Starfish (Asterias rubens) as a New Source of Marine Lipids: Effect of Season, Size and Oil Extraction Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.1.1. Starfish
2.1.2. Starfish Meal
2.2. Pretreatment of the Starfish before Analysis
2.3. Dry Matter
2.4. Oil Content
2.5. Total Fatty Acid Composition: Fatty Acid Methyl Esters (FAME)
2.6. Lipid Classes
2.7. Tocopherols
2.8. Astaxanthin, Astaxanthin Esters, and Other Pigments
2.8.1. Astaxanthin and Its Esters
2.8.2. Other Pigments Only Measured in One Sample (Starfish Meal December 2019)
2.9. Peroxide Value (PV)
2.10. Free Fatty Acids (FFAs)
2.11. Oil Extraction from Starfish and Starfish Meal
2.11.1. Heat and Centrifugation
2.11.2. Enzyme Extraction
2.11.3. Extraction by Heat and Ethanol
2.11.4. Supercritical Carbon Dioxide (Sc-CO2) Extraction with Ethanol
2.12. Statistics
3. Results and Discussion
3.1. Compositions and Compositional Variation
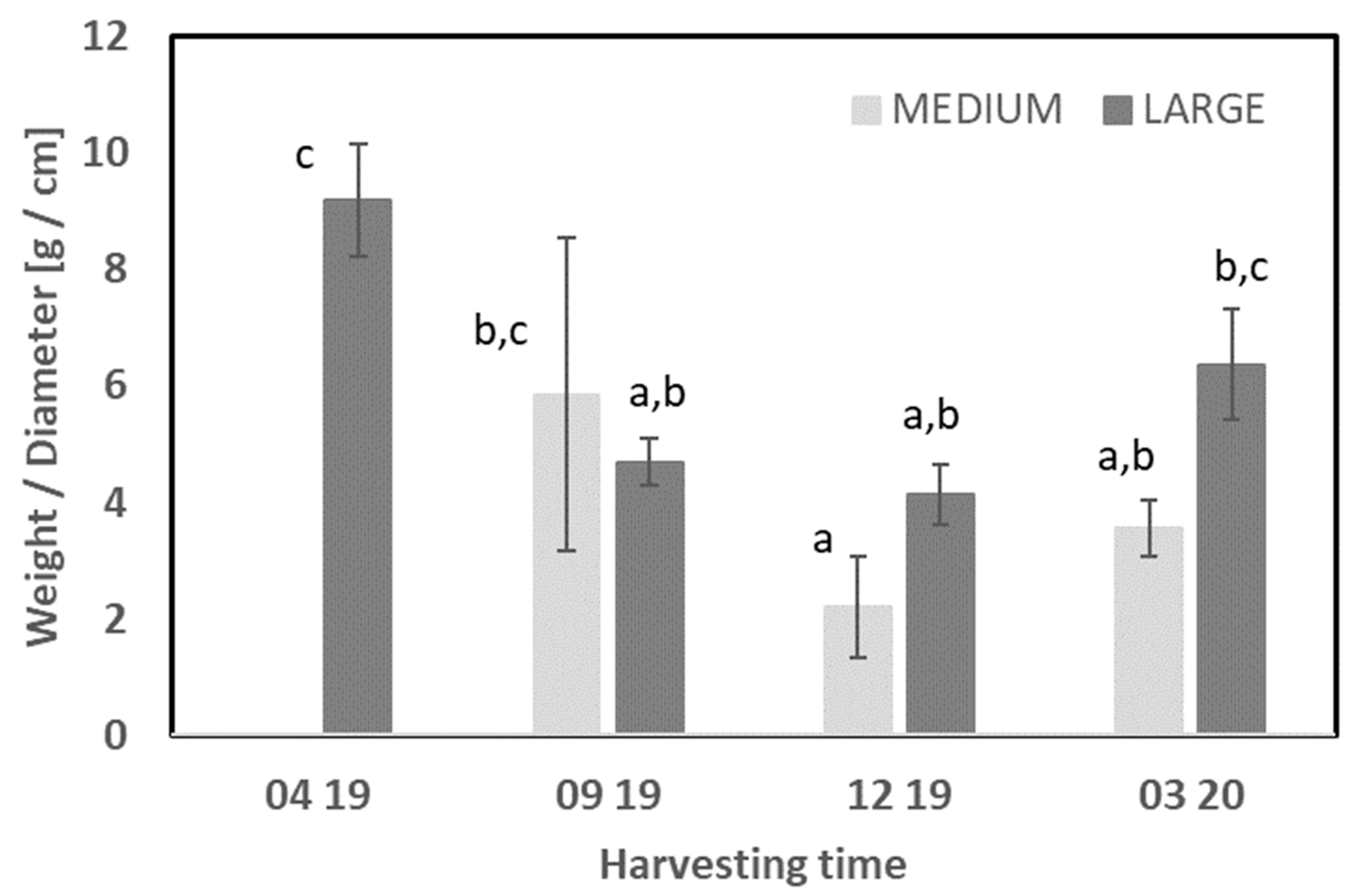
3.1.1. Starfish
3.1.2. Starfish Meal
3.1.3. Summary and Potential as a New Source of Marine Oil
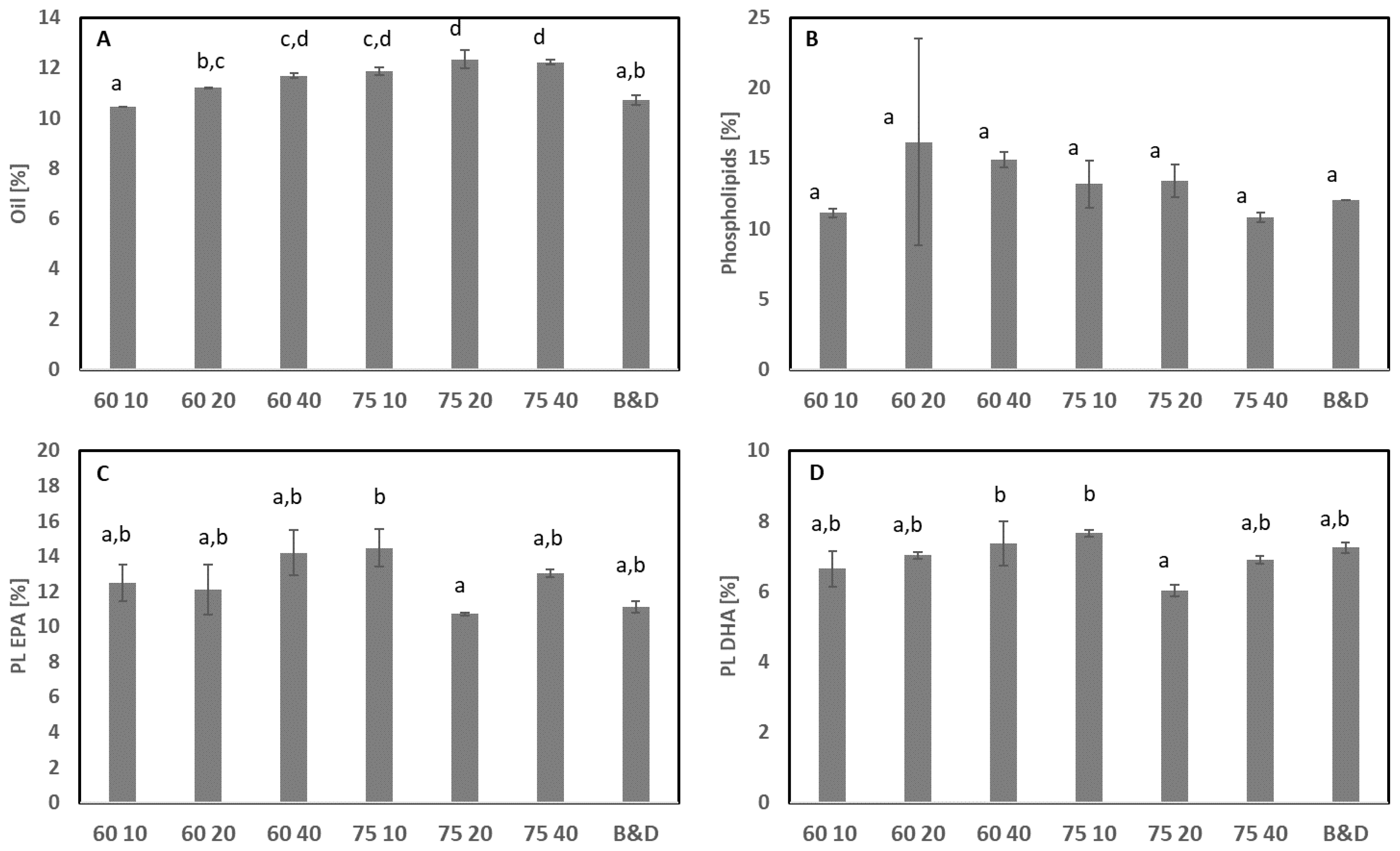
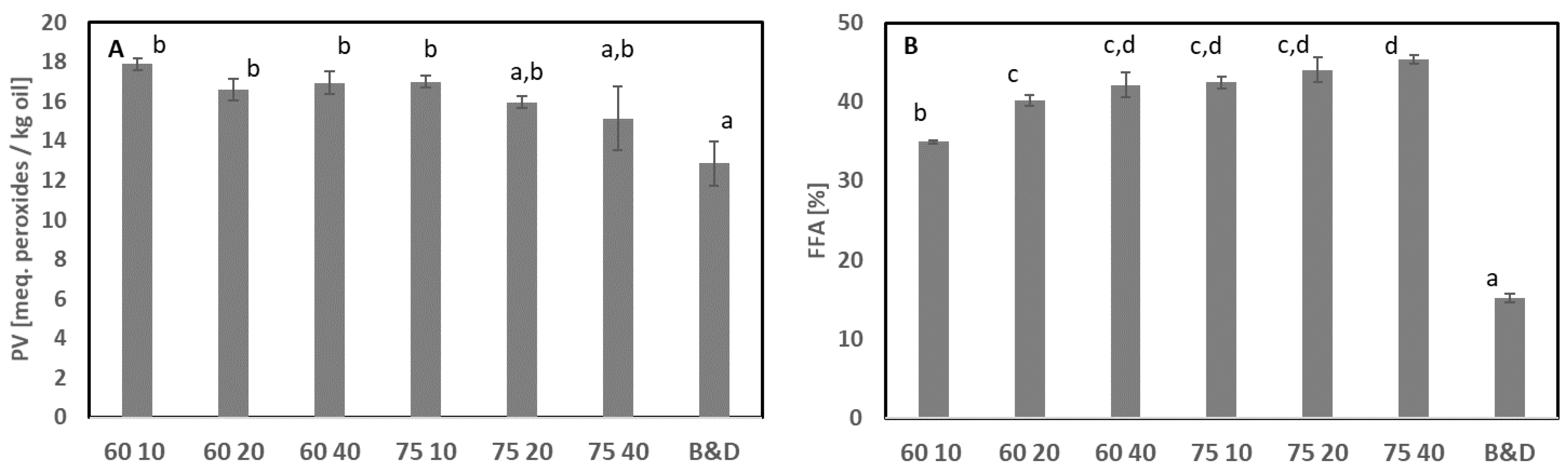
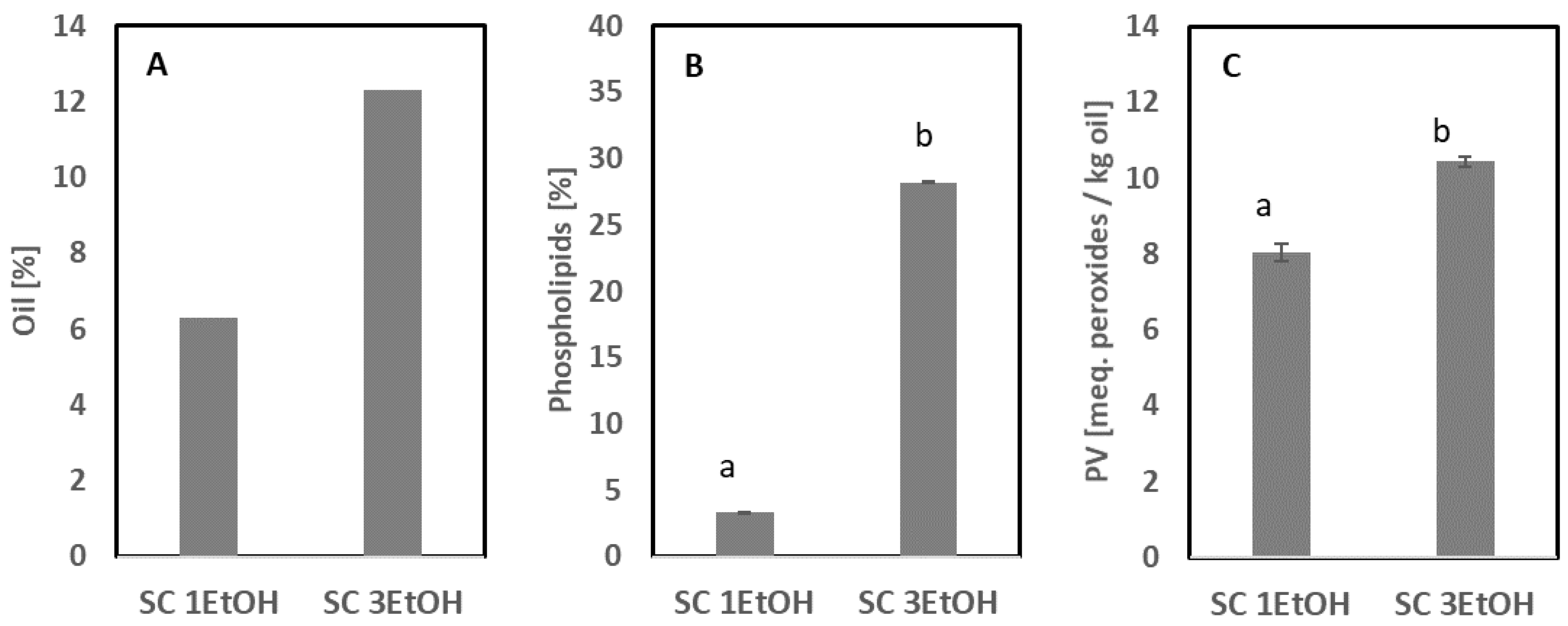
3.2. Extraction of Oil
3.2.1. Traditional Extraction: Heat and Separation
3.2.2. Combination of Solvent and Heat
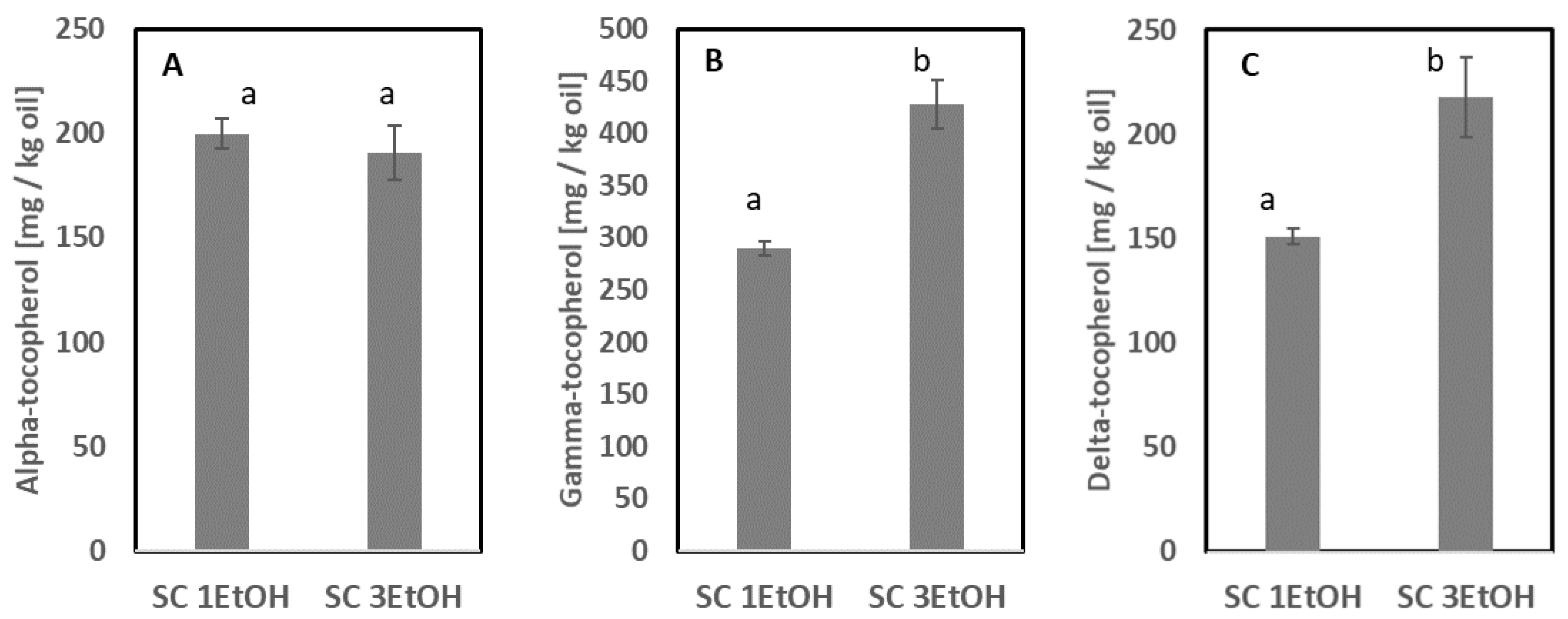
3.2.3. Supercritical CO2 Extraction without and with Co-Solvent
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DHA | Docosahexaeenoic acid |
| EPA | Eicosapentanoic acid |
| EtOH | Ethanol |
| FAME | Fatty acid methyl ester |
| FFA | Free fatty acid |
| LC | Long chain |
| NL | Neutral lipid |
| PL | Polar lipid |
| PUFA | Polyunsaturated fatty acid |
| PV | Peroxide value |
| Sc-CO2 | Super critical CO2 |
References
- GlobeNewswire. Fish Oil Market to Reach USD 5.42 Billion by 2026, Reports and Data. 2020. Available online: https://www.globenewswire.com/news-release/2019/04/09/1799956/0/en/Fish-Oil-Market-To-Reach-USD-5-42-Billion-By-2026-Reports-And-Data.html (accessed on 9 June 2020).
- Bimbo, A. Sources of omega-3 fatty acids. In Food Enrichment with Omega-3 Fatty Acids; Jacobsen, C., Nielsen, N.S., Horn, A.F., Sørensen, A.-D.M., Eds.; Woodhead Publishing: Sawston, UK, 2013. [Google Scholar]
- Petersen, J.K.; Gislason, H.; Fitridge, I.; Saurel, C.; Degel, H.; Nielsen, C.F. Fiskeri Efter Søstjerner i Limfjorden. Fagligt Grundlag for en Forvaltningsplan; Institut for Akvatiske Resourcer, Danmarks Tekniske Universitet: Lyngby, Denmark, 2016. [Google Scholar]
- Ministry of Environment of Denmark; Enviromental Protection Agency. De invasive Arter. Available online: https://mst.dk/ (accessed on 1 December 2021).
- Shah, A.K.M.A.; Kurihara, H.; Takahashi, K. Seasonal variations of lipid content and composition in starfish Asterias amurensis Lütken. Eurasian Chem. Technol. J. 2013, 15, 45–50. [Google Scholar] [CrossRef]
- Van der Heide, M.E.; Møller, L.F.; Petersen, J.K.; Nørgaard, J.V. Annual variation in the composition of major nutrients of the common starfish (Asterias Rubens). Anim. Feed. Sci. Technol. 2018, 238, 91–97. [Google Scholar] [CrossRef]
- Wang, Q.; Ikegame, K.; Takahashi, K.; Xue, C.; Zhang, W.; Wang, H.; Hou, W.; Wang, Y. Comparison of lipids in organs of the starfish Asterias amurensis associated with different treatments. J. Ocean. Univ. China (Ocean. Costal Sea Res.) 2013, 12, 413–417. [Google Scholar] [CrossRef]
- Cansell, M. Marine phospholipids as dietary carriers of long-chain polyunsaturatedfatty acids. Lipid Technol. 2010, 22, 223–226. [Google Scholar] [CrossRef]
- Rossmeisl, M.; Jilkova, M.; Kuda, O.; Jelenik, T.; Medrikova, D.; Stankova, B.; Kristinsson, B.; Haraldsson, G.G.; Svensen, H.; Stokens, I.; et al. Metabolic effects of n-3 PUFA as phospholipids are superior to triglycerides in mice fed a high-fat diet: Possible role of endocannabinoids. PLoS ONE 2012, 7, e38834. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Safafar, H.; Hass, M.Z.; Møller, P.; Holdt, S.L.; Jacobsen, C. High-EPA biomass from Nannochloropsis salina cultivated in a flat-panel photo-bioreactor on a process water-enriched growth medium. Mar. Drugs 2016, 14, 144. [Google Scholar] [CrossRef] [PubMed]
- AOCS. AOCS Official Method Ce 1b-89. In Official Methods and Recommended Practice of the AOCS; AOCS: Champaign, IL, USA, 1998. [Google Scholar]
- Safafar, H.; Ljubic, A.; Møller, P.; Jacobsen, C. Two-step direct transesterification as a rapid method for the analysis of fatty acids in microalgae biomass. Eur. J. Lipid Sci. Technol. 2019, 121, 1700409. [Google Scholar] [CrossRef]
- AOCS. AOCS Official Method Ce 8-89. In Official Methods and Recommended Practice of the AOCS; AOCS: Champaign, IL, USA, 1997. [Google Scholar]
- 74A:1991; Anhydrous Milkfat: Determination of Peroxide Value. International IDF Standard: Wilmington, DE, USA, 1991.
- Araujo, J.; Sica, P.; Costa, C.; Márquez, M.C. Enzymatic hydrolysis of fish waste as an alternative to produce high value-added products. Waste Biomass Valorization 2021, 12, 847–855. [Google Scholar] [CrossRef]
- Aitta, E.; Marsol-Vall, A.; Damerau, A.; Yang, B. Enzyme-assisted extraction of fish oil from whole fish and by-products of Baltic Herring (Clupea harengus membras). Foods 2021, 10, 1811. [Google Scholar] [CrossRef]
- Haq, M.; Chun, B.S. Characterization of phospholipids extracted from Atlantic salmon by-product using supercritical CO2 with ethanol as co-solvent. J. Clean. Prod. 2018, 178, 186–195. [Google Scholar] [CrossRef]
- Bimbo, A.P. Guidelines for characterizing food-grade fish oils. Inform 1998, 9, 473–483. [Google Scholar]
- Jacobsen, C. Starfish and discard from cod: A source of healthy omega-3 fatty acids? Open Access Gov. J. 2021, 2021, 432. [Google Scholar]
- Lu, F.S.H.; Bruheim, I.; Ale, M.T.; Jacobsen, C. The effect of thermal treatment towards the quality changes of Antartic krill meal during its manufacturing process. High processing temperatures decrease product quality. Eur. J. Lipid. Sci. Technol. 2015, 117, 411–420. [Google Scholar] [CrossRef]
- Wang, L.; Yang, F.; Rong, Y.; Yuan, Y.; Ding, Y.; Shi, W.; Wang, Z. Effects of different proteases enzymatic extraction on the yield and quality of Antarctic krill oil. Food. Sci. Nutr. 2019, 7, 2224–2230. [Google Scholar] [CrossRef]
- Xie, D.; Jin, J.; Sun, J.; Liang, L.; Wang, X.; Zhang, W.; Wang, X.; Jin, Q. Comparison of solvents for extraction of krill oil from krill meal: Lipid yield, phospholipids content, fatty acids composition and minor components. Food Chem. 2017, 233, 434–441. [Google Scholar] [CrossRef]
- Yin, F.-W.; Zhou, D.-Y.; Liu, Y.-F.; Zhao, Q.; Liu, Z.-Y.; Song, L.; Zhou, X.; Zhang, J.-R.; Zhu, B.-W. Extraction and characterization of phospholipid-enriched oils from Antarctic krill (Euphausia Superba) with different solvents. J. Aquat. Food Prod. Technol. 2018, 27, 292–304. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Liu, J.; Lin, S.; Wang, Z.; Wang, C.; Wang, E.; Zhang, Y.; Liu, J. Supercritical fluid extraction of flavonoids from Maydis stigma and its nitrite-scavenging ability. Food Bioprod. Process. 2011, 89, 333–339. [Google Scholar] [CrossRef]
- Gifford, M.; Biancani, E.; Kearsley, W.; Maluchnik, W.; Farrell, S.; Savelski, M.J.; Hesketh, R.P. Economic Feasibility Study on the Supercritical Fluid Extraction of Edible Oils; College of Engineering, Rowan University: Glassboro, NJ, USA, 2015; Available online: https://www.supercriticalfluids.com/wp-content/uploads/AP-105-Economic-Feasibility-on-SFE-of-Edible-Oils1.pdf (accessed on 22 May 2020).
- Zuknik, M.H.; Norulaini, N.N.; Omar, A.M. Supercritical carbon dioxide extraction of lycopene: A review. J. Food Eng. 2012, 112, 253–262. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Murakami, M.; Nakano, H.; Konosu, S.; Kokura, T.; Yamamoto, H.; Kosaka, M.; Hata, K. Supercritical carbon dioxide extraction of oils from Antarctic krill. J. Agric. Food Chem. 1986, 34, 904–907. [Google Scholar] [CrossRef]
- Reyes, F.A.; Mendiola, J.A.; Ibáñez, E.; del Valle, J.M. Astaxanthin extraction from Haematococcus pluvialis using CO2-expanded ethanol. J. Supercrit. Fluids 2014, 92, 75–83. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Mendiola, J.A.; Fontecha, J.; van den Broek, L.A.M.; Sijtsma, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Downstream processing of Isochrysis galbana: A step towards microalgal biorefinery. Green Chem. 2015, 17, 4599–4609. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Mendiola, J.A.; van den Broek, L.A.M.; Houweling-Tan, B.; Sijtsma, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Green compressed fluid technologies for downstream processing of Scenedesmus obliquus in a biorefinery approach. Algal Res. 2017, 24, 111–121. [Google Scholar] [CrossRef]
| Sample Code | Replicate | Month | Year | Weight (g) | Diameter (cm) |
|---|---|---|---|---|---|
| LA 04 19 | 1 | April | 2019 | 174 | 21.5 |
| 2 | April | 2019 | 191 | 20.0 | |
| 3 | April | 2019 | 198 | 20.0 | |
| ME 09 19 | 1 | September | 2019 | 45.9 | 12.5 |
| 2 | September | 2019 | 74.3 | 8.4 | |
| 3 | September | 2019 | 67.3 | 13.4 | |
| LA 09 19 | 1 | September | 2019 | 89.2 | 19.3 |
| 2 | September | 2019 | 89.7 | 17.5 | |
| 3 | September | 2019 | 71.2 | 16.5 | |
| ME 12 19 | 1 | December | 2019 | 10.1 | 8.0 |
| 2 | December | 2019 | 31.3 | 13.0 | |
| 3 | December | 2019 | 34.0 | 11.5 | |
| LA 12 19 | 1 | December | 2019 | 56.8 | 16.0 |
| 2 | December | 2019 | 67.0 | 15.0 | |
| 3 | December | 2019 | 79.4 | 18.0 | |
| ME 03 20 | 1 | March | 2020 | 38.3 | 12.0 |
| 2 | March | 2020 | 43.6 | 13.0 | |
| 3 | March | 2020 | 57.2 | 14.0 | |
| LA 03 20 | 1 | March | 2020 | 89.3 | 16.0 |
| 2 | March | 2020 | 122 | 20.0 | |
| 3 | March | 2020 | 163 | 22.0 | |
| SM 03 19 | March | 2019 | |||
| SM 04 19 | April | 2019 | |||
| SM 09 19 | September | 2019 | |||
| SM 12 19 | December | 2019 | |||
| SM 03 20 | March | 2020 |
| Dry Matter 1 | Oil | EPA | DHA | Lipid Classes | Tocopherols 2 | Astaxanthins 2 | PV | FFA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PL EPA | PL DHA | NL + FFA EPA | NL + FFA DHA | Alpha | Gamma | Delta | Free | Esters | |||||||
| Units | (%) | (%) | (%) of Total Fatty Acids | (%) of Total Fatty Acids | (%) of Total Fatty Acids in the Different Lipid Classes | (µg/g) | (µg/g) | (meq. ROOH/kg oil) | (%) of Fatty Acids | ||||||
| STARFISH | |||||||||||||||
| LA 04 19 | 23.7 ± 1.7 a | 3.4 ± 0.1 d | 15.0 ± 1.5 c | 5.69 ± 0.5 a | 28.8 ± 2.6 bc | 7.66 ± 1.3 a | 6.27 ± 0.4 a | 4.60 ± 0.3 a | 20.4 ± 3.3 b | ND | ND | 0.42 ± 02 ab | 1.80 ± 0.1 a | 9.47 ± 1.3 b | 8.09 ± 0.4 c |
| ME 09 19 | 26.8 ± 0.5 ab | 3.2 ± 0.1 cd | 6.46 ± 0.5 ab | 5.37 ± 0.2 a | 14.0 ± 1.2 a | 8.18 ± 0.3 a | 3.30 ± 0.1 a | 4.21 ± 0.2 a | 30.8 ± 0.4 c | ND | ND | 0.77 ± 0.1 c | 2.01 ± 0.1 a | 7.66 ± 0.3 ab | 6.05 ± 0.5 b |
| LA 09 19 | 26.6 ± 0.2 a | 4.0 ± 0.1 e | 7.38 ± 2.1 ab | 5.17 ± 0.1 a | 15.4 ± 4.0 a | 7.76 ± 0.6 a | 4.96 ± 2.0 a | 4.36 ± 0.2 a | 35.4 ± 3.0 c | ND | ND | 0.63 ± 0.0 bc | 2.38 ± 0.3 a | 6.22 ± 0.4 a | 5.49 ± 0.0 b |
| ME 12 19 | 27.2 ± 1.7 ab | 2.9 ± 0.0 ab | 6.29 ± 0.6 a | 5.38 ± 0.3 a | 13.9 ± 2.2 a | 9.34 ± 0.5 a | 3.48 ± 0.2 a | 4.86 ± 0.2 a | 8.16 ± 0.3 a | ND | ND | 0.71 ± 0.1 c | 2.80 ± 0.9 a | 9.92 ± 0.8 b | 15.7 ± 0.6 e |
| LA 12 19 | 31.1 ± 2.7 b | 4.1 ± 0.1 e | 5.11 ± 0.5 a | 5.16 ± 0.0 a | 13.9 ± 1.0 a | 9.69 ± 0.7 ab | 2.47 ± 0.3 a | 4.03 ± 0.4 a | 17.6 ± 0.7 b | ND | ND | 0.55 ± 0.1 abc | 2.84 ± 0.3 a | 5.22 ± 0.2 a | 12.7 ± 0.1 d |
| ME 03 20 | 24.5 ± 1.8 a | 2.7 ± 0.1 a | 11.2 ± 0.7 bc | 9.15 ± 0.8 b | 24.7 ± 3.6 b | 12.6 ± 0.9 b | 6.97 ± 2.0 a | 9.39 ± 1.2 b | 14.2 ± 7.4 ab | ND | ND | 0.43 ± 0.0 ab | 1.82 ± 0.0 a | 24.9 ± 1.6 c | 3.09 ± 0.4 a |
| LA 03 20 | 25.5 ± 0.6 a | 3.0 ± 0.1 bc | 14.6 ± 3.6 c | 6.59 ± 2.1 a | 33.1 ± 3.8 c | 8.35 ± 2.1 a | 8.38 ± 5.5 a | 6.86 ± 2.4 ab | 17.8 ± 1.5 b | ND | ND | 0.30 ± 0.0 a | 2.40 ± 0.3 a | 23.8 ± 1.6 c | 3.33 ± 0.3 a |
| STARFISH MEAL | |||||||||||||||
| SM 03 19 | 90.6 ± 0.0 a | 11.6 ± 0.4 ab | 7.92 ± 0.3 b | 3.70 ± 0.0 a | 18.0 ± 1.9 b | 6.40 ± 0.6 a | 5.24 ± 0.2 d | 3.16 ± 0.1 a | 1.25 ± 0.3 a | 18.6 ± 1.6 a | 24.0 ± 0.9 a | 0.32 ± 0.1 ab | 4.76 ± 0.3 c | 0.27 ± 0.32 a | 32.1 ± 0.5 c |
| SM 04 19 | 90.6 ± 0.0 a | 11.8 ± 0.0 ab | 8.05 ± 0.0 b | 3.85 ± 0.0 a | 18.8 ± 0.4 b | 6.66 ± 0.1 a | 5.20 ± 0.1 cd | 3.03 ± 0.1 a | 1.47 ± 0.1 a | 19.0 ± 0.5 a | 25.2 ± 0.5 a | 0.28 ± 0.1 a | 4.44 ± 0.1 bc | 0.09 ± 0.1 a | 31.9 ± 0.4 c |
| SM 09 19 | 96.6 ± 0.1 d | 13.1 ± 0.4 c | 5.23 ± 0.1 a | 4.79 ± 0.1 c | 12.1 ± 0.0 a | 8.65 ± 0.0 b | 4.51 ± 0.1 bc | 4.47 ± 0.0 b | 24.0 ± 0.3 b | ND | ND | 0.58 ± 0.0 ab | 3.69 ± 0.3 b | 4.89 ± 0.3 b | 15.4 ± 0.3 b |
| SM 12 19 | 95.8 ± 0.0 b | 10.7 ± 0.2 a | 5.09 ± 0.0 a | 4.32 ± 0.0 b | 11.1 ± 0.3 a | 7.24 ± 0.1 a | 4.41 ± 0.0 b | 4.06 ± 0.0 b | 22.5 ± 1.0 b | ND | ND | 0.62 ± 0.0 b | 4.61 ± 0.0 c | 12.9 ± 1.1 d | 14.8 ± 0.5 b |
| SM 03 20 | 96.1 ± 0.0 c | 12.1 ± 0.3 bc | 8.79 ± 0.2 c | 4.96 ± 0.1 c | 25.4 ± 0.2 c | 8.98 ± 0.0 b | 3.71 ± 0.3 a | 4.07 ± 0.2 b | 265 ± 9.0 c | ND | ND | 1.03 ± 0.1 c | 2.58 ± 0.1 a | 10.7 ± 0.3 c | 7.36 ± 0.2 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sørensen, A.-D.M.; Getachew, A.T.; Jacobsen, C. Starfish (Asterias rubens) as a New Source of Marine Lipids: Effect of Season, Size and Oil Extraction Methods. Foods 2022, 11, 2998. https://doi.org/10.3390/foods11192998
Sørensen A-DM, Getachew AT, Jacobsen C. Starfish (Asterias rubens) as a New Source of Marine Lipids: Effect of Season, Size and Oil Extraction Methods. Foods. 2022; 11(19):2998. https://doi.org/10.3390/foods11192998
Chicago/Turabian StyleSørensen, Ann-Dorit Moltke, Adane Tilahun Getachew, and Charlotte Jacobsen. 2022. "Starfish (Asterias rubens) as a New Source of Marine Lipids: Effect of Season, Size and Oil Extraction Methods" Foods 11, no. 19: 2998. https://doi.org/10.3390/foods11192998
APA StyleSørensen, A.-D. M., Getachew, A. T., & Jacobsen, C. (2022). Starfish (Asterias rubens) as a New Source of Marine Lipids: Effect of Season, Size and Oil Extraction Methods. Foods, 11(19), 2998. https://doi.org/10.3390/foods11192998








