Nutritional Quality and Oxidative Stability during Thermal Processing of Cold-Pressed Oil Blends with 5:1 Ratio of ω6/ω3 Fatty Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Heating Procedure
2.3. Fatty Acid Composition Analysis
2.4. Tocochromanols Analysis
2.5. Total Polar Compounds (TPC) Analysis
2.6. Polymerized Triacylglycerols (PTG) Analysis
2.7. Calculated Iodine Value (CIV)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Oil Blends
3.2. Tocochromanols
3.3. Formation of Polar Compounds during Heating
3.4. Polymerized Triacylglycerols (PTG)
3.5. Principal Components Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chew, S.C. Cold-pressed rapeseed (Brassica napus) oil: Chemistry and functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef]
- Siger, A.; Nogala-Kalucka, M.; Lampart-Szczapa, E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipids 2008, 15, 137–149. [Google Scholar] [CrossRef]
- Damude, H.G.; Kinney, A.J. Enhancing Plant Seed Oils for Human Nutrition. Plant Physiol. 2008, 147, 962–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tvrzicka, E.; Kremmyda, L.-S.; Stankova, B.; Zak, A. Fatty Acids as Biocompounds: Their Role in Human Metabolism, Health and Disease—A Review. Part 1: Classification, Dietary Sources and Biological Functions. Biomed. Pap. 2011, 155, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.C. Cold pressed rapeseed (Brassica napus) oil. In Cold Pressed Oils; Elsevier: Amsterdam, The Netherlands, 2020; pp. 65–80. [Google Scholar]
- Bozdoğan Konuşkan, D. Minor bioactive lipids in cold pressed oils. In Cold Pressed Oils; Elsevier: Amsterdam, The Netherland, 2020; pp. 7–14. [Google Scholar]
- Mildner-Szkudlarz, S.; Różańska, M.; Siger, A.; Kowalczewski, P.Ł.; Rudzińska, M. Changes in chemical composition and oxidative stability of cold-pressed oils obtained from by-product roasted berry seeds. LWT 2019, 111, 541–547. [Google Scholar] [CrossRef]
- Różańska, M.B.; Kowalczewski, P.Ł.; Tomaszewska-Gras, J.; Dwiecki, K.; Mildner-Szkudlarz, S. Seed-Roasting Process Affects Oxidative Stability of Cold-Pressed Oils. Antioxidants 2019, 8, 313. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, S.V.; Pandiselvam, R.; Thushara, R.; Manikantan, M.R.; Hebbar, K.B.; Beegum, S.; Mathew, A.C.; Neenu, S.; Shil, S. Engineering intervention for production of virgin coconut oil by hot process and multivariate analysis of quality attributes of virgin coconut oil extracted by various methods. J. Food Process Eng. 2020, 43, e13395. [Google Scholar] [CrossRef]
- Zheng, L.; Jin, J.; Huang, J.; Wang, Y.; Korma, S.A.; Wang, X.; Jin, Q. Effects of heat pretreatment of wet-milled corn germ on the physicochemical properties of oil. J. Food Sci. Technol. 2018, 55, 3154–3162. [Google Scholar] [CrossRef]
- WHO and FAO Joint Consultation: Fats and Oils in Human Nutrition. Nutr. Rev. 2009, 53, 202–205. [CrossRef]
- Davis, B.C.; Kris-Etherton, P.M. Achieving optimal essential fatty acid status in vegetarians: Current knowledge and practical implications. Am. J. Clin. Nutr. 2003, 78, S640–S646. [Google Scholar] [CrossRef] [Green Version]
- Innis, S.M. Omega-3 Fatty Acid Biochemistry: Perspectives from Human Nutrition. Mil. Med. 2014, 179, 82–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmadfa, I.; Kornsteiner, M. Fats and Fatty Acid Requirements for Adults. Ann. Nutr. Metab. 2009, 55, 56–75. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Evolutionary Aspects of Diet: The Omega-6/Omega-3 Ratio and the Brain. Mol. Neurobiol. 2011, 44, 203–215. [Google Scholar] [CrossRef]
- Young, K. Omega-6 (n-6) and omega-3 (n-3) fatty acids in tilapia and human health: A review. Int. J. Food Sci. Nutr. 2009, 60, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Woo, J.; Chen, Z.; Leung, S.; Peng, X. Serum fatty acid, lipid profile and dietary intake of Hong Kong Chinese omnivores and vegetarians. Eur. J. Clin. Nutr. 2000, 54, 768–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolić, M.; Jovanović, M.; Nikolić, K. Advantages and disadvantages of vegetarian nutrition. Zdr. Zast. 2019, 48, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Al-Saleh, I.A.; Billedo, G.; El-Doush, I.I. Levels of selenium, dl-α-tocopherol, dl-γ-tocopherol, all-trans-retinol, thymoquinone and thymol in different brands of Nigella sativa seeds. J. Food Compos. Anal. 2006, 19, 167–175. [Google Scholar] [CrossRef]
- Khatana, C.; Saini, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic Insights into the Oxidized Low-Density Lipoprotein-Induced Atherosclerosis. Oxid. Med. Cell. Longev. 2020, 2020, 5245308. [Google Scholar] [CrossRef]
- Pardauil, J.J.R.; Souza, L.K.C.; Molfetta, F.A.; Zamian, J.R.; Rocha Filho, G.N.; da Costa, C.E.F. Determination of the oxidative stability by DSC of vegetable oils from the Amazonian area. Bioresour. Technol. 2011, 102, 5873–5877. [Google Scholar] [CrossRef] [PubMed]
- Van Hoed, V.; De Clercq, N.; Echim, C.; Andjelkovic, M.; Leber, E.; Dewettinck, K.; Verhé, R. Berry seeds: A source of specialty oils with high content of bioactives and nutritional value. J. Food Lipids 2009, 16, 33–49. [Google Scholar] [CrossRef]
- Meyer, K.; Kinney, A.J. Biosynthesis and Biotechnology of Seed Lipids Including Sterols, Carotenoids and Tocochromanols. In Lipids in Photosynthesis. Advances in Photosynthesis and Respiration; Wada, H., Murata, N., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 407–444. [Google Scholar]
- Falk, J.; Munné-Bosch, S. Tocochromanol functions in plants: Antioxidation and beyond. J. Exp. Bot. 2010, 61, 1549–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebler, D.C. The Role of Metabolism in the Antioxidant Function of Vitamin E. Crit. Rev. Toxicol. 1993, 23, 147–169. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phyther. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [Green Version]
- Gramza, A.; Korczak, J. Tea constituents (Camellia sinensis L.) as antioxidants in lipid systems. Trends Food Sci. Technol. 2005, 16, 351–358. [Google Scholar] [CrossRef]
- Maszewska, M.; Florowska, A.; Dłużewska, E.; Wroniak, M.; Marciniak-Lukasiak, K.; Żbikowska, A. Oxidative Stability of Selected Edible Oils. Molecules 2018, 23, 1746. [Google Scholar] [CrossRef] [Green Version]
- Laguerre, M.; Lecomte, J.; Villeneuve, P. Evaluation of the ability of antioxidants to counteract lipid oxidation: Existing methods, new trends and challenges. Prog. Lipid Res. 2007, 46, 244–282. [Google Scholar] [CrossRef]
- Saguy, I.S.; Dana, D. Integrated approach to deep fat frying: Engineering, nutrition, health and consumer aspects. J. Food Eng. 2003, 56, 143–152. [Google Scholar] [CrossRef]
- Bansal, G.; Zhou, W.; Barlow, P.J.; Lo, H.-L.; Neo, F.-L. Performance of palm olein in repeated deep frying and controlled heating processes. Food Chem. 2010, 121, 338–347. [Google Scholar] [CrossRef]
- Crosa, M.J.; Skerl, V.; Cadenazzi, M.; Olazábal, L.; Silva, R.; Suburú, G.; Torres, M. Changes produced in oils during vacuum and traditional frying of potato chips. Food Chem. 2014, 146, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Saleh, A.S.M.; Chen, J.; Shen, Q. Chemical alterations taken place during deep-fat frying based on certain reaction products: A review. Chem. Phys. Lipids 2012, 165, 662–681. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Ghorbani, M.; Meshginfar, N.; Mahoonak, A.S. A Review on Frying: Procedure, Fat, Deterioration Progress and Health Hazards. J. Am. Oil Chem. Soc. 2016, 93, 445–466. [Google Scholar] [CrossRef]
- Kmiecik, D.; Fedko, M.; Kobus-Cisowska, J.; Kulczyński, B.; Przeor, M.; Szczepaniak, O.; Gramza-Michałowska, A. Cold-Pressed Oils Blends with Pro-Health and Technological Features. Polish Patent Application No. P.432047, 12 August 2019. [Google Scholar]
- Kmiecik, D.; Fedko, M.; Kobus-Cisowska, J.; Kulczyński, B.; Przeor, M.; Gramza-Michałowska, A. Cold-Pressed Oils Blends with Pro-Health and Technological Features. Polish Patent Application No. P.432048, 12 August 2019. [Google Scholar]
- AOCS. Official Method Ce 1h-05. Determination of cis-, trans-, Saturated, Monounsaturated and Polyunsaturated Fatty Acids in Vegetable or Non-ruminant Animal Oils and Fats by Capillary GLC, 6th ed.; American Oil Chemists’ Society: Champaign, IL, USA, 2009. [Google Scholar]
- Siger, A.; Michalak, M.; Rudzińska, M. Canolol, tocopherols, plastochromanol-8, and phytosterols content in residual oil extracted from rapeseed expeller cake obtained from roasted seed. Eur. J. Lipid Sci. Technol. 2016, 118, 1358–1367. [Google Scholar] [CrossRef]
- AOCS. Official Method 982.27. Polar Components in Frying Fats, 6th ed.; American Oil Chemists’ Society: Champaign, IL, USA, 2009. [Google Scholar]
- AOCS. Official Method 993.25. Polymerized Triglycerides in Oils and Fats, 6th ed.; American Oil Chemists’ Society: Champaign, IL, USA, 2009. [Google Scholar]
- AOCS. Official Method Cd 1c-85. Calculated Iodine Value, 6th ed.; American Oil Chemists’ Society: Champaign, IL, USA, 2009. [Google Scholar]
- Mason, R.P.; Libby, P.; Bhatt, D.L. Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1135–1147. [Google Scholar] [CrossRef]
- Ghasemi Fard, S.; Wang, F.; Sinclair, A.J.; Elliott, G.; Turchini, G.M. How does high DHA fish oil affect health? A systematic review of evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 1684–1727. [Google Scholar] [CrossRef] [Green Version]
- Barceló-Coblijn, G.; Murphy, E.J. Alpha-linolenic acid and its conversion to longer chain n−3 fatty acids: Benefits for human health and a role in maintaining tissue n−3 fatty acid levels. Prog. Lipid Res. 2009, 48, 355–374. [Google Scholar] [CrossRef]
- Park, H.G.; Lawrence, P.; Engel, M.G.; Kothapalli, K.; Brenna, J.T. Metabolic fate of docosahexaenoic acid (DHA; 22:6n-3) in human cells: Direct retroconversion of DHA to eicosapentaenoic acid (20:5n-3) dominates over elongation to tetracosahexaenoic acid (24:6n-3). FEBS Lett. 2016, 590, 3188–3194. [Google Scholar] [CrossRef]
- Burdge, G.C.; Calder, P.C. Conversion of α-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef]
- Pieszka, M.; Migdał, W.; Gąsior, R.; Rudzińska, M.; Bederska-Łojewska, D.; Pieszka, M.; Szczurek, P. Native Oils from Apple, Blackcurrant, Raspberry, and Strawberry Seeds as a Source of Polyenoic Fatty Acids, Tocochromanols, and Phytosterols: A Health Implication. J. Chem. 2015, 2015, 659541. [Google Scholar] [CrossRef] [Green Version]
- Munteanu, A.; Zingg, J.-M.; Azzi, A. Anti-atherosclerotic effects of vitamin E—myth or reality? J. Cell. Mol. Med. 2004, 8, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Szymańska, R.; Cela, J.; Munne-Bosch, S. Plastochromanol-8: Fifty years of research. Phytochemistry 2014, 108, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.H. Ground nut oil. In Encyclopedia of Food Sciences and Nutrition; Academic Press: Cambridge, MA, USA, 2003; pp. 2967–2974. ISBN 978-0-12-227055-0. [Google Scholar]
- Birringer, M.; Siems, K.; Maxones, A.; Frank, J.; Lorkowski, S. Natural 6-hydroxy-chromanols and -chromenols: Structural diversity, biosynthetic pathways and health implications. RSC Adv. 2018, 8, 4803–4841. [Google Scholar] [CrossRef] [Green Version]
- Rokosik, E.; Dwiecki, K.; Siger, A. Nutritional quality and phytochemical contents of cold pressed oil obtained from chia, milk thistle, nigella, and white and black poppy seeds. Grasas Aceites 2020, 71, e368. [Google Scholar] [CrossRef]
- Ahsan, H.; Ahad, A.; Siddiqui, W.A. A review of characterization of tocotrienols from plant oils and foods. J. Chem. Biol. 2015, 8, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Barrera-Arellano, D.; Ruiz-Mendez, V.; Marquez Ruiz, G.; Dobarganes, C. Loss of tocopherols and formation of degradation compounds in triacylglycerol model systems heated at high temperature. J. Sci. Food Agric. 1999, 79, 1923–1928. [Google Scholar] [CrossRef]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and Tocotrienols—Bioactive Dietary Compounds; What Is Certain, What Is Doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry of Deep-Fat Frying Oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef]
- Rudzińska, M.; Hassanein, M.M.M.; Abdel-Razek, A.G.; Kmiecik, D.; Siger, A.; Ratusz, K. Influence of composition on degradation during repeated deep-fat frying of binary and ternary blends of palm, sunflower and soybean oils with health-optimised saturated-to-unsaturated fatty acid ratios. Int. J. Food Sci. Technol. 2018, 53, 1021–1029. [Google Scholar] [CrossRef]
- Grajzer, M.; Szmalcel, K.; Kuźmiński, Ł.; Witkowski, M.; Kulma, A.; Prescha, A. Characteristics and Antioxidant Potential of Cold-Pressed Oils-Possible Strategies to Improve Oil Stability. Foods 2020, 9, 1630. [Google Scholar] [CrossRef]
- Kmiecik, D.; Gramza-Michałowska, A.; Korczak, J. Anti-polymerization activity of tea and fruits extracts during rapeseed oil heating. Food Chem. 2018, 239, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Seppanen, C.M.; Song, Q.; Saari Csallany, A. The Antioxidant Functions of Tocopherol and Tocotrienol Homologues in Oils, Fats, and Food Systems. J. Am. Oil Chem. Soc. 2010, 87, 469–481. [Google Scholar] [CrossRef]
- Górnaś, P.; Radenkovs, V.; Pugajeva, I.; Soliven, A.; Needs, P.W.; Kroon, P.A. Varied Composition of Tocochromanols in Different Types of Bran: Rye, Wheat, Oat, Spelt, Buckwheat, Corn, and Rice. Int. J. Food Prop. 2016, 19, 1757–1764. [Google Scholar] [CrossRef] [Green Version]
- Nogala-Kałucka, M.; Dwiecki, K.; Siger, A.; Górnaś, P.; Polewski, K.; Ciosek, S. Antioxidant synergism and antagonism between tocotrienols, quercetin and rutin in model system. Acta Aliment. 2013, 42, 360–370. [Google Scholar] [CrossRef]
- Kim, I.-H.; Kim, C.-J.; Kim, D.-H. Physicochemical Properties of Methyl Linoleate Oxidized at Various Temperatures. Korean Soc. Food Sci. Technol. 1999, 31, 600–605. [Google Scholar]
- Vonk, R.J.; Kalivianakis, M.; Minich, D.M.; Bijleveld, C.M.A.; Verkade, H.J. The metabolic importance of unabsorbed dietary lipids in the colon. Scand. J. Gastroenterol. 1997, 32, 65–67. [Google Scholar] [CrossRef]
- Yoon, S.H.; Jung, M.Y.; Min, D.B. Effects of thermally oxidized triglycerides on the oxidative stability of soybean oil. J. Am. Oil Chem. Soc. 1988, 65, 1652–1656. [Google Scholar] [CrossRef]
- Alvis, A.; Vélez, C.; Rada-Mendoza, M.; Villamiel, M.; Villada, H.S. Heat transfer coefficient during deep-fat frying. Food Control 2009, 20, 321–325. [Google Scholar] [CrossRef]
- Martin, J.C.; Dobarganes, M.C.; Nour, M.; Marquez-Ruiz, G.; Christie, W.W.; Lavillonnière, F.; Sébédio, J. Effect of fatty acid positional distribution and triacylglycerol composition on lipid by-products formation during heat treatment: I. polymer formation. J. Am. Oil Chem. Soc. 1998, 75, 1065–1071. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Salta, F.N.; Chiou, A.; Andrikopoulos, N.K. Formation and distribution of oxidized fatty acids during deep- and pan-frying of potatoes. Eur. J. Lipid Sci. Technol. 2007, 109, 1111–1123. [Google Scholar] [CrossRef]
- Lampi, A.-M.; Kamal-Eldin, A. Effect of α- and γ-tocopherols on thermal polymerization of purified high-oleic sunflower triacylglycerols. J. Am. Oil Chem. Soc. 1998, 75, 1699–1703. [Google Scholar] [CrossRef]
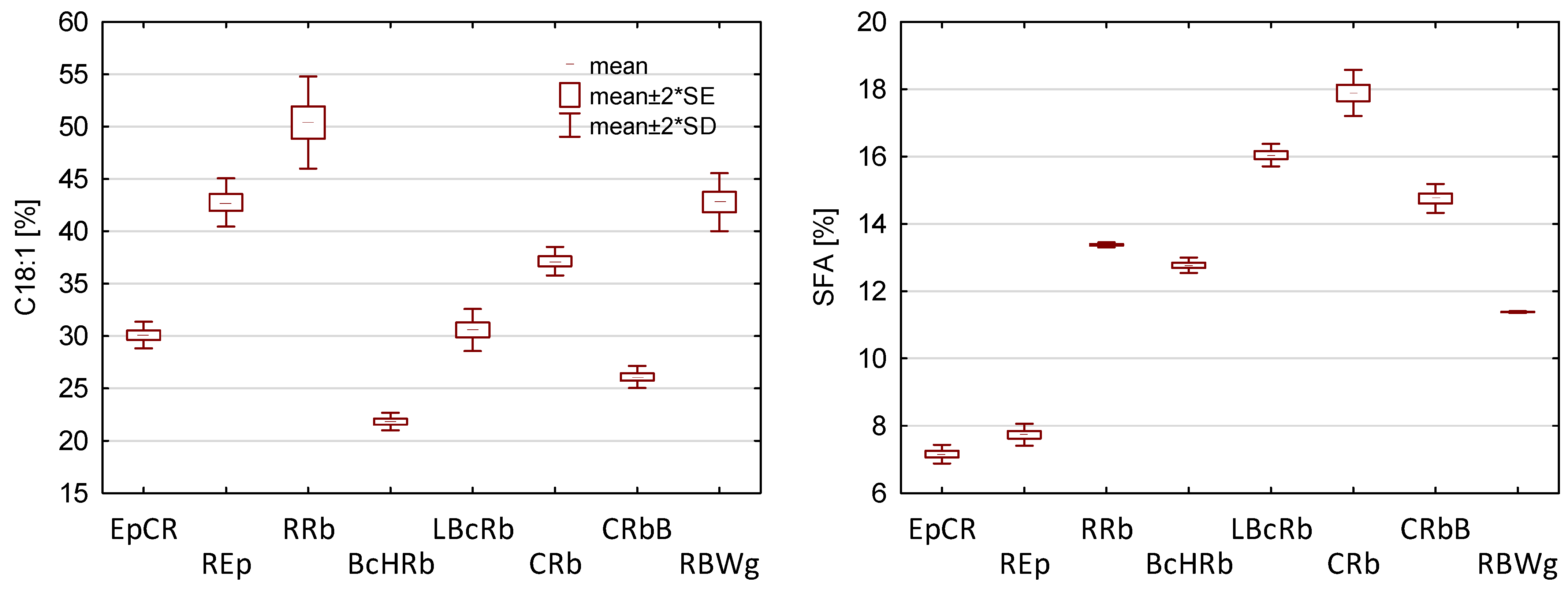
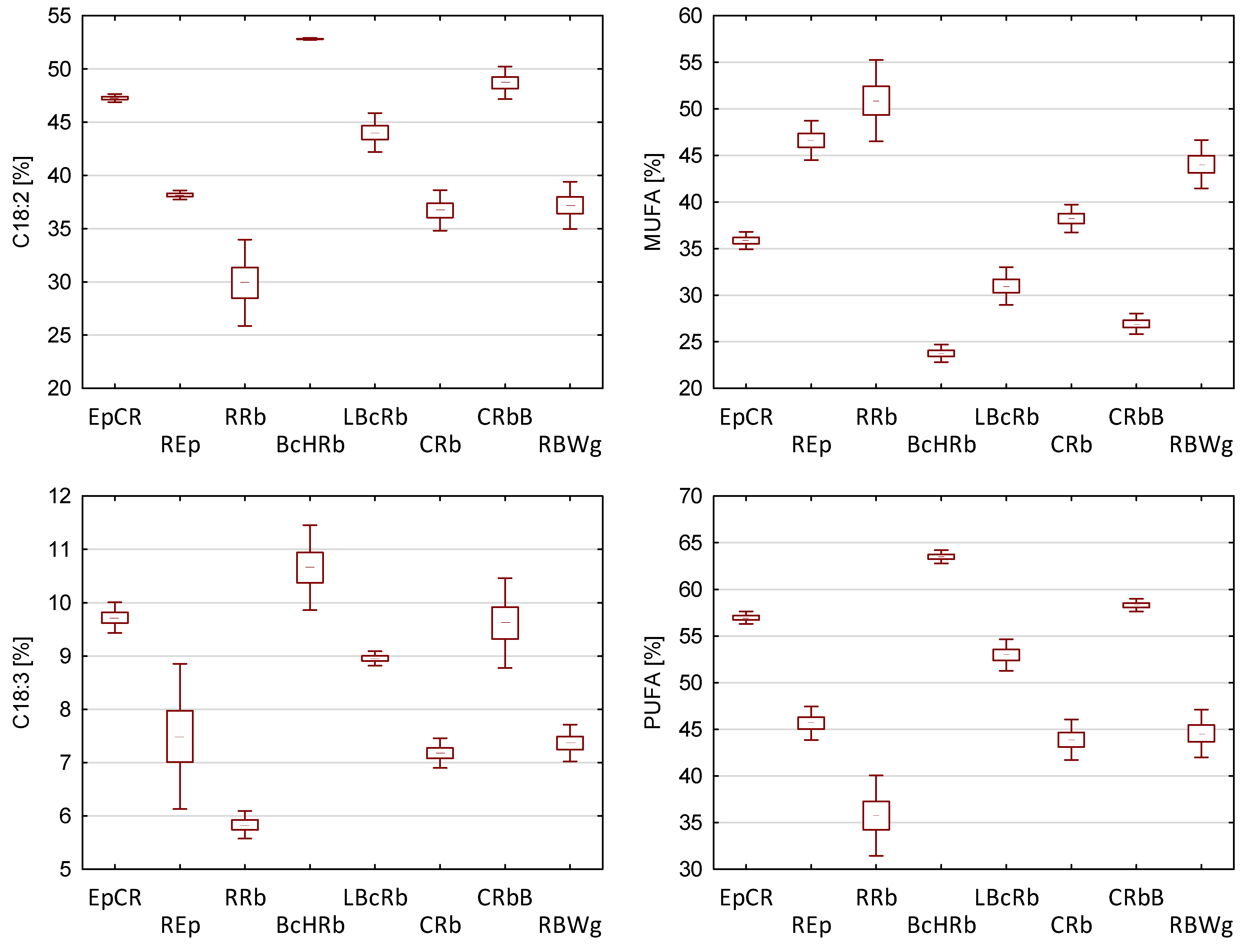
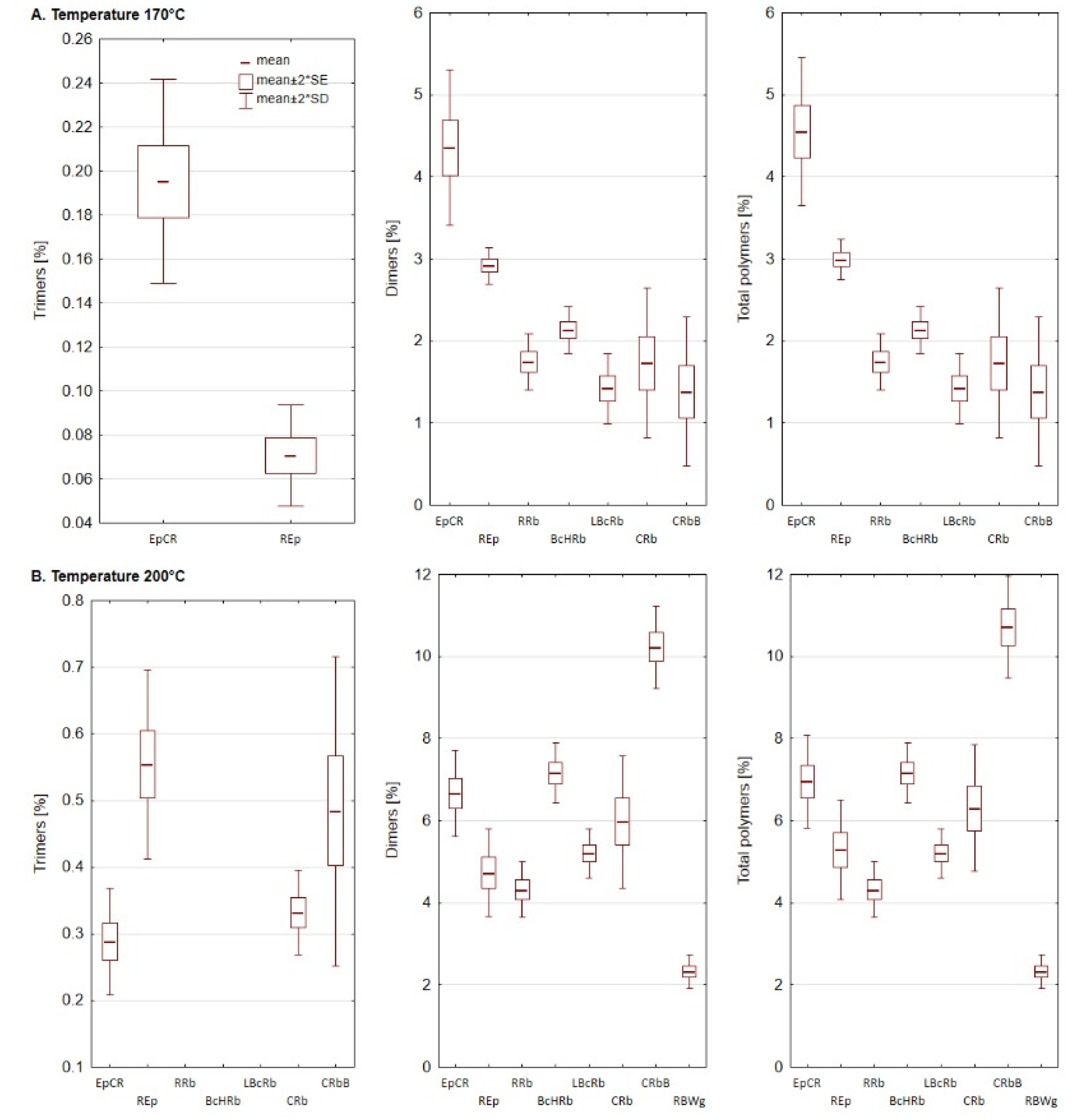

| No. | Code | Type of Oil | Share [%] | Ratio ω6/ω3 | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | EpCR | Evening primrose oil | Camelina oil | Rapeseed oil | 50 | 10 | 40 | 4.86:1 |
| 2 | REp | Rapeseed oil | Evening primrose oil | 65 | 35 | - | 5.11:1 | |
| 3 | RRb | Rapeseed oil | Rice bran oil | 45 | 55 | - | 5.13:1 | |
| 4 | BcHRb | Black cumin oil | Hemp oil | Rice bran oil | 25 | 55 | 20 | 4.96:1 |
| 5 | LBcRb | Linseed oil | Black cumin oil | Rice bran oil | 15 | 45 | 40 | 4.92:1 |
| 6 | CRb | Camelina oil | Rice bran oil | 12 | 88 | - | 5.11:1 | |
| 7 | CRbB | Camelina oil | Rice bran oil | Black cumin oil | 18 | 10 | 72 | 5.07:1 |
| 8 | RBWg | Rapeseed oil | Black cumin oil | Wheat germ oil | 50 | 30 | 20 | 5.05:1 |
| Type of Blends 1 | PC-8 | Total Tocopherols | Total Tocotrienols | CIV |
|---|---|---|---|---|
| EpCR | 1.15 ± 0.06 b | 60.25 ± 0.56a | nd | 137.69 ± 0.29 d |
| REp | 1.38 ± 0.03 d | 57.16 ± 0.66a | nd | 125.51 ± 1.24 bc |
| RRb | 0.40 ± 0.01 a | 37.84 ± 1.09e | 11.47 ± 0.36 c | 110.81 ± 1.96 a |
| BcHRb | nd | 44.92 ± 0.63 f | 7.17 ± 0.04 b | 139.67 ± 0.56 d |
| LBcRb | nd | 14.34 ± 0.18 b | 20.12 ± 0.17 d | 126.32 ± 0.54 c |
| CRb | 0.8 ± 0.01 c | 26.19 ± 0.27 d | 24.12 ± 0.07 f | 115.15 ± 1.37 a |
| CRbB | 0.50 ± 0.12 a | 18.09 ± 0.42 c | 21.46 ± 0.36 e | 132.60 ± 0.26 e |
| RBWg | 1.03 ± 0.03 b | 90.5 ± 2.52 g | 2.71 ± 0.03 a | 121.52 ± 1.26 b |
| Tocopherols | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Blends 1 | α-T | β-T | γ-T | δ-T | ||||||||
| Not Heated | Heating Temperature | Not Heated | Heating Temperature | Not Heated | Heating Temperature | Not Heated | Heating Temperature | |||||
| 170 [°C] | 200 [°C] | 170 [°C] | 200 [°C] | 170 [°C] | 200 [°C] | 170 [°C] | 200 [°C] | |||||
| EpCR | 18.62 ± 0.24 cd | 0.24 ± 0.04 a | nd | 0.06 ± 0.01 a | nd | nd | 40.89 ± 0.35 b | 4.50 ± 0.04 d | 3.88 ± 0.04 e | 0.69 ± 0.02 b | 0.42 ± 0.06 c | 0.39 ± 0.01 c |
| REp | 20.61 ± 0.36 d | nd | nd | 0.04 ± 0.00 a | nd | nd | 35.86 ± 0.31 f | 0.22 ± 0.01 b | nd | 0.64 ± 0.01 ab | 0.24 ± 0.01 b | 0.16 ± 0.01 b |
| RRb | 16.61 ± 0.45 c | nd | nd | 0.23 ± 0.01 a | nd | nd | 20.61 ± 0.66 e | 1.21 ± 0.08 a | 0.14 ± 0.02 ab | 0.40 ± 0.03 ab | 0.05 ± 0.00 a | nd |
| BcHRb | 3.67 ± 0.18 ab | nd | nd | 0.19 ± 0.01 a | nd | nd | 39.64 ± 0.28 b | 8.41 ± 0.05 e | 2.08 ± 0.05 d | 1.42 ± 0.15 c | 0.40 ± 0.02 c | nd |
| LBcRb | 4.33 ± 0.01 b | nd | nd | 0.40 ± 0.07 a | nd | nd | 9.47 ± 0.21 c | 0.81 ± 0.04 ab | 0.57 ± 0.02 c | 0.14 ± 0.03 a | nd | nd |
| CRb | 9.71 ± 0.01 e | nd | nd | 0.30 ± 0.00 a | nd | nd | 15.87 ± 0.23 a | 2.83 ± 0.11 c | 2.28 ± 0.04 d | 0.31 ± 0.06 ab | 0.14 ± 0.02 ab | 0.11 ± 0.1 a |
| CRbB | 1.70 ± 0.03 a | 0.07 ± 0.00 a | nd | 0.58 ± 0.06 a | nd | nd | 15.57 ± 0.35 a | 1.18 ± 0.01 a | 0.41 ± 0.01 bc | 0.24 ± 0.01 ab | 0.11 ± 0.04 ab | nd |
| RBWg | 51.19 ± 1.44 f | 48.58 ± 0.04 b | 42.66 ± 0.09 | 13.75 ± 0.38 b | 11.71 ± 0.04 | 10.38 ± 0.18 | 18.60 ± 0.35 d | 14.39 ± 0.45 f | 9.45 ± 0.28 f | 6.96 ± 0.35 d | 4.14 ± 0.06 d | 1.12 ± 0.01 d |
| Tocotrienols | ||||||||||||
| Type of Blends 1 | α-T3 | β-T3 | γ-T3 | δ-T3 | ||||||||
| Not Heated | Heating Temperature | Not Heated | Heating Temperature | Not Heated | Heating Temperature | Not Teated | Heating Temperature | |||||
| 170 [°C] | 200 [°C] | 170 [°C] | 200 [°C] | 170 [°C] | 200 [°C] | 170 [°C] | 200 [°C] | |||||
| EpCR | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| REp | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| RRb | 0.50 ± 0.02 a | nd | nd | 1.83 ± 0.04 c | nd | nd | 9.14 ± 0.38 c | 0.515 ± 0.02 a | nd | nd | nd | nd |
| BcHRb | 1.18 ± 0.03 c | nd | nd | 3.40 ± 0.08 a | 1.12 ± 0.08 b | 0.54 ± 0.04 b | 2.09 ± 0.00 a | 0.45 ± 0.03 a | 0.09 ± 0.01 a | 0.51 ± 0.09 c | 0.53 ± 0.03 | nd |
| LBcRb | 2.11 ± 0.11 e | nd | nd | 11.14 ± 0.04 d | 1.62 ± 0.08 c | 1.56 ± 0.08 d | 6.70 ± 0.22 b | 0.58 ± 0.06 a | 0.43 ± 0.01 b | 0.18 ± 0.03 a | nd | nd |
| CRb | 0.83 ± 0.03 b | nd | nd | 3.05 ± 0.02 a | nd | nd | 19.98 ± 0.12 d | 2.26 ± 0.16 c | 2.15 ± 0.11 c | 0.27 ± 0.00 ab | nd | nd |
| CRbB | 3.41 ± 0.01 f | 0.22 ± 0.01 | nd | 15.59 ± 0.33 e | 2.12 ± 0.08 d | 1.33 ± 0.01 c | 2.13 ± 0.01 a | 1.05 ± 0.00 b | nd | 0.33 ± 0.01 b | nd | nd |
| RBWg | 1.44 ± 0.01 d | nd | nd | 1.28 ± 0.04 b | 0.65 ± 0.00 a | 0.34 ± 0.03 a | nd | nd | nd | nd | nd | nd |
| Type of Blends 1 | Not Heated | Heating Temperature | |
|---|---|---|---|
| 170 [°C] | 200 [°C] | ||
| EpCR | 4.75 ± 0.07 aB | 8.85 ± 0.07 bD | 13.54 ± 0.27 cE |
| REp | 4.21 ± 0.17 aB | 9.85 ± 0.17 bE | 15.74 ± 0.04 cF |
| RRb | 5.85 ± 0.16 aD | 7.52 ± 0.25 bA | 10.85 ± 0.07 cB |
| BcHRb | 3.47 ± 0.00 aA | 6.95 ± 0.38 bA | 11.74 ± 0.17 cC |
| LBcRb | 3.25 ± 0.13 aA | 6.04 ± 0.06 bB | 9.74 ± 0.38 cA |
| CRb | 1.98 ± 0.14 aC | 4.57 ± 0.07 bC | 8.45 ± 0.10 cD |
| CRbB | 4.31 ± 0.33 aB | 7.05 ± 0.06 bA | 11.21 ± 0.11 cBC |
| RBWg | 3.41 ± 0.01 aA | 5.85 ± 0.14 bB | 9.85 ± 0.20 cA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kmiecik, D.; Fedko, M.; Siger, A.; Kowalczewski, P.Ł. Nutritional Quality and Oxidative Stability during Thermal Processing of Cold-Pressed Oil Blends with 5:1 Ratio of ω6/ω3 Fatty Acids. Foods 2022, 11, 1081. https://doi.org/10.3390/foods11081081
Kmiecik D, Fedko M, Siger A, Kowalczewski PŁ. Nutritional Quality and Oxidative Stability during Thermal Processing of Cold-Pressed Oil Blends with 5:1 Ratio of ω6/ω3 Fatty Acids. Foods. 2022; 11(8):1081. https://doi.org/10.3390/foods11081081
Chicago/Turabian StyleKmiecik, Dominik, Monika Fedko, Aleksander Siger, and Przemysław Łukasz Kowalczewski. 2022. "Nutritional Quality and Oxidative Stability during Thermal Processing of Cold-Pressed Oil Blends with 5:1 Ratio of ω6/ω3 Fatty Acids" Foods 11, no. 8: 1081. https://doi.org/10.3390/foods11081081
APA StyleKmiecik, D., Fedko, M., Siger, A., & Kowalczewski, P. Ł. (2022). Nutritional Quality and Oxidative Stability during Thermal Processing of Cold-Pressed Oil Blends with 5:1 Ratio of ω6/ω3 Fatty Acids. Foods, 11(8), 1081. https://doi.org/10.3390/foods11081081









