3.1. Principal Component Analysis of All Data
The raw data consist of 278 samples, grouped together according to species and sampling months. Samples from July 2016 were not included because the storage condition of these samples was unknown. To provide a graphical overview of the relationship and patterns in the variation in the data, a principal component analysis (PCA) on all data was performed.
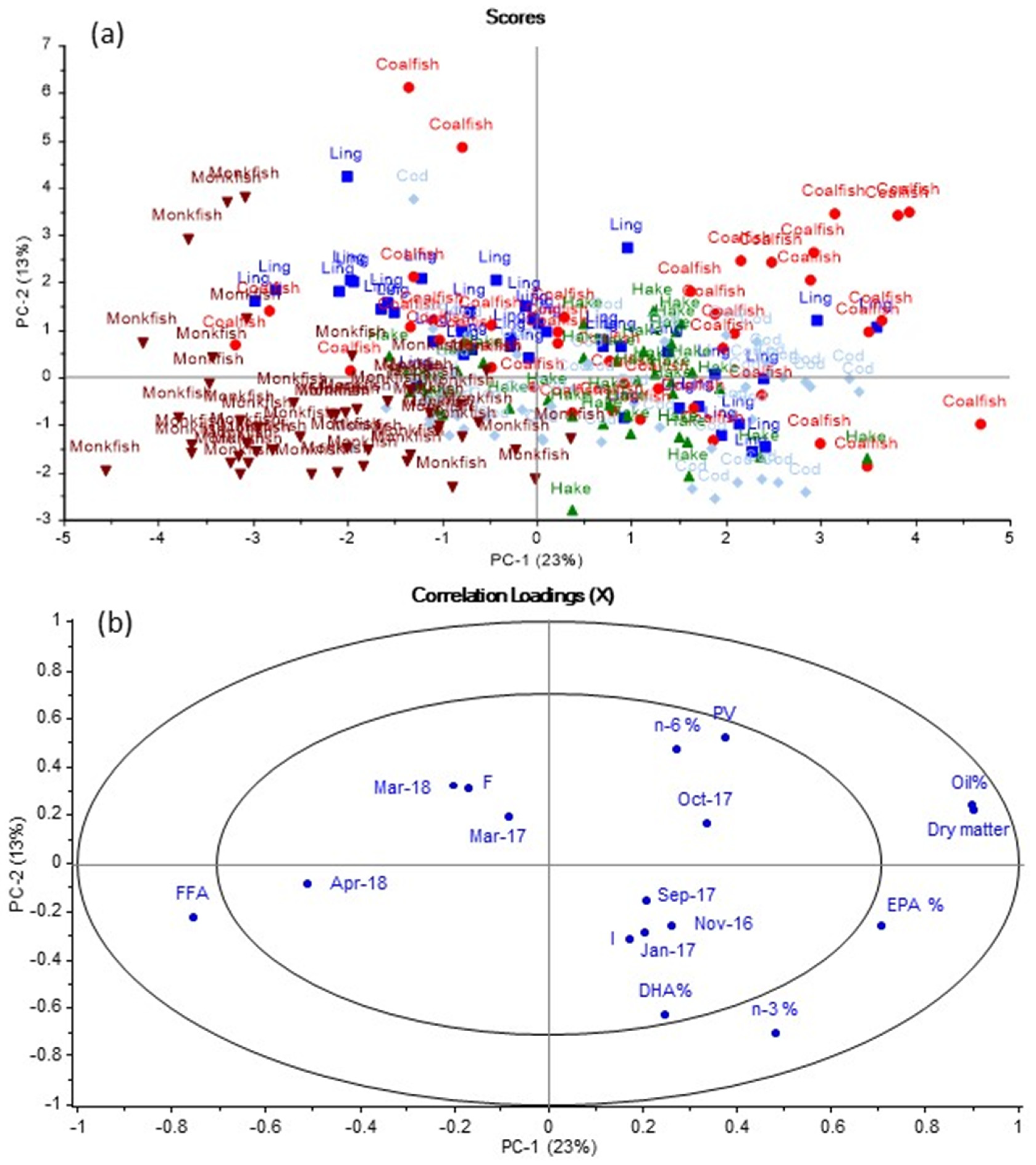
The PCA plot for the data set including all mean values of the livers from the five fish species caught per month. In the scores plot in
Figure 1a, clusters of the different species of fish are observed with most monkfish samples located to the left and most coalfish samples located to the right. Overlaps between the different species are observed, indicating similarities between the five species. By comparing
Figure 1a with the loadings plot in
Figure 1b, it is found that monkfish generally had high FFA content, but lower dry matter and oil content than the other species. The opposite was observed for coalfish. Some coalfish samples were located in the same direction as PV, suggesting that PV was high in these coalfish samples. Based on
Figure 1, cod, coalfish, ling and hake were found to generally have higher content of EPA and DHA than monkfish. The different sampling months were located within the inner ellipse of the loadings plot, indicating that different sampling months contributed less to explaining the variation in the data than other variables (measured variables such as PV, FFA, oil, EPA and DHA), which were located between the inner and outer ellipses.
3.2. Effect of Storage Conditions
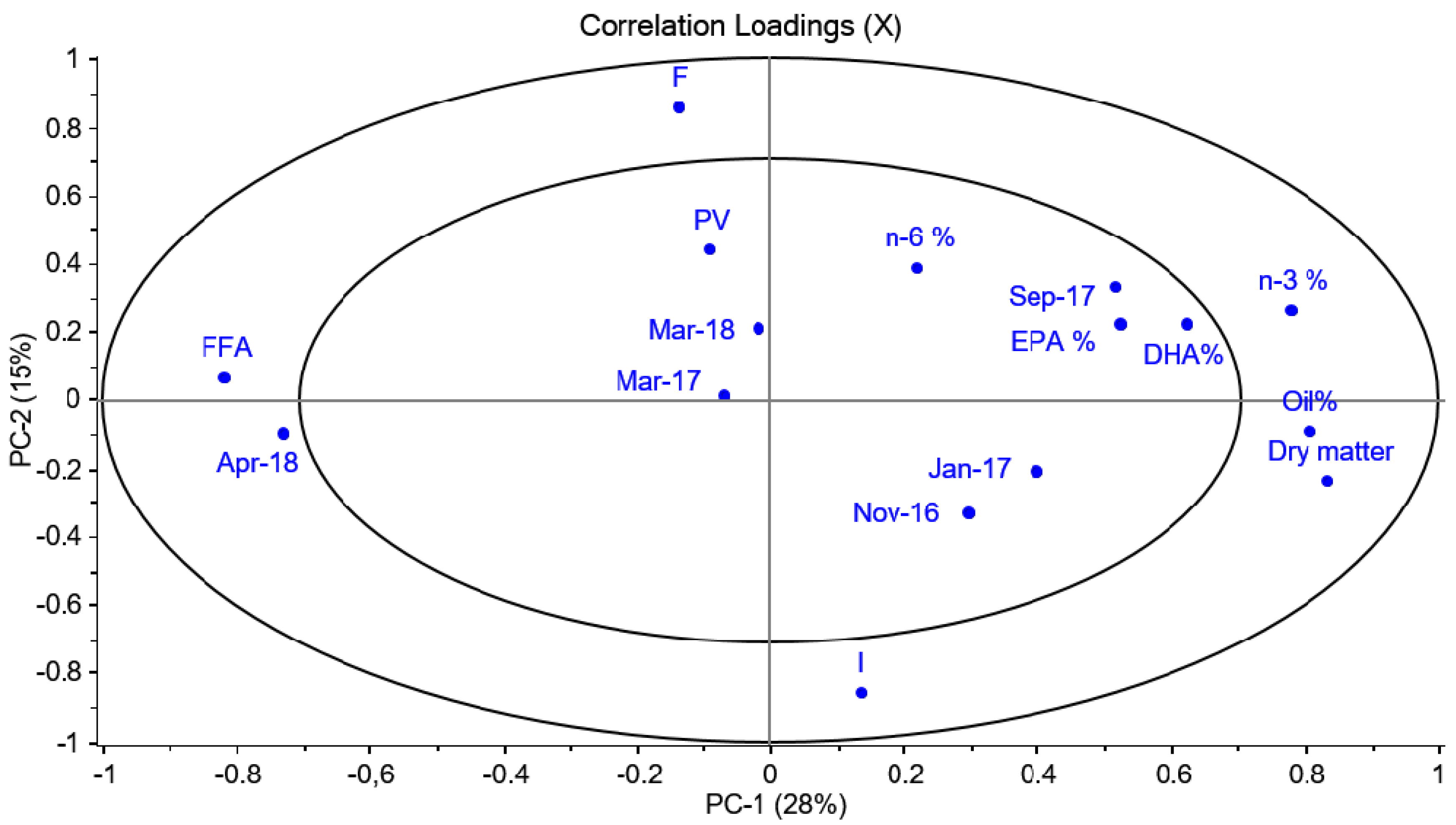
A PCA was also performed to analyse whether storage conditions (on ice (I) or frozen (F)) had any effect on fatty acid composition, FFA or PV for all the fish species. However, no correlation between the measured variables and storage conditions was observed (
Figure A1).
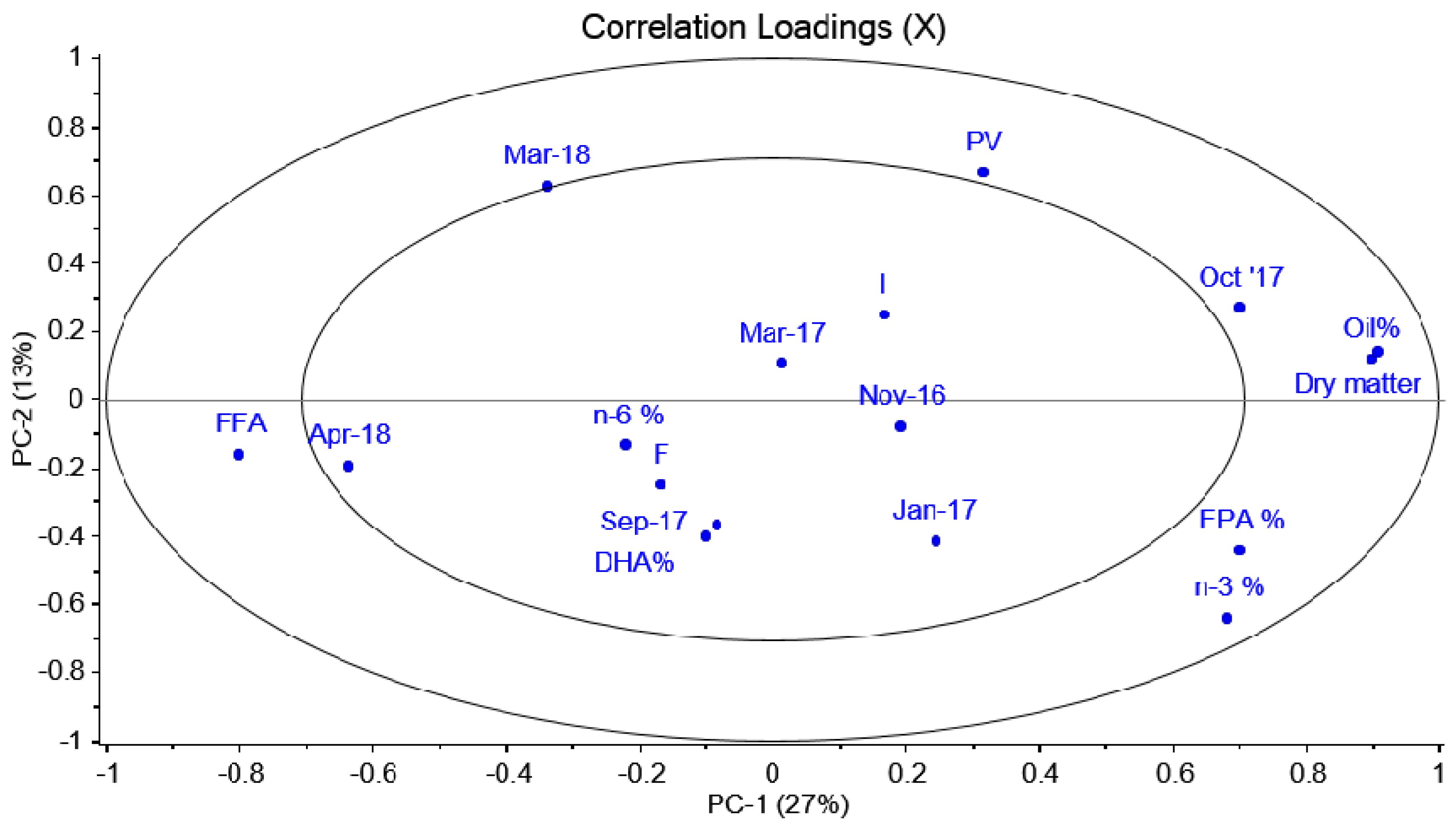
To further study whether storage conditions affected individual species, PCA models were constructed for each fish species. It was observed that ling, hake and coalfish had indications of slight effects on storage conditions. For ling specifically, it was observed that the PV was generally higher for samples that had been stored in frozen conditions only (
Figure A2). However, the opposite was observed for cod for which fish stored on ice at sea generally had a higher PV (
Figure A3). Furthermore, it was observed from the PCA model that the days of storage at sea did not affect the oil composition nor the peroxide value and percentage of free fatty acids in the fish liver. Due to these observations, the effect of storage condition and time at sea was neglected when plotting and examining the data of the individual species and their corresponding PCA plots, as shown in
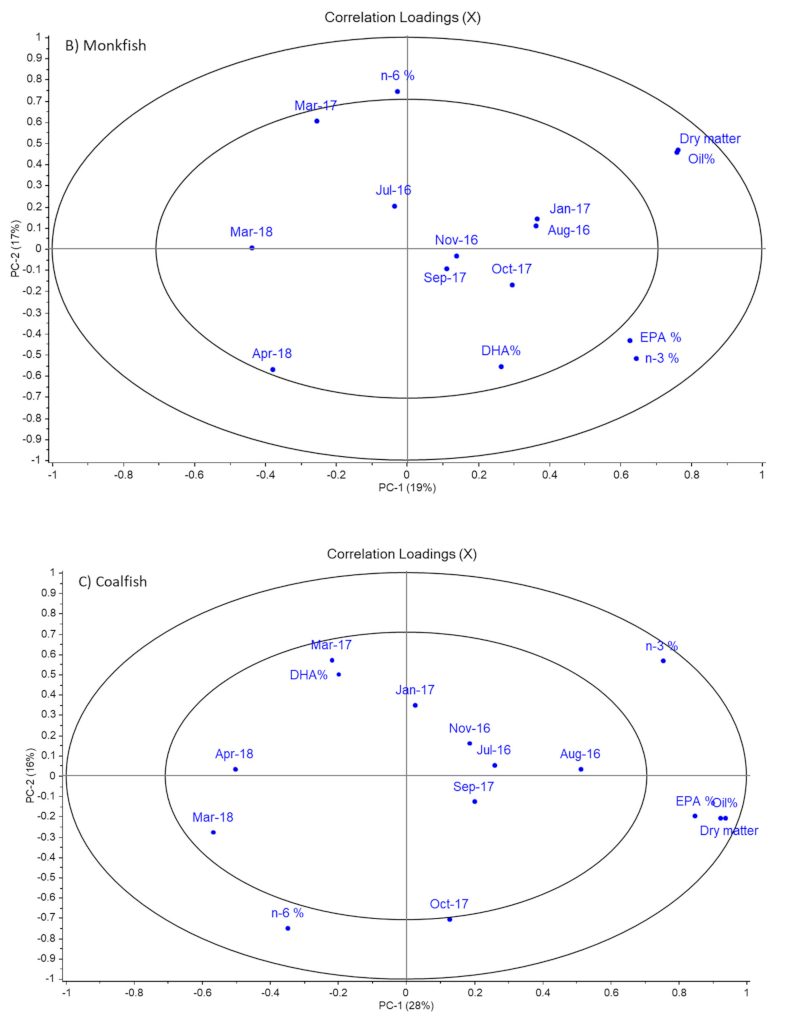
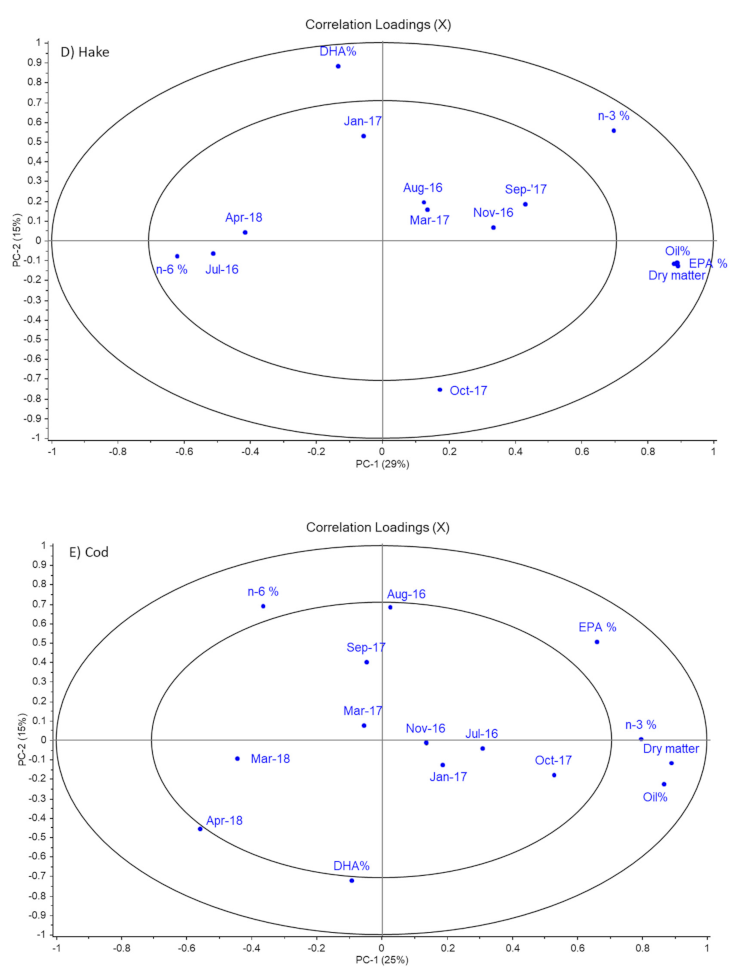
Figure 2.
Figure 2 shows a clear correlation between dry matter and lipid content in all fish species. Such a correlation is expected as oil content contributes to the total dry matter content. This interpretation is further confirmed by the raw data in
Table 2, which shows that the patterns in the seasonal variation in dry matter and lipid content are quite similar. Comparison of the values of dry matter and lipid content also shows that the lipids constituted between 72% to 95% of the dry matter content. This was in accordance with a study by Eliassen and Vahl [
17], who investigated seasonal variations in water and fat content of cod livers. In the PCA plot in
Figure 2, there is a repeating pattern of April 2018 being negatively correlated to dry matter and oil content. This finding suggests that the values of these variables in this specific month were lower compared to samples from other months. The observations regarding low dry matter and lipid content in April 2018 were, in general, confirmed by the raw data in
Table 2. However, due to large biological variation differences between values obtained for livers from April 2018 and other sampling months were not significant for all species, but there was a clear trend that both dry matter and lipid content were lowest or second lowest in April 2018 for all species. Furthermore, dry matter content for monkfish was lower compared to the other fish species, but relatively stable throughout the year with a content of 44.1–54.3%. Although the differences were not always significant, ling, coalfish, hake and cod all followed a similar pattern, with a trend to a slight increase in dry matter content observed during autumn and a decrease in spring followed by the previously mentioned low dry matter content in April 2018, of approximately 60%. Moreover, for the lipid content, differences between sampling points were often not significant due to large biological variation, but the general trends were similar to those described for dry matter content. For example, lipid content for ling increased from 55.6% in July 2016 to 63.2% in January 2017, whereafter it decreased to 55.4% in March 2017. Then, it increased significantly to 65.7% in September 2017, whereafter it significantly decreased to approximately 52% in March and April 2018.
The lipid content for monkfish was observed to be the lowest, ranging from content between 31.1% in April 2018 and 43.4% in January 2017, while the other four fish species generally had a lipid content ranging between 42.4–75.9%. The lipid content for coalfish was the highest in six out of nine sampling points and ranged between 48.9% in April 2018 to 75.9% in August 2016.
Røjbæk et al. [
9] reported that the lipid content of Baltic cod varied between 50% to 60%, which was in accordance with the levels observed in the present study (49.7–64.3%). Falch et al. [
18] compared lipid contents in livers from cod caught in spring, autumn and summer in the Barents Sea (59% to 76%), Icelandic waters (45% to 65%) and southern coast of Ireland (30% to 40%). The livers of the present study, thus, resembled livers from Icelandic waters the most. Icelandic waters are closer to the fishing grounds along the southern part of the Norwegian coast—where the fish in this study were caught—than the other fishing grounds in the study by Falch et al. [
18]. They also reported the lipid content in coalfish (saithe) and ling caught from the same fishing grounds, as mentioned above, except that no ling were caught in the Barents Sea. Again, the values obtained for livers from Icelandic waters (46% to 72% for coalfish and 47% to 74% for ling) were more similar to the values obtained in this study (48.9% to 75.9% for coalfish and 51.4% to 65.7% for ling) than the values obtained from other fishing grounds. Falch et al. [
18] performed a two-way analysis of variance on the effect of fishing ground and season. They found that the effect of fishing ground was significant for all fish species, whereas season significantly influenced lipid content in ling and coalfish, but not in cod. Dominguez-Petit et al. [
19] reported lipid content as varying between 62% to 75% in European hake, which also corresponded well with the observations in our study.
The obvious difference in both dry matter and oil content between monkfish and coalfish, ling, hake and cod may be due to monkfish being from the Lophiiformes order while the other four are from the Gadiformes order of fish. Likewise, according to United States Department of Agriculture, the overall fat content in raw monkfish is found to be 1.52%, while it is 0.67% for cod [
20]. This difference and the possibility of monkfish storing its fat differently from cod species (e.g., less fat in the liver) may be the cause for differences observed in the data.
3.3. Fatty Acid Composition
The scope of this study is to evaluate the potential of the livers as a source of oils rich in long-chain omega-3 fatty acids. Therefore, the focus will mainly be on the sum of all omega-3 PUFA and omega-6 PUFA as well as EPA and DHA. Nevertheless, the total fatty acid composition of livers from the five fish species from the sampling in November 2016 is shown in
Table 3.
Overall, the fatty acid composition of the livers from the different fish species followed the same pattern. The fatty acid present in highest concentration was C18:1 n-9, which constituted between 12.6% (coalfish) and 15.8% (ling) of the total fatty acids. Røjbek et al. [
9] also found that C18:1 n-9 was the most prominent fatty acid in cod liver, and it constituted 20.6–24.1% of the fatty acids in triglycerides. DHA was the second most prominent fatty acid and constituted approximately 13% of the total fatty acids. The sum of C20:1 n-9 and C20:1 n-11 was another group of monounsaturated fatty acids present in high amounts (9.8% in monkfish to 12.0% in coalfish). In total, monounsaturated fatty acids (MUFAs) constituted ca. 42% to 44% of the fatty acids, which was slightly higher than the results reported by Falch et al. in livers from saithe (coalfish), ling and cod from Icelandic waters caught in the autumn (35.5–41.0%) [
18]. PUFAs constituted between 30% to 31% of the fatty acids, which was slightly lower than found in the study by Falch et al. [
18] (32.9–34.8%). The major part of PUFA were omega-3 fatty acids, and the omega-3 to omega-6 ratio varied between 9.2 (monkfish) to 11.6 (coalfish). It has been proposed that in order to have an intake of PUFA that benefits health and prevents disease, the intake of omega-6 PUFA should not be more than two times higher than the omega-3 PUFA intake [
21]. This corresponds to an omega-3:omega-6 ratio of >0.5. Therefore, liver oils from these five fish species have a highly health beneficial omega-3:omega-6 ratio.
The total sum of saturated fatty acids was between 19.4% to 21.7%, with C16:0 constituting the major part (from 11.7% in cod to 13.2% in monkfish).
3.4. Sum of Omega-6 PUFA
With a few exceptions,
Figure 2 did not show strong correlations between sampling months and content of omega-6 PUFA. This was confirmed by the raw data in
Table 4, which showed that all fish types had a similar omega-6 PUFA content ranging from 2.35–3.50% until March 2017. Thereafter, the differences between species became larger, with hake and monkfish experiencing a decrease in September 2017 to 1.46% and 1.96%, respectively. The omega-6 PUFA content continued to decrease significantly for monkfish until April 2018. In contrast, the omega-6 PUFA content increased significantly between September 2017 to October 2017 for hake, to 2.86%. Thereafter, the omega-6 content for hake was at the same levels as those of ling, coalfish and cod (2.3–3.6%) for the rest of the sampling period. High standard deviations were observed for some of the data points due to large variation between individual samples of fish.
Røjbek et al. [
9] reported that the total content of omega-6 PUFA in cod livers varied during the season from 4.8–5.3% in triacylglycerols and 3.2–4.5% in phospholipids. Méndez [
22] reported a seasonal variation of omega-6 PUFA (C18:2 n-6) in hake from 2.0% to 2.8%. McGill and Moffat [
23] studied the fatty acid composition in different commercial liver oils. They found that monkfish and coalfish liver oils contained 1.7% and 1.6% omega-6 PUFA (C18:2 n-6). These values were all within the ranges observed for the different species in the current study.
3.5. Sum of Omega-3 PUFA in % of Total Fatty Acids
The omega-3 PUFA content in the livers ranged from 19.0% to 29.1% and were relatively stable during the seasons (
Table 5). However, a slight decrease was observed in both March 2017 and March 2018 compared to the previous months. This could also be observed in
Figure 2, where a negative correlation between omega-3 PUFA content and March 2017 and/or March/April 2018 were observed for all fish species. The decrease was, however, only significant for coalfish and cod and only in March 2018. Overall, cod liver was found to have the highest values of omega-3 PUFA in the sampling period. Thus, cod liver had the highest or second highest omega-3 PUFA content at eight out of the nine sampling points. The omega-3 PUFA content reached the lowest levels in ling and coalfish (only approximately 20%). These low levels of omega-3 PUFA were found towards the end of the sampling period.
Røjbek et al. [
9] found that omega-3 PUFA levels in cod livers from Baltic Sea varied during the season from 35–42% in triacylglycerols and from 46% to 51% in phospholipids. These values were substantially higher than those found in our study. The fatty acid composition in livers varies with the diet of the fish, which may be different between cods caught in the Baltic Sea and in the North Sea. This could explain the large difference in the omega-3 PUFA content between the two studies. Variations in diet, most likely, also explained the minor fluctuations in the omega-3 PUFA content observed in March in both 2017 and 2018. Moreover, seasonal variations are also due to the reproductive cycle, at least in female species [
9]. It is known that these five fish species generally have spawning season around spring (ranging between January and May) [
24]. Spawning season has, in previous studies, been shown to cause reduced muscle tissue in favor of gonad maturation in farmed Atlantic cod [
25,
26]. The growth of fish is normalized post-spawning season (around October). In gadoid species such as cod, the liver is the primary energy reserve as the liver lipids are mobilized when more energy is required [
27]. It is a possibility that the extra energy used before and during spawning season results in lower lipid content in the liver, meaning less oil in the liver will be present for extraction. However, the livers in this study are both from females and males, so the effect of the spawning season might be lower than if the livers had only been from females. Nevertheless, results in
Table 2 show lower lipid content in the samples for April and March, which may support the mentioned hypothesis. To fully investigate a correlation between seasons (including spawning period) and liver oil attributes, more data are required. This means that more data from the summer seasons and, generally, more data from the same seasons in different years would be beneficial.
3.6. Content of EPA in % of Total Fatty Acids
The livers from all five species of fish were, in general, found to follow a similar pattern, with respect to their EPA as that observed for the omega-3 PUFA content in
Figure 2; this means that levels of EPA were, in general, stable throughout the seasons, but with the lowest levels in March 2017 and/or March/April 2018. However,
Table 6 showed that EPA levels were not significantly lower at these sampling points than at most other sampling points.
Table 6 also confirmed that cod liver had a relatively stable EPA content, which ranged between 5.6% to 8.6%, and it generally contained the highest amount of EPA compared to the other species (at seven out of the nine sampling points). Falch et al. [
18] found that EPA content in cod livers from Icelandic waters varied between 10.1% in the spring to 8.5% in the autumn. In our study, the EPA content in cod livers tended to be highest in the autumn and lowest in the early spring. Monkfish liver was found to have the lowest EPA content, which agreed with the interpretation from the PCA in
Figure 1. The EPA content in monkfish livers varied between 4.6% to 6.4%. McGill and Moffat [
23] observed an EPA content of 7.7% in commercial monkfish liver oil, which is a little higher than observed in our study.
The EPA content in livers from ling and hake varied between the levels observed for livers from monkfish and cod. Falch et al. [
18] found that the EPA content in ling livers from Icelandic waters was lowest in the autumn (4.8%) and highest in the spring (6.3%). Again, this was in contrast to the pattern observed in our study, where ling livers tended to have the highest values in the early autumn. We found slightly higher levels of EPA in our study. EPA content in hake livers from Argentina–Uruguay varied from 5.0% to 8.4%, with the highest values observed in late summer and the lowest in winter [
22]. In our study, there was no clear seasonal effect on the EPA content of hake livers.
Coalfish livers had a large variation in their EPA content (4.4% to 9.0%). Although a similar large variation was not observed for coalfish livers caught in Icelandic waters (6.3% to 9.0%), the EPA levels were almost in the same range.
3.7. Content of DHA in Livers as % of Total Fatty Acids
The values of DHA in the livers from the five species generally ranged between 10.3% to 14.6% DHA (
Table 7). In
Figure 2, the pattern of DHA was almost similar to that of EPA for ling and monkfish. The location of DHA in the loadings plots for these two species suggested that DHA levels were low in March 2017 (monkfish) or April 2018 (ling). This was confirmed by the results in
Table 7. However, DHA content was not significantly different between any sampling points for these two species. High standard deviations for monkfish and ling were observed in March 2017, meaning that the low DHA % in this month may be caused by a random and unexplainable variation within samples of the two species in that month.
Figure 2 also showed that for the other three species, DHA levels behaved differently from EPA levels. For hake and coalfish, high levels of DHA were found in January 2017 (14.6%) and March 2017 (13.3%), respectively, whereas for cod a high level was observed in April 2018 (14.4%). For these species, the DHA levels were significantly lower in July and August 2016 for coalfish (10.5% and 10.8%, respectively), October 2017 (11.7%) for hake and in August 2016 (11.2%) for cod compared to the months mentioned above for the high DHA levels for the corresponding fish species.
The highest values were, in general, observed for cod livers (five out of the nine sampling times). The observations in
Figure 1, that monkfish livers had lower DHA content than livers from the other fish species, were only confirmed by the data in
Table 7 in March 2017 and March 2018.
The study by Falch et al. [
18] reported lower DHA values for livers from cod (6.0% to 9.7%), ling (5.4% to 9.4%) and coalfish (6.2% to 9.4%) when these species were caught in Icelandic waters compared to the findings in this study. In contrast, livers from hake caught in waters of Argentina–Uruguay had slightly higher levels of DHA (12.7–17.6%) than found in our study [
22]. Moreover, McGill and Moffat [
23] found DHA levels of 12.4% and 14.2% in commercial coalfish and monkfish liver oils. These values are within the ranges found in the present study. Differences between fatty acid compositions reported in the different studies may be due to the different catching grounds and, thereby, the different diets of the fish.
3.8. Liver Oils as a New Source of Fish Oils
Moving the focus back to the possibilities of utilizing the fishing waste from the five species of fish in the production of marine oil for human consumption, the recommended values of EPA and DHA in fish liver oil are 7–16% and 6–18%, respectively [
28]. The most suitable species for the production of liver oil are, therefore, cod and hake, as they generally had a high content of both EPA and DHA. Coalfish livers were also observed to have high contents of EPA and DHA. However, the livers from this species seemed to vary more during the seasons, and the EPA content dropped to ca. 5% and below in some months. This may make coalfish less suitable for all-year production than cod and hake. Ling livers only had higher EPA levels than recommended for two of the sampling months, but DHA levels were within the recommended levels in all sampling months. Monkfish livers had lower EPA levels than recommended in all sampling months, but DHA levels were within the recommended levels. Since monkfish livers had the lowest lipid content throughout the sampling period, this was the least suitable fish for liver oil production.
In a high-quality fish oil ready for human consumption, the omega-6 PUFA content should be as low as possible to combat the average consumer’s generally higher intake of omega-6 PUFA compared to omega-3 PUFA. In a typical cod liver oil, the total omega-6 fatty acid content is 3.6% while the omega-3 content is approximately 24% [
29]. A similar ratio between omega-6 and omega-3 PUFA was observed for all fish species throughout the sampling period. This showed that the oils in the fish livers have great potential for production of a fish oil, with a quality similar to cod liver oil based on the ratio between omega-6 and omega-3 PUFA.