Abstract
Nowadays, there is an increased interest in coffee derivatives (green beans, roasted beans, and coffee by-products (Cascara and Silverskin)) due to their particular chemical composition. This study aimed to compare the content of dry matter, total fat, fatty acids, and fiber (ADF, NDF) of coffee by-products (Cascara and Silverskin) and coffee beans (green and roasted under different conditions). Coffee beans and their by-products were obtained from 100% C. arabica coffee cherries from Panama by dry process. The lowest concentrations of fat corresponded to Cascara 4.24 g·kg−1 and Silverskin 23.70 g·kg−1, respectively. The major fatty acids detected in all samples were palmitic, stearic, oleic, and linoleic acids, the latter two being essential fatty acids. LDA showed that 89.01% of the variability between beans and by-products was explained by lignoceric, myristic, behenic, tricosanoic, arachidic, and heneicosanoic acids. Silverskin appeared to be a good source of lignoceric, myristic, and behenic acids and had a higher concentration of dietary fiber (314.95 g·kg−1) than Cascara (160.03 g·kg−1). Coffee by-products (Silverskin and Cascara) are low-fat products enriched in dietary fiber. Their incorporation, after adjustment, into the global diet may contribute to nutrition security, the sustainability of the coffee sector, and human health.
Keywords:
coffee; Cascara; dietary fiber; dry matter; total fat; fatty acids; coffee beans; roasting process; coffee Silverskin 1. Introduction
With constantly increasing consumption, coffee beans are one of the most traded commodities globally [1]. According to Iriondo-DeHond et al. (2019) [2], a coffee cherry has various anatomic parts characterized by unique chemical and bioactive compositions. A coffee cherry consists of the outer skin, usually green in unripe fruit and red (some particular genotypes can be orange or yellow) in ripe fruit, which covers a soft and sweet flesh, following the highly hydrated layer of mucus (pectin layer), a thin yellowish endocarp, parchment, and finally the Silverskin covering the green coffee beans [3,4].
The post-harvest processing aims to obtain green coffee beans. The first step of processing starts with the pulp and hull removal using either a wet or dry method [5]. The dry and wet processing methods affect the composition of coffee beans and their by-products [6]. Harvested coffee cherries are dried in the sun and hulled using the mechanical system during the dry process. The dried husk, consisting of skin, pulp, mucilage, and parchment, is removed, together with as much Silverskin as possible [4]. Wet processing depulper removes the pulp. Then, the fermentation takes place to eliminate mucilage. Next, the beans covered by the parchment are washed, drained, and dried until the moisture reaches around 10%. In the final step, the parchment is removed using a hulling machine. Out of 100 kg of mature coffee cherries, 39 kg of pulp and skin is produced [7].
The chemical composition of the green beans primarily depends on genetic variation, post-harvest processing techniques, bean maturation, agricultural practices, and environmental gradients. The roasting process determines coffee sensory characteristics [8,9,10]. During the roasting, hundreds of physical and chemical reactions occur.
The coffee industry produces by-products rich in bioactive compounds such as polyphenols, alkaloids, chlorogenic acids, antioxidants, carbohydrates, proteins, and dietary fiber. These by-products can be a problem for the environment, roasters, growers, and producers, given that they have no further use [2]. Approximately 90% of the coffee cherry parts are discarded and classified as agricultural waste. Cascara or husk, mucilage, parchment, Silverskin, and spent coffee grounds are the main by-products [3]. The latter is produced during the brewing process, and it is also composed of nutrients present in roasted coffee beans such as fatty acids and insoluble dietary fiber (cellulose and hemicellulose) [11,12,13]. Spent coffee grounds also contain micronutrients (potassium, calcium, magnesium, sulfur, phosphorus, iron, manganese, boron, and copper) [14]. Consequently, they have been exploited as a value-added food product [15].
Based on Figure 1 [16] it is essential to distinguish between the cherry pulp and cherry husk (Cascara). These products are obtained by using different methods of coffee bean processing [1]. Coffee by-products have been designed as a potential sustainable source of macronutrients, micronutrients, and bioactive compounds. Due to the chemical composition of green coffee beans, a similar composition could be assumed for coffee by-products. Cascara and Silverskin are considered to be good sources of dietary fiber. Cascara has potential as a natural sustainable source of nutrients, which include proteins (8–11%), minerals (3–7%), lipids (0.5–3%), and total carbohydrates (58–85%), cellulose (24.5%), hemicellulose (29.7%), and lignin (23.7%) [1,3,17].

Figure 1.
Schematic representation of coffee processing and by-products generation adapted from [16].
Silverskin is detached from green beans and represents the only by-products produced during the roasting process. One hundred and twenty kilograms of green coffee generate approx. 2.5 kg of Silverskin [6]. Silverskin contains a higher dietary fiber level (cellulose, hemicellulose, and lignin) compared to coffee beans. Xylose, arabinose, galactose, and mannose are the main monosaccharides in Silverskin [18]. Dietary fiber (55%) is the significant component of Silverskin, which includes soluble (10%) and insoluble (45%) fiber. Part of the dietary fiber composing coffee Silverskin is soluble, which gives the potential to develop functional foods [3]. Other significant components are proteins (19%), carbohydrates (6%), and fat (6%). Silverskin is also a rich source of polyphenols, chlorogenic acid, and antioxidants [2,11].
Several studies describe coffee by-products’ health benefits, mainly antioxidant, antimicrobial, anti-inflammatory, and anti-aging effects, while very few data have been provided on the nutritional value of them. Moreover, to the best of our knowledge, there are no previous papers available comparing the nutritional composition of unroasted and roasted coffee beans and the by-products generated during post-harvest processes such as depulping (Cascara) and roasting (Silverskin). Previous studies on the content of food contaminants, (ochratoxin A) and acrylamide [18,19,20], support the food safety of coffee by-products for human consumption. Consequently, the EFSA (2021) [21] has considered Cascara a traditional food that can be commercialized in Europe for human consumption, starting from January 2022. Therefore, the upcycling of coffee by-products allows for the reduction of their environmental impact and turns them into nutritious ingredients and foods that can contribute to nutrition security and sustainable health.
The aim of the present research is to provide novel information on the nutritional composition of coffee beans and the by-products generated during coffee post-harvest processing. We applicated a statistical comparison of the fatty acid profile in green coffee beans, their roasted forms (light, medium, and dark), and coffee by-products (Cascara and Silverskin). A comparative study on their chemical composition was performed with the end to stimulate their inclusion as part of a daily healthy diet for humans. At the same time, the paper aims to contribute to the sustainability of the coffee sector and nutrition security. The intake of the chemicals selected for analysis includes nutrients (fats and dietary fiber) that should be controlled to avoid the risk of non-communicable chronic diseases considered pandemics of the 21st century.
2. Materials and Methods
2.1. Material
For this research, Cascara and green bean samples were obtained from Coffea arabica cherries from Central America, Panama, Hartmann micro-lot, Santa Clara, by dry method (natural) of post-harvest processing. Silverskin was obtained as mixture from light, medium, and dark roasting processes. The samples were obtained from the local company dedicated to importing and distributing coffee, Caffé Oro Ltd. (Zvolen, Slovakia). All measurements were performed in triplicate and results are shown in g·kg−1 of dry matter.
2.2. Roasting Process
Green coffee was roasted using traditional barrel roaster technology with a gas heating system using Toper TKSMX 10 machine (Toper, Izmir, Turkey). Roasting programs were set as follows: Light roast (Cinnamon)—temperature 180 °C for 3 min, then 207 °C for 2 min and 50 s. Medium roast (Full City++)—the initial temperature was 180 °C for 3 min, then after 4 min the first crack occurred at 205 °C and ended at 215 °C. The second crack started at 225–230 °C (Full City +). Five seconds later, the beans reached 232 °C for 15 s. Dark roast (French)—the initial temperature was 180 °C for 3 min, then after 4 min the first crack occurred at 205 °C and ended at 215 °C. The second crack started at 225–230 °C. Then, we kept the beans at 235 °C for 45 s; samples of Silverskin were obtained from the roasting process. Silverskin was collected after each roasting process, and coffee beans were placed in a metal container to cool to room temperature, then moved to odor-free plastic bags with a CO2 de-gassing valve obtained from Caffé Oro Ltd. Samples were analyzed the next day. To ensure that the right roasting level was reached, roasted samples were measured using RoastRite, Coffee Roast Analyzer (RoastRiteTM, Taipei City, Taiwan). A detailed description of samples is shown in Table 1.

Table 1.
Detailed description of analyzed samples.
2.3. Grinding
Given the hard structure of green beans, samples were frozen at −20 °C for 30 min before the analysis to ensure easier homogenization. Samples were homogenized using Grindomix GM 200 (Retsch, Haan, Germany) for 60 s (roasted beans, Cascara, and Silverskin) and 120 s (green beans) at 10,000 rpm to achieve the required grinding thickness. The homogenized samples were then sieved through a sieve with a mesh diameter of 1 mm.
2.4. Determination of Dry Matter
The dry matter was determined by moisture analyzer KERN DAB 100-3 (KERN & SOHN GmbH, Balingen, Germany) and expressed in g·kg−1.
2.5. Fat and Fatty Acids Profile
Total fat content was quantified by Soxhlet extraction with petroleum ether following the procedure described by the ISO 12966-2:2017: preparation of methyl esters of fatty acids, animal and vegetable fats, and oils [22]. Gas chromatography of fatty acid methyl esters was employed for the analysis of the individual profile. The fat content and fatty acids methyl ester profile are in g·kg−1 of dry matter.
- Chemical reagents
Petroleum ether 40–65 °C (Sigma-Aldrich, Steinheim, Germany), filter medium Hyflo Super Cel (diatomaceous earth) (Sigma-Aldrich, Steinheim, Germany), methanol for chromatography (Sigma-Aldrich, Steinheim, Germany), KOH (Centralchem, Bratislava, Slovakia), HCl 33–36% (Centralchem, Bratislava, Slovakia), NaHSO4 H2O (Centralchem, Bratislava, Slovakia), Na2SO4 (Centralchem, Bratislava, Slovakia), anhydrous N-hexane for chromatography (Sigma-Aldrich, Steinheim Germany), 37 component standard FAME mix 10 mg.mL−1 in methylene chloride containing C4-C24 FAME (2–4% relative concentration), manufacturer Supelco, catalog number 47885-U chromatography (Sigma-Aldrich, Steinheim, Germany).
- Apparatus
1. Tecator Soxtec System HT 1043 Extraction unit (Gemini, Apeldoom, the Netherlands) apparatus at a flow rate of 10 cycles/h or 10 mL·min−1;
2. Vacuum oven, (Fisher Scientific, Waltham, MA, USA) temperature adjustable (100 ± 3 °C).
Preparation of Fatty Acid Methyl Esters (FAME)
The fat used must be liquid, dry, and clean. Approximately 200 mg of fat was weighed into a ground glass tube. A total of 5 mL of n-hexane was added to the fat by pipette. The fat was dissolved by stirring. In total, 1 mL of 2 M KOH in methanol was added with a micropipette. The tube contents were shaken vigorously and placed in a water bath set at 60 °C for 1 min and 30 s. Then, 2 mL of 1 M HCl was added, and the tube contents were gently mixed. After equilibration and separation of the layers, the upper layer containing FAME was carefully removed by pipette, filtered through anhydrous Na2SO4, and used for GC-FID analysis. Prior to loading into the column, the FAME solution was diluted in a vial, usually at a ratio of 50 μL FAME solution/950 μL n-hexane.
GC-FID was performed using Agilent 6890 GC with FID (flame ionization detector), and analytical column: 60 m × 250 μm × 0.15 μm DB-23 (Agilent 122-2361) was used. Experimental conditions were set as follows: injector temperature: 250 °C; injected volume: 1 μL; dividing ratio: 1/10; carrier gas: helium; head pressure: 238.96 kPa (2.225 mL·min−1); temperature program: 50 °C for 1 min, 25 °C min−1 till the system reached 175 °C, then 2 °C min−1 to 230 °C; detector temperature: 280 °C; gas detector: hydrogen: 35 mL·min−1, air: 350 mL·min−1, nitrogen; make-up gas: 30 mL·min−1.
The quality indicator was the elution time of the separated analytes. The chromatograms of the samples were compared with the chromatogram of the standard. The quantity indicator is the area under the peak of the analyte of interest.
The internal normalization method was used for quantitative evaluation, i.e., provided that all components of the sample were recorded on the chromatogram, representing a total peak area of 100%, and the areas under the individual peaks, i.e., the individual fatty acid methyl esters, represented the weight percentage of a given fatty acid of the total fatty acid content present in the sample [23].
2.6. Determination of Dietary Fiber, Neutral Detergent Fiber (NDF), and Acid Detergent Fiber (ADF)
The analysis was performed according to the Decree of the Ministry of Justice of the Slovak Republic no. 2145/2004-100 of 23.8.2004 on official sampling and laboratory testing and evaluation of feeding stuff [23]. The procedure is described in detail in the Supplementary Material.
2.7. Statistical Analysis
For the summarizing and describing of our results, descriptive statistics was performed. The ANOVA (Duncan test and REGWQ) and linear discriminant analysis (LDA) were used to describe any possible significant differences between the analyzed samples. This statistical analysis was performed using Microsoft Office Excel 365 for iOS and XLStat, Addinsoft (2022). XLStat is a statistical and data analysis solution (New York, NY, USA).
LDA is a statistical method based on reduction of a classification. This common technique provides class separability by drawing a decision region between the different classes. This advanced method focuses on pattern recognition and machine learning to find a linear combination of characteristics that separates observations into classes, which is used for dimensionality reduction and better classification (maximizing the distance between the means of two classes, minimizing the variation between each category) [24,25].
3. Results and Discussion
3.1. Dry Matter (DM)
Green beans reached an average DM of 932.20 g·kg−1. Our results indicated a relationship between roasting degree and DM values. Under dark roasting conditions, the highest values of DM were obtained, being on average 983.10 g·kg−1. Medium and light values were 981.20 and 959.85 g·kg−1, respectively. Our previous research showed that medium roasted coffee could have from 967.7 to 998.2 g·kg−1 of DM [26]. Results agreed with those reported by Król et al. (2019) [27], which were of 962.4 ± 8.7 g·kg−1 for light, 982.8 ± 10.4 g·kg−1 for medium, and 987.4 ± 9.1 g·kg−1 dark roasting conditions. Cascara DM value was of 890.5 g·kg−1, similar to 911.3 ± 9.66 g·kg−1 reported by Ameca et al. (2018) (911.3 ± 9.66 g·kg−1) [28]. The Silverskin mix showed an average DM value of 920.80 g·kg−1. Significant differences were observed between each analyzed group (p < 0.0001).
3.2. Fat and Fatty Acids Methyl Esters (FAME)
The fat content of the samples in decreasing order was as follows: DRC (135.84 g·kg−1) > GC (99.42 g·kg−1) > MRC (73.22 g·kg−1) > LRC (68.64 g·kg−1) > SS (23.70 g·kg−1) > CC (4.24 g·kg−1). The results indicated that fat content increased in relation to the roasting process. Endeshaw and Belay (2020) [29] showed a similar trend, with the fat content in dark roasted coffee beans being higher than those found in medium or light roasted coffees. Liu and Kitts (2011) [30] associated this behavior to the degradation of carbohydrates and the evaporation of volatile chemicals.
Regarding coffee by-products, our results are in accordance with the findings presented by Iriondo-DeHond et al. (2020) [3], who reported low levels of fat in Cascara and Silverskin samples. Costa et al. (2018) [31] described that Silverskin contains 24.2 g·kg−1 of fat. Similar results were obtained in our research. Results and significant differences based on ANOVA Duncan and REGWQ tests are shown in Table 2. Chromatograms of GC-FID analysis of the coffee beans and their by-products are shown in Supplementary Material Figure S1.

Table 2.
Average content of dry matter, fat, and profile of fatty acids determined by GC-FID.
Thirteen acids of the 40 analyzed were detected in the coffee and/or its by-products (Table 2). The rest—arachidonic acid, butyric acid, capric acid, caproic acid, caprylic acid, cis-10-heptadecenoic acid, cis-10-pentadecenoic acid, cis-11,14,17-eicosatrienoic acid, cis-11,14-eicosadienoic acid, cis-13,16-docosadienoic acid, cis-5,8,11,14,17-eicosapentaenoic acid (EPA), cis-8,11,14-eicosatrienoic acid, elaidic acid, erucic acid, lauric acid, linolelaidic acid, myristoleic acid, nervonic acid, cis-4,7,10,13,16,19-docosahexaenoic acid (DHA), palmitoleic acid, pentadecanoic acid, tridecanoic acid, undecanoic acid, and γ-linolenic acid—were not identified in the coffee beans nor in the by-products.
Linoleic acid was the most abundant fatty acid in the samples under study, which in decreasing order are as follows: (60.56 g·kg−1) > GC (43.67 g·kg−1) > MRC (32.54 g·kg−1) > LRC (30.19 g·kg−1) > SS (5.88 g·kg−1) > CC (1.32 g·kg−1) and palmitic acid DRC (47.61 g·kg−1) > GC (34.93 g·kg−1) > MRC (25.39 g·kg−1) > LRC (24.40 g·kg−1) > SS (6.03 g·kg−1) > CC (1.34 g·kg−1). Dong et al. (2015) [32] stated that linoleic, palmitic, oleic, and arachidic acid are typical for coffee. Other acids identified in coffee are myristic, stearic, and linolenic [33]. Our results are in accordance with those previously reported [34], which indicated as major components palmitic and linoleic acids in Silverskin. The same study identified low values of heptadecanoic, lignoceric, and myristic acids in this by-product.
We found similar concentrations of oleic and stearic acids in green coffee beans: 7.23 g·kg−1 of oleic and 6.96 g·kg−1 of stearic acid, respectively. After light and medium roasting processes, we observed a significant decrease of both fatty acids. However, under dark roasting conditions their concentration significantly increased compared to the green beans. Coffee by-products contained significantly lower values (Cascara 0.24 g·kg−1 and Silverskin 1.12 g·kg−1) than those found in green and roasted coffee beans. Tsegay et al., 2020 [35], found a content of 55.5 g·kg−1 of palmitic acid, 8.92 g·kg−1 of oleic acid, and 51.6 g·kg−1 of linoleic acid in green coffee beans. They reported a relationship between the concentration of fatty acids and geographical origin, namely the altitude of the growing area. Our results are in accordance with this research (Table 2). The roasting process caused a significant decrease in arachidic acid. Similarly, Koshima et al., 2020 [33], observed the same effect. Interestingly, Silverskin contained 13.70% significantly higher concentrations of this acid compared with that found in the green beans.
The chemical composition of green, roasted coffee and its by-products is crucial from a nutrition point of view and for further describing potential differences between coffee and its by-products to determine the possible application in producing novel, innovative foods. To better understand mutual correlations, variability, and pattern recognition, we subjected data to linear discriminant analysis. This type of statistics was previously used for similar purposes, mainly for pattern recognition and differences description regarding geographical origin [36,37]. Wilks’s lambda test showed that at least one mean vector is different with strong significance (p < 0.0001). According to our results, 98.11%, thus the majority, of variability can be described by two factors (Figure 2a). Vectors showed in Figure 2a are responsible for the location of each analyzed group in the 2D space. The higher the content of individual acids is, the stronger their corresponding vectors are in sLDA.
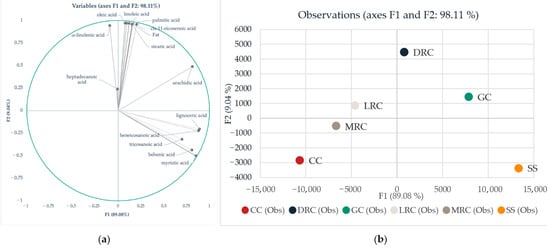
Figure 2.
Differentiation of coffee and its by-products based on FAME using linear discriminant analysis (LDA): (a) variables representation and mutual correlation in 2-dimensional LDA and (b) LDA map where: GC = green coffee, LRC = light roast coffee, MRC = medium roast coffee, DRC = dark roast coffee, CC = Cascara, SS = Silverskin.
Factor F1 explains 89.08% of the variability and correlates with lignoceric, myristic, behenic, tricosanoic, arachidic, and heneicosanoic acids. Furthermore, Factor F2 explains almost the rest of the variability, 9.04%, and correlates with fat content, heptadecanoic, α-linolenic, oleic, linoleic, palmitic, cis-11-eicosenoic, and stearic acids (Figure 2a).
According to LDA (Figure 2), green coffee is a good source of arachidic acid, compared to roasted coffee. On the other hand, light and medium roast processing caused a significant decrease in each identified fatty acid. Only behenic, cis-11-eicosenoic, and lignoceric acids showed stability in their content under light and medium roasting conditions. Variable representation showed that dark roasted beans are a better source of fat, stearic, palmitic, linoleic, oleic, cis-11-eicosenoic, and α-linolenic acids, compared to the green, light, and medium roasted beans. On the contrary, our results suggest that coffee Cascara is not a rich source of the above-mentioned acids. Cascara was defined mostly by linoleic and palmitic acids in smaller concentrations than those found in green and roasted beans. However, Silverskin appeared to be a good source of lignoceric, myristic, and behenic acids.
The identified fatty acids were divided into three groups (PUFA, MUFA, and SFA). Polyunsaturated fatty acids (PUFA) are defined as hydrocarbon chains containing two or more double bonds. The characterization either as an n-3 PUFA or n-6 PUFA refers to the position of the first double bond relative to the methyl end of the fatty acid. In nature, double bonds are usually in the cis form [38]. Monounsaturated fatty acids (MUFA) are chemically classified as fatty acids containing a single, double bond [39]. On the other hand, saturated fatty acids (SFA) have no double bond; the human body can synthesize this type of fat [40].
Figure 3 summarizes differences in fatty acid profiles of the samples. As can be observed, PUFA (linoleic and α-linoleic), MUFA (oleic and cis 11-eicosenoic acids), and SFA (myristic, palmitic, heptadecanoic, stearic, arachidic, heneicosanoic, behenic, tricosanoic, and lignoceric acids) contents were significantly higher (p < 0.001) in coffee beans compared to their by-products.

Figure 3.
ANOVA differences in PUFA, MUFA, SFA, ratio Σn3/Σn6, ratio Σn6/Σn3, and ratio PUFA/SFA. Different letters denote significant statistical differences (p ≤ 0.0001) between samples for a particular group of lipids. GC = green coffee, LRC = light roast coffee, MRC = medium roast coffee, DRC = dark roast coffee, CC = Cascara, SS = Silverskin.
PUFA content in green coffee was 45.05 g·kg−1. The content of PUFA in roasted beans increased during the roasting process (LRC < MRC < DRC). The lowest concentration of PUFA was observed in coffee by-products (Cascara 2.20 g·kg−1; Silverskin 6.35 g·kg−1). Omega-3 and omega-6 fatty acids are essential PUFA (polyunsaturated fatty acids). These acids must be a part of our diet, given that they cannot be synthesized in the human body. The balance between dietary n-6 and n-3 fatty acids is crucial regarding human health [41,42]. PUFA, especially linoleic and α-linolenic acids, affect the function and responsiveness of cell membranes and tissue metabolism, to hormonal and other signals. These biological activities may be grouped as the regulation of membrane structure and function, regulation of intracellular signaling pathways, transcription factor activity together with gene expression, and regulation of the production of bioactive lipid mediators. Through these actions, fatty acids affect health, well-being, and the risk of developing disease [41,42,43].
Green coffee beans contained 7.56 g·kg−1 of monounsaturated fatty acids (MUFA). The roasting process significantly increased their content. The highest concentration of MUFA reached beans roasted on dark level (11.60 g·kg−1). As expected, Silverskin (1.21 g·kg−1) and Cascara (0.24 g·kg−1) showed low levels of them. Schwingshackl and Hoffmann (2012) [39] stated that data from meta-analyses exploring evidence from long-term perspective cohort studies provide ambiguous results with respect to the effects of MUFA on the risk of coronary heart disease (CHD). However, several studies have indicated an increase of HDL cholesterol and a corresponding decrease in triacylglycerols following a MUFA-rich diet.
The values of SFA copied the following decreasing order: DRC (61.06 g·kg−1) > GC (46.09 g·kg−1) > MRC (33.16 g·kg−1) > LRC (31.46 g·kg−1) > SS (16.01 g·kg−1) > CC (1.80 g·kg−1).
The Σn6/Σn3 ratio in green coffee reached 31.57 g·kg−1. The range of the Σn6/Σn3 ratio in the roasted samples ranged from 30.17 g·kg−1 (LRC) to 31.48 g·kg−1 (MRC). Silverskin contained higher values (12.29 g·kg−1) of Σn6/Σn3 ratio compared to the Cascara sample. The values of the n3/n6 ratio in green, light, medium, and dark were identical (0.03 g·kg−1). The observed difference of the ratio n3/n6 was in coffee by-products (Silverskin 12.29 g·kg−1; Cascara 0.67 g·kg−1).
The PUFA/SFA ratio is known to be a crucial parameter regarding human health and nutrition, given that specific saturated and polyunsaturated fatty acids might have different effects in metabolism. Foods with a low PUFA/SFA rate are considered unhealthy and may induce hypercholesterolemia. When this ratio is analyzed separately, there are some restrictions, considering all SFA can increase blood cholesterol, thus ignoring the hypocholesterolemic effect of MUFA [44]. Foods with Σ PUFA/Σ SFA ratios below 0.45 have been considered undesirable for human diet because of their potential to induce a cholesterol increase in the blood [45]. The ratio in green and light roasted samples reached 0.97 and 0.99, respectively. Given that the medium roasting process caused significant changes in fatty acids (Table 2), the PUFA/SFA ratio in medium and dark roasted beans increased from 1.01 (medium) to 1.02 (dark), but the changes were not significant (Figure 3). Among the by-products, Cascara reached the highest ratio of PUFA/SFA, 1.23, while Silverskin showed a significantly lower value of 0.44. Consequently, the lipid profile of the different coffee products studied in the present investigation can be considered healthy.
Figure 4 shows the graphical representation of the LDA of fatty acid profiles and PUFA ratios. In this case, Wilks’s lambda test shows that at least one mean vector is different with strong significance (p < 0.0001). Two factors are needed to explain the majority (95.24%) of the variability between green, roasted coffee beans, and their by-products (Silverskin and Cascara). Factor F1 explains 82.28% of the variability and correlates with PUFA, MUFA, SFA, and ratio Σn6/Σn3. Factor F2 is responsible for 12.96% variability and correlates with Σn3/Σn6 (Figure 4a). Based on the LDA map, it was evident that green and roasted coffees are well separated from their by-products, Cascara and Silverskin (Figure 4b). Given the vector attributes, Figure 4a suggests that PUFA, MUFA, and SFA are more characteristics for coffee beans (green beans and dark roast coffee beans).
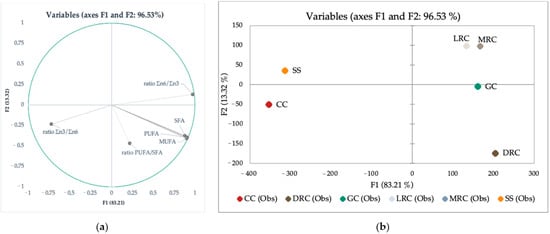
Figure 4.
LDA map for PUFA, MUFA, SFA, ratio Σn3/Σn6, ratio Σn6/Σn3, and ratio PUFA/SFA): (a) variable’s representation and mutual correlation in 2-dimensional LDA and (b) LDA map where: GC = green coffee, LRC = light roast coffee, MRC = medium roast coffee, DRC = dark roast coffee, CC = Cascara, SS = Silverskin.
3.3. Determination of Dietary Fiber, ADF, and NDF
Table 3 shows data on the dietary fiber of the samples under study. Green coffee beans from Panama contained 167.29 g·kg−1 of dietary fiber. We observed the increase caused by the roasting degree, with the medium level (MRC) having the highest values, 284.59 g·kg−1, which is higher by 25% than the dark roasting level (DRC 213.45 g·kg−1). Similarly, Endeshaw and Belay (2020) [29] reported that the fiber contents in roasted coffee could range from 13.13 to 33.10%, and the fiber content of dark roasted coffee was lower than in medium and light roasted coffee. The results of the fiber by Ranic et al. (2015) [46] showed that in roasted coffee it is 26.5 ± 2.8 g·100 g−1.

Table 3.
Average content of fiber and acid and neutral detergent fiber in green and roasted C. arabica and its by-products.
Costa et al., 2018 [31], determined that Silverskin has approximately 564 g·kg−1 of fiber. Iriondo-DeHond et al. (2020) [3] reported that Cascara and Silverskin are main sustainable sources of dietary fiber. Our results showed that the highest fiber content reached was with Silverskin (SE 314.95 g·kg−1); however, Cascara had only 88.34 g·kg−1 of fiber.
Holtzapple 2003 [47] stated that acid-detergent fiber is a residue containing cellulose, lignin, and insoluble minerals (mainly silica). Neutral detergent fiber (NDF) is the total cell wall, which is comprised of the ADF fraction plus hemicellulose. Acid detergent fiber (ADF) refers to the cell wall portions that are made up of cellulose and lignin [47,48].
A similar pattern as in the fiber was observed within ADF and NDF, which means that the roasting process caused a significant increase (p < 0.0001) in both parameters. Nevertheless, the dark roast caused a significant decrease (p < 0.0001). The medium roasting level reached the highest value of ADF and NDF, 41.82 g·kg−1 and 60.45 g·kg−1, respectively.
The by-products reached relatively high values of ADF and NDF too. When comparing the content of ADF, we observed that Silverskin contained 38.64 g·kg−1, whereas Cascara only contained 18.03 g·kg−1. The same pattern was observed for NDF. Ameca et al. (2018) [28] evaluated ADF and NDF in the Cascara obtained from C. arabica from Mexico. They observed that samples contained 52.14% of ADF and 55.19% of NDF. However, their samples of Cascara were obtained using wet post-harvest processing. Klingel et al. (2020) [18] had similar results when focusing on the fiber in Silverskin and Cascara. Rios et al. (2020) [49] also identified total dietary fiber (474.44 ±18.5 g·kg−1), insoluble dietary fiber (313.2 ± 15.1 g·kg−1), and soluble dietary fiber (161.2 ± 11.5 g·kg−1) in Cascara. Guglielmetii et al. (2019) [50] state that Silverskin extract is a good source of total dietary fiber (36.06 ± 1.67 g.100g−1).
4. Conclusions
The roasting degree differently affected the composition of the green beans. Dark roasted coffee showed the highest values of dry matter, fat, and the major fatty acids among the beans under study. Our results confirmed that coffee beans contain mainly linoleic, palmitic, oleic, and stearic acids. In conclusion, the analysis of the selected chemical parameters allowed for differentiation between green and roasted beans submitted to different degrees of roasting and coffee by-products. The factors obtained by LDA proved that 89.08% of the variability between coffee beans and their by-products correlates with lignoceric, myristic, behenic, tricosanoic, arachidic, and heneicosanoic acids. Light and medium roasting caused a significant decrease in the identified fatty acids. Only behenic, cis-11-eicosenoic, and lignoceric acids showed stability in their content with light and medium roasting temperatures.
Silverskin appeared to be a good source of lignoceric, myristic, and behenic acids and had a higher concentration of dietary fiber than Cascara. Silverskin showed the highest values of the dietary fiber among the studied samples and the second lowest in fat after Cascara. In general, the data confirmed that coffee beans (green and roasted) and their by-products are healthy foods attending to their composition in nutrients, including those studied in the present research. The results suggest that, given the fatty acids content and dietary fiber, coffee by-products might be used for the production of enriched and innovative foods.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods11081082/s1 Detailed description of the fiber determination methodology, Figure S1: Chromatograms of fatty acids measurements by GC-FID.
Author Contributions
Conceptualization, A.B. and M.B.; methodology, I.N.; formal analysis, I.N.; investigation, K.P.; resources, A.B.; data curation, A.D. and L.J.; writing—original draft preparation, K.P.; writing—review and editing, Ľ.B.; visualization, A.D.; supervision, B.G.; project administration, A.B.; M.D.d.C.—review and editing; A.I.-D.—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education, Science, Research, and Sport of the Slovak Republic, grant number APVV-19-0180.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was supported by The Ministry of Education, Science, Research and Sport of the Slovak Republic, grant VEGA 1/0734/20, by the Slovak Research and Development Agency, grant APVV-19-0180, and the Spanish Ministry of Science and Innovation, PID2019-11510RB-I00.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ADF: acid detergent fiber; CC: Cascara coffee; DM: dry matter; DRC: dark roast coffee; EFSA: European Food Safety Authority; FAME: fatty acid methyl esters profile; GC: green coffee; GC-FID: gas chromatography with flame ionization detection; LRC: light roast coffee; Mamsl: meters above mean sea level; MRC: medium roast coffee; MUFA: monounsaturated fatty acids; NDF: neutral detergent fiber; PUFA: polyunsaturated fatty acids; REGWQ: the Ryan, Einot, Gabriel, Welsh, studentized Range Q test; SFA: saturated fatty acids.
References
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Aparicio García, N.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Velázquez Escobar, F.; Blanch, G.P.; Velázquez Escobar, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Iriondo-DeHond, A.; Iriondo-DeHond, M.; Del Castillo, M. Applications of Compounds from Coffee Processing By-Products. Biomolecules 2020, 10, 1219. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Chaudhry, A.F.; Aniol, H.; Shegos, C.J. Congenital Hypothyroidism Due to Thyroid Agenesis. Multidiscip. Med. Inf. Netw. 2020, 60, 25–27. [Google Scholar] [CrossRef]
- Kleinwächter, M.; Selmar, G.B.D. Chapter 9—Coffee Beans and Processing. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 73–81. ISBN 9780124095175. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, Composition, and Application of Coffee and Its Industrial Residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef] [Green Version]
- Sualeh, A.; Tolessa, K.; Mohammed, A. Biochemical composition of green and roasted coffee beans and their association with coffee quality from different districts of southwest Ethiopia. Heliyon 2020, 6, e05812. [Google Scholar] [CrossRef]
- Sharma, H. A Detail Chemistry of Coffee and Its Analysis. In Coffee-Production and Research; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Diviš, P.; Pořízka, J.; Kříkala, J. The effect of coffee beans roasting on its chemical composition. Potravinarstvo Slovak J. Food Sci. 2019, 13, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Perrone, D.; Donangelo, R.; Donangelo, C.M.; Farah, A. Modeling Weight Loss and Chlorogenic Acids Content in Coffee during Roasting. J. Agric. Food Chem. 2010, 58, 12238–12243. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Barbosa, H.M.A.; Passos, C.P.; Silva, C.M. Supercritical fluid extraction of spent coffee grounds: Measurement of extraction curves, oil characterization and economic analysis. J. Supercrit. Fluids 2014, 86, 150–159. [Google Scholar] [CrossRef]
- Passos, C.P.; Rudnitskaya, A.; Neves, J.M.; Lopes, G.R.; Evtuguin, D.V.; Coimbra, M.A. Structural features of spent coffee grounds water-soluble polysaccharides: Towards tailor-made microwave assisted extractions. Carbohydr. Polym. 2019, 214, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Bhosale, R.; Shobana, S.; Banu, J.R.; Pugazhendhi, A.; Mahmoud, E.; Sirohi, R.; Bhatia, S.K.; Atabani, A.; Mulone, V.; et al. A review on valorization of spent coffee grounds (SCG) towards biopolymers and biocatalysts production. Bioresour. Technol. 2020, 314, 123800. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.-W.; Kuo, C.-J.; Kao, L.-H.; Lin, G.-Y.; Chen, P.-Y. Polysaccharidic spent coffee grounds for silver nano-particle immobilization as a green and highly efficient biocide. Int. J. Biol. Macromol. 2019, 140, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Passos, C.P.; Ferreira, P.; Coimbra, M.A.; Gonçalves, I. Coffee By-Products and Their Suitability for Developing Active Food Packaging Materials. Foods 2021, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, A.; Rios, M.B.; Herrera, T.; Rodriguez-Bertos, A.; Nuñez, F.; San Andres, M.I.; Sanchez-Fortun, S.; Del Castillo, M.D. Coffee Silverskin Extract: Nutritional Value, Safety and Effect on Key Biological Functions. Nutrients 2019, 11, 2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klingel, T.; Kremer, J.I.; Gottstein, V.; De Rezende, T.R.; Schwarz, S.; Lachenmeier, D.W. A Review of Coffee By-Products Including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef]
- Gottstein, V.; Bernhardt, M.; Dilger, E.; Keller, J.; Breitling-Utzmann, C.M.; Schwarz, S.; Kuballa, T.; Lachenmeier, D.; Bunzel, M. Coffee Silver Skin: Chemical Characterization with Special Consideration of Dietary Fiber and Heat-Induced Contaminants. Foods 2021, 10, 1705. [Google Scholar] [CrossRef]
- dos Santos, M.; de Macedo, L.M.; Tundisi, L.L.; Ataide, J.A.; Camargo, G.A.; Alves, R.C.; Oliveira, M.B.P.; Mazzola, P.G. Coffee by-products in topical formulations: A review. Trends Food Sci. Technol. 2021, 111, 280–291. [Google Scholar] [CrossRef]
- Technical Report on the Notification of Cherry Pulp from Coffea Arabica L. and Coffea Canephora Pierre ex A. Froehner as a Traditional Food from a Third Country following Article 14 of Regulation (EU) 2015/2283. EFSA Supporting Publications, 18(5). Available online: https://www.efsa.europa.eu/en/supporting/pub/en-6657 (accessed on 3 January 2022). [CrossRef]
- International Organization for Standardization. Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Me-thyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids (ISO/DIS Standard No. 12966-2). 2017. Available online: https://www.iso.org/standard/72142.html (accessed on 3 January 2022).
- Decree of the Ministry of Agriculture of the Slovak Republic of 23 August 2004 no. 2145/2004-100, Which Amends the De-cree of the Ministry of Agriculture of the Slovak Republic of 7 October 1997 no. 1497/4/1997-100 on Official Sampling and Laboratory Testing and Evaluation of Feed, as Amended by the Decree of the Ministry of Agriculture of the Slovak Republic of 12 February 2003 no. 149/2 / 2003-100. Available online: https://www.mpsr.sk/index.php?navID=126&year=2004 (accessed on 3 January 2022).
- Zhang, D.; Lu, G. Shape-based image retrieval using generic Fourier descriptor. Signal Process. Image Commun. 2002, 17, 825–848. [Google Scholar] [CrossRef]
- Vaibhaw; Sarraf, J.; Pattnaik, P.K. Chapter 2—Brain–computer interfaces and their applications. In An Industrial IoT Approach for Pharmaceutical Industry Growth; Academic Press: Cambridge, MA, USA, 2020; Volume 2, pp. 31–54. [Google Scholar] [CrossRef]
- Bobková, A.; Jakabová, S.; Belej, L.; Jurčaga, L.; Čapla, J.; Bobko, M.; Demianová, A. Analysis of caffeine and chlorogenic acids content regarding the preparation method of coffee beverage. Int. J. Food Eng. 2021, 17, 403–410. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Tatarak, A.; Hallmann, E. The content of polyphenols in coffee beans as roasting, origin and storage effect. Eur. Food Res. Technol. 2019, 246, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Ameca, G.M.; Cerrilla, M.E.O.; Córdoba, P.Z.; Cruz, A.D.; Hernández, M.S.; Haro, J.H. Chemical composition and antioxidant capacity of coffee pulp. Ciência Agrotecnologia 2018, 42, 307–313. [Google Scholar] [CrossRef]
- Endeshaw, H.; Belay, A. Optimization of the roasting conditions to lower acrylamide content and improve the nutrient composition and antioxidant properties of Coffea arabica. PLoS ONE 2020, 15, e0237265. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kitts, D.D. Confirmation that the Maillard reaction is the principle contributor to the antioxidant capacity of coffee brews. Food Res. Int. 2011, 44, 2418–2424. [Google Scholar] [CrossRef]
- Costa, A.S.; Alves, R.C.; Vinha, A.F.; Costa, E.; Costa, C.S.; Nunes, M.A.; Almeida, A.A.; Santos-Silva, A.; Oliveira, M.B.P. Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. Food Chem. 2018, 267, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Tan, L.; Zhao, J.; Hu, R.; Lu, M. Characterization of Fatty Acid, Amino Acid and Volatile Compound Compositions and Bioactive Components of Seven Coffee (Coffea robusta) Cultivars Grown in Hainan Province, China. Molecules 2015, 20, 16687–16708. [Google Scholar] [CrossRef]
- Koshima, Y.; Kitamura, Y.; Islam, M.; Kokawa, M. Quantitative and Qualitative Evaluation of Fatty Acids in Coffee Oil and Coffee Residue. Food Sci. Technol. Res. 2020, 26, 545–552. [Google Scholar] [CrossRef]
- Angeloni, S.; Scortichini, S.; Fiorini, D.; Sagratini, G.; Vittori, S.; Neiens, S.D.; Steinhaus, M.; Zheljazkov, V.D.; Maggi, F.; Caprioli, G. Characterization of Odor-Active Compounds, Polyphenols, and Fatty Acids in Coffee Silverskin. Molecules 2020, 25, 2993. [Google Scholar] [CrossRef]
- Tsegay, A.; Araya, T.; Amare, K.; G/tsadik, F. Knowledge, Attitude, and Practice on Cervical Cancer Screening and Associated Factors Among Women Aged 15–49 Years in Adigrat Town, Northern Ethiopia, 2019: A Community-Based Cross-Sectional Study. Int. J. Women’s Health 2021, 12, 1283–1298. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Cui, C.; Fisk, I.D. Variability of single bean coffee volatile compounds of Arabica and robusta roasted coffees analysed by SPME-GC-MS. Food Res. Int. 2018, 108, 628–640. [Google Scholar] [CrossRef]
- Demianová, A.; Bobková, A.; Lidiková, J.; Jurčaga, L.; Bobko, M.; Belej, Ľ.; Kolek, E.; Poláková, K.; Iriondo-DeHond, A.; del Castillo, M.D. Volatiles as chemical markers suitable for identification of the geographical origin of green Coffea arabica L. Food Control 2022, 136, 108869. [Google Scholar] [CrossRef]
- Minihane, A.-M.; Lovegrove, J.A. Health benefits of polyunsaturated fatty acids. In Improving the Fat Content of Foods; Williams, C.M., Buttriss, J., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2006. [Google Scholar]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated Fatty Acids and Risk of Cardiovascular Disease: Synopsis of the Evidence Available from Systematic Reviews and Meta-Analyses. Nutrients 2012, 4, 1989–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eshak, E.S.; Yamagishi, K.; Iso, H. Dietary Fat and Risk of Cardiovascular Disease. Encycl. Cardiovasc. Res. Med. 2018, 1, 60–89. [Google Scholar] [CrossRef]
- Husted, K.S.; Bouzinova, E.V. The importance of n-6/n-3 fatty acids ratio in the major depressive disorder. Medicina 2016, 52, 139–147. [Google Scholar] [CrossRef]
- Wijendran, V.; Hayes, K.C. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu. Rev. Nutr. 2004, 24, 597–615. [Google Scholar] [CrossRef]
- Zárate, R.; El Jaber-Vazdekis, N.; Tejera, N.; Pérez, J.A.P.; Rodríguez, C. Significance of long chain polyunsaturated fatty acids in human health. Clin. Transl. Med. 2017, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Melo, J.; Ferreira, F.; Da Silva, T.L.; Nascimento, K.; de Oliveira, V.; Junior, J.L.B.; Barbosa, M.I.M.J.; Saldanha, T. Nutritional quality and functional lipids in the free-range egg yolks of Brazilian family farmers. Rev. Chil. Nutr. 2019, 46, 420–428. [Google Scholar] [CrossRef] [Green Version]
- Mapiye, C.; Chimonyo, M.; Dzama, K.; Hugo, A.; Strydom, P.; Muchenje, V. Fatty acid composition of beef from Nguni steers supplemented with Acacia karroo leaf-meal. J. Food Compos. Anal. 2011, 24, 523–528. [Google Scholar] [CrossRef]
- Ranić, M.; Konić-Ristić, A.; Takić, M.; Glibetić, M.; Pavlović, Z.; Dimitrijević-Branković, S. Nutrient profile of black coffee consumed in Serbia: Filling a gap in the food composition database. J. Food Compos. Anal. 2015, 40, 61–69. [Google Scholar] [CrossRef]
- Holtzapple, M.T. Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 3535–3542. ISBN 9780122270550. [Google Scholar] [CrossRef]
- Sharpe, P. Nutritional Value of Pasture Plants for Horses. Horse Pasture Manag. 2019, 1, 37–64. [Google Scholar] [CrossRef]
- Rios, M.B.; Iriondo-DeHond, A.; Iriondo-DeHond, M.; Herrera, T.; Velasco, D.; Gómez-Alonso, S.; Callejo, M.J.; Del Castillo, M.D. Effect of Coffee Cascara Dietary Fiber on the Physicochemical, Nutritional and Sensory Properties of a Gluten-Free Bread Formulation. Molecules 2020, 25, 1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guglielmetti, A.; Fernandez-Gomez, B.; Zeppa, G.; Del Castillo, M.D. Nutritional Quality, Potential Health Promoting Properties and Sensory Perception of an Improved Gluten-Free Bread Formulation Containing Inulin, Rice Protein and Bioactive Compounds Extracted from Coffee Byproducts. Pol. J. Food Nutr. Sci. 2019, 69, 157–166. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).