 0.67 | 105.01 ± 0.46 | 194.94 ± 2.73 | 18.63 ± 1.20 | |
| C | 35.78 ± 0.99 | 55.96 ± 4.36 | 92.84 ± 3.00 | 216.00 ± 24.76 | 42.65 ± 1.07 | |
| February 2020 | A | 5.74 ± 0.18 | 6.31 ± 0.59 | 97.67 ± 1.71 | 125.72 ± 13.67 | 15.94 ± 1.64 |
| B | 8.41 ± 0.48 | 11.71 ± 0.75 | 98.94 ± 5.98 | 184.92 ± 6.47 | 19.40 ± 5.22 | |
| C | 36.40 ± 0.19 | 57.04 ± 1.96 | 90.00 ± 11.43 | 188.85 ± 1.99 | 44.42 ± 1.00 | |
| June 2020 | A | 7.40 ± 0.18 | 7.22 ± 0.16 | 95.33 ± 2.26 | 108.82 ± 8.50 | 16.32 ± 0.28 |
| B | 9.38 ± 0.57 | 12.76 ± 0.91 | 91.73 ± 11.47 | 178.15 ± 17.65 | 20.76 ± 1.33 | |
| C | 36.59 ± 1.31 | 57.17 ± 2.12 | 85.67 ± 9.61 | 182.22 ± 6.60 | 47.25 ± 0.67 | |
| July 2020 | A | 7.52 ± 0.59 | 7.72 ± 0.87 | 71.36 ± 16.28 | 87.42 ± 9.13 | 16.44 ± 4.12 |
| B | 10.14 ± 0.50 | 13.97 ± 0.23 | 89.15 ± 6.18 | 165.78 ± 6.77 | 23.83 ± 0.57 | |
| C | 38.73 ± 1.92 | 57.45 ± 1.89 | 79.76 ± 8.72 | 160.95 ± 8.91 | 50.71 ± 4.62 | |
| September 2020 | A | 7.98 ± 0.12 | 8.03 ± 0.49 | 62.31 ± 5.46 | 86.42 ± 5.36 | 17.85 ± 3.68 |
| B | 10.77 ± 0.15 | 14.10 ± 0.22 | 86.56 ± 4.05 | 162.87 ± 9.20 | 26.40 ± 1.81 | |
| C | 40.29 ± 2.53 | 58.30 ± 3.58 | 76.02 ± 13.20 | 159.92 ± 20.37 | 50.89 ± 1.40 | |
| October 2020 | A | 8.68 ± 0.56 | 8.51 ± 0.23 | 61.05 ± 7.18 | 82.93 ± 0.53 | 17.89 ± 0.76 |
| B | 11.08 ± 0.34 | 14.46 ± 0.60 | 75.88 ± 11.64 | 140.88 ± 19.27 | 28.90 ± 1.36 | |
| C | 33.71 ± 0.80 | 58.58 ± 1.30 | 66.60 ± 3.96 | 135.52 ± 0.06 | 51.50 ± 8.51 | |
| November 2020 | A | 8.85 ± 0.22 | 8.59 ± 1.01 | 49.98 ± 6.12 | 81.20 ± 3.01 | 19.09 ± 0.61 |
| B | 11.42 ± 0.57 | 15.58 ± 0.48 | 60.02 ± 0.37 | 119.80 ± 13.71 | 33.16 ± 0.20 | |
| C | 33.50 ± 1.98 | 58.60 ± 2.77 | 55.39 ± 5.96 | 110.86 ± 5.88 | 52.00 ± 7.34 | |
| December 2020 | A | 8.90 ± 0.04 | 8.88 ± 0.20 | 49.98 ± 5.90 | 80.29 ± 0.40 | 20.03 ± 2.43 |
| B | 13.18 ± 0.02 | 17.45 ± 0.80 | 55.63 ± 9.77 | 106.64 ± 11.16 | 33.80 ± 0.25 | |
| C | 32.89 ± 0.07 | 61.73 ± 3.28 | 51.24 ± 4.67 | 91.75 ± 15.06 | 52.68 ± 4.02 | |
| March 2021 | A | 10.10 ± 0.91 | 9.80 ± 1.09 | 37.74 ± 4.14 | 55.34 ± 4.36 | 21.16 ± 1.42 |
| B | 13.74 ± 0.25 | 17.52 ± 0.25 | 39.57 ± 6.39 | 72.65 ± 0.53 | 37.39 ± 9.10 | |
| C | 31.61 ± 3.04 | 62.70 ± 2.73 | 43.66 ± 4.57 | 89.27 ± 2.60 | 67.83 ± 5.55 |
| Month | EVOO | Hydroxytyrosol | Tyrosol | Oleacein | Oleocanthal | Oleocanthalic Acid |
|---|---|---|---|---|---|---|
| December 2019 | A | 4.72 ± 0.11 | 5.47 ± 0.25 | 116.09 ± 18.39 | 148.93 ± 3.04 | 10.61 ± 2.96 |
| B | 5.67 ± 0.01 | 7.28 ± 0.01 | 110.69 ± 9.80 | 226.36 ± 13.30 | - | |
| C | 34.11 ± 0.57 | 55.62 ± 3.10 | 94.49 ± 3.75 | 216.12 ± 36.44 | 39.72 ± 4.59 | |
| January 2020 | A | 27.30 ± 0.74 | 47.80 ± 1.21 | 42.72 ± 3.10 | 72.45 ± 10.51 | 13.70 ± 0.17 |
| B | 14.53 ± 0.34 | 19.73 ± 2.43 | 83.22 ± 19.83 | 173.17 ± 11.17 | 37.54 ± 1.51 | |
| C | 36.85 ± 1.70 | 59.15 ± 2.53 | 72.24 ± 7.05 | 176.47 ± 13.33 | 51.07 ± 4.20 | |
| February 2020 | A | 27.09 ± 0.07 | 51.66 ± 1.33 | 40.96 ± 3.99 | 43.38 ± 5.26 | 17.48 ± 1.92 |
| B | 16.34 ± 0.18 | 22.14 ± 0.63 | 65.30 ± 16.09 | 134.09 ± 4.94 | 38.75 ± 1.66 | |
| C | 37.06 ± 0.04 | 61.43 ± 0.02 | 66.23 ± 12.31 | 149.64 ± 20.87 | 58.72 ± 6.79 | |
| June 2020 | A | 29.89 ± 0.05 | 51.74 ± 0.28 | 16.20 ± 1.05 | 31.58 ± 1.15 | 20.37 ± 0.95 |
| B | 18.93 ± 2.77 | 27.70 ± 0.61 | 55.30 ± 19.06 | 88.56 ± 13.35 | 41.28 ± 7.31 | |
| C | 38.44 ± 3.91 | 65.76 ± 0.03 | 52.96 ± 7.33 | 98.93 ± 15.17 | 63.13 ± 3.07 | |
| July 2020 | A | 28.64 ± 0.54 | 52.63 ± 4.18 | N.D. | N.D. | 20.64 ± 0.55 |
| B | 22.20 ± 4.19 | 27.78 ± 1.00 | 43.45 ± 11.00 | 74.50 ± 11.13 | 45.26 ± 2.39 | |
| C | 40.29 ± 0.35 | 67.69 ± 2.35 | 36.93 ± 2.29 | 91.33 ± 9.36 | 67.71 ± 0.46 | |
| September 2020 | A | 27.36 ± 1.34 | 53.14 ± 0.13 | N.D. | N.D. | 22.04 ± 0.67 |
| B | 21.28 ± 0.17 | 28.43 ± 0.57 | 43.37 ± 18.58 | 73.59 ± 8.80 | 46.70 ± 1.22 | |
| C | 41.78 ± 0.73 | 67.89 ± 1.89 | 33.72 ± 19.08 | 89.72 ± 7.23 | 75.11 ± 1.23 | |
| October 2020 | A | 27.36 ± 0.42 | 53.66 ± 1.00 | N.D. | N.D. | 22.39 ± 3.43 |
| B | 20.86 ± 0.47 | 29.29 ± 0.71 | 35.62 ± 10.56 | 71.31 ± 11.69 | 54.95 ± 0.83 | |
| C | 34.06 ± 0.88 | 69.77 ± 0.96 | 33.44 ± 0.40 | 77.73 ± 11.28 | 77.16 ± 7.27 | |
| November 2020 | A | 25.30 ± 0.01 | 54.18 ± 0.91 | N.D. | N.D. | 23.11 ± 1.25 |
| B | 20.36 ± 0.90 | 33.09 ± 0.34 | N.D. | N.D. | 56.64 ± 0.92 | |
| C | 31.95 ± 6.21 | 72.39 ± 0.90 | N.D. | 66.34 ± 10.36 | 95.56 ± 5.21 | |
| December 2020 | A | 19.22 ± 0.92 | 54.51 ± 0.34 | N.D. | N.D. | 23.96 ± 2.98 |
| B | 20.18 ± 0.63 | 33.54 ± 2.72 | N.D. | N.D. | 60.39 ± 0.68 | |
| C | 31.88 ± 7.04 | 73.47 ± 2.78 | N.D. | N.D. | 89.11 ± 0.15 | |
| March 2021 | A | 17.91 ± 2.80 | 54.76 ± 2.25 | N.D. | N.D. | 24.00 ± 6.85 |
| B | 18.50 ± 0.29 | 36.63 ± 0.19 | N.D. | N.D. | 67.23 ± 3.84 | |
| C | 30.49 ± 1.24 | 74.38 ± 0.06 | N.D. | N.D. | 83.27 ± 7.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito Salsano, J.; Digiacomo, M.; Cuffaro, D.; Bertini, S.; Macchia, M. Content Variations in Oleocanthalic Acid and Other Phenolic Compounds in Extra-Virgin Olive Oil during Storage. Foods 2022, 11, 1354. https://doi.org/10.3390/foods11091354
Esposito Salsano J, Digiacomo M, Cuffaro D, Bertini S, Macchia M. Content Variations in Oleocanthalic Acid and Other Phenolic Compounds in Extra-Virgin Olive Oil during Storage. Foods. 2022; 11(9):1354. https://doi.org/10.3390/foods11091354
Chicago/Turabian StyleEsposito Salsano, Jasmine, Maria Digiacomo, Doretta Cuffaro, Simone Bertini, and Marco Macchia. 2022. "Content Variations in Oleocanthalic Acid and Other Phenolic Compounds in Extra-Virgin Olive Oil during Storage" Foods 11, no. 9: 1354. https://doi.org/10.3390/foods11091354
APA StyleEsposito Salsano, J., Digiacomo, M., Cuffaro, D., Bertini, S., & Macchia, M. (2022). Content Variations in Oleocanthalic Acid and Other Phenolic Compounds in Extra-Virgin Olive Oil during Storage. Foods, 11(9), 1354. https://doi.org/10.3390/foods11091354






