Antimicrobial Activity of Red Alga Flour (Gelidium sp.) and Its Effect on Quality Retention of Scomber scombrus during Refrigerated Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Alga Flour
2.2. In Vitro Analysis of Microbial Activity
2.3. Study of Microbial Activity Inhibition in Chilled Fish
2.3.1. Preparation of Icing Media Including the Alga Flour Extract
2.3.2. Raw Fish, Chilled Storage, and Sampling Procedure
2.3.3. Microbial Analyses
2.3.4. Chemical Analyses
2.3.5. Statistical Analysis
3. Results
3.1. Study on In Vitro Microbial Activity
3.2. Study on Microbial Activity Inhibition during Fish Chilling
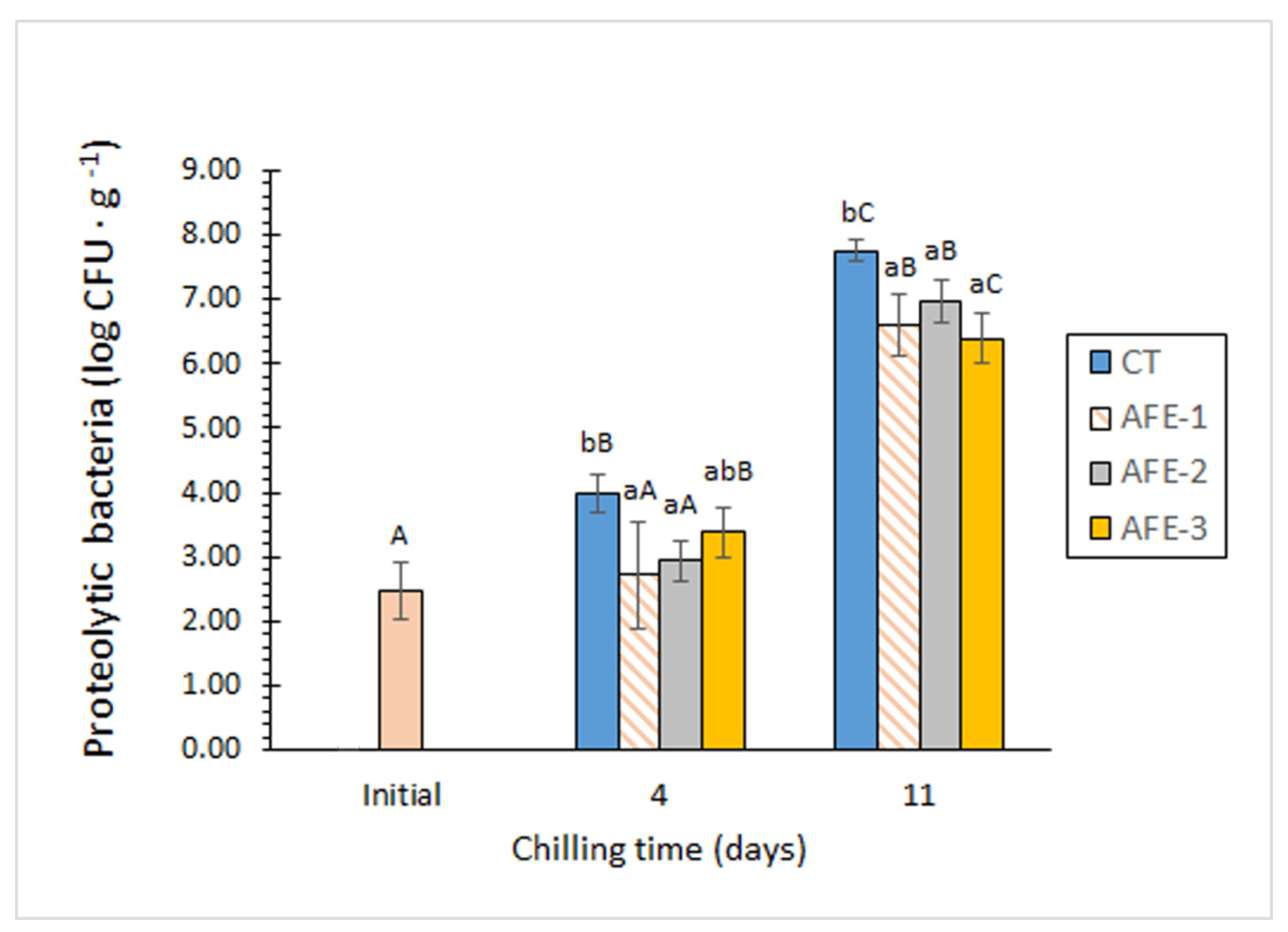
3.2.1. Microbial Parameters
3.2.2. Chemical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sikorski, Z.E.; Kolakowski, E. Endogenous enzyme activity and seafood quality: Influence of chilling, freezing, and other environmental factors. In Seafood Enzymes. Utilization and Influence on Postharvest Seafood Quality; Haard, N.F., Simpson, B.K., Eds.; Marcel Dekker: New York, NY, USA, 2000; pp. 451–487. [Google Scholar]
- Ghali, A.; Dave, D.; Budge, S.; Brooks, M. Fish spoilage mechanisms and preservation: Review. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef] [Green Version]
- Aubourg, S.P. Fish: Processing. In Encyclopedia of Food and Health; Caballero, B., Finglas, P., Toldrá, F., Eds.; Elsevier Ltd.: Oxford, UK, 2016; pp. 710–715. [Google Scholar]
- Campos, C.A.; Gliemmo, M.; Aubourg, S.P.; Barros-Velázquez, J. Novel technologies for the preservation of chilled aquatic food products. In Novel Technologies in Food Science; McElhatton, A., Amaral Sobral, P., Eds.; Springer: New York, NY, USA, 2012; pp. 299–323. [Google Scholar]
- Gokoglu, N. Novel natural food preservatives and applications in seafood preservation: A review. J. Sci. Food Agric. 2019, 99, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Ma, X.; Xie, J. Review on natural preservatives for extending fish shelf life. Foods 2019, 8, 490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabanelli, G.; Barbieri, F.; Montanari, C.; Gardini, F. Application of natural antimicrobial strategies in seafood preservation. In Innovative Technologies in Seafood Processing; Özogul, Y., Ed.; CRC Press, Taylor and Francis: Boca Raton, FL, USA, 2020; pp. 243–262. [Google Scholar]
- Smit, A. Medicinal and pharmaceutical uses of seaweed natural products: A Review. J. App. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Grosso, C.; Vinholes, J.; Valentão, P.; Andrade, P.B. Halogenated compounds from seaweed, a biological overview. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Columbus, F., Ed.; Nova Science Publishers: New York, NY, USA, 2011; pp. 1–25. [Google Scholar]
- Anis, M.; Ahmed, S.; Hasan, M. Algae as nutrition, medicine and cosmetic: The forgotten history, present status and future trends. World J. Pharm. Pharm Sci. 2017, 6, 1934–1959. [Google Scholar]
- European Council Regulation, European Community (EC). Concerning Novel Foods and Novel Food Ingredients. CELEX-EUR Off. J. 1997, L-43, 1–7. [Google Scholar]
- Miranda, J.M.; Trigo, M.; Barros-Velázquez, J.; Aubourg, S.P. Quality enhancement of chilled lean fish by previous active dipping in Bifurcaria bifurcata alga extract. Food Bioprocess Technol. 2018, 11, 1662–1673. [Google Scholar] [CrossRef] [Green Version]
- Oucif, H.; Miranda, J.M.; Mehidi, S.A.; Abi-Ayad, S.M.; Barros-Velázquez, J.; Aubourg, S.P. Effectiveness of a combined ethanol-aqueous extract of alga Cystoseira compressa for the quality enhancement of a chilled fatty fish species. Eur. Food Res. Technol. 2018, 244, 291–299. [Google Scholar] [CrossRef] [Green Version]
- García-Soto, B.; Miranda, J.; Rodríguez-Bernaldo, A.d.Q.; Sendón, R.; Rodríguez-Martínez, A.; Barros-Velázquez, J.; Aubourg, S.P. Effect of biodegradable film (lyophilised alga Fucus spiralis and sorbic acid) on quality properties of refrigerated megrim (Lepidorhombus whiffiagonis). Int. J. Food Sci. Technol. 2015, 50, 1891–1900. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Arulkumar, A.; Rosemary, T.; Paramasivam, S.; Rajendran, R.B. Phytochemical composition, in vitro antioxidant, antibacterial potential and GC-MS analysis of red seaweeds (Gracilaria corticata and Gracilaria edulis) from Palk Bay, India. Biocatal. Agricult. Biotechnol. 2018, 15, 63–71. [Google Scholar] [CrossRef]
- Ortiz-Viedma, J.; Aguilera, J.M.; Flores, M.; Lemus-Mondaca, R.; Larrazabal, M.J.; Miranda, J.M.; Aubourg, S.P. Protective effect of red algae (Rhodophyta) extracts on essential dietary components of heat-treated salmon. Antioxidants 2021, 10, 1108. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jónsdóttir, R.; Kristinsson, H.G.; Hreggvidsson, G.O.; Jónsson, J.O.; Thorkelsson, G.; Ólafsdóttir, G. Enzyme-enhanced extraction of antioxidant ingredients from red alga Palmaria palmata. LWT-Food Sci. Technol. 2010, 43, 1387–1393. [Google Scholar] [CrossRef]
- Agarwal, P.; Kayala, P.; Chandrasekaran, N.; Mukherjee, A.; Shah, S.; Thomas, J. Antioxidant and antibacterial activity of Gelidium pusillum (Stackhouse) against Aeromonas caviae and its applications in aquaculture. Aquac. Intern. 2021, 29, 845–858. [Google Scholar] [CrossRef]
- Corato, U.D.; Salimbeni, R.; De Pretis, A.; Avella, N.; Patruno, G. Antifungal activity of crude extracts from brown and red seaweeds by a supercritical carbon dioxide technique against fruit postharvest fungal diseases. Postharv. Biol. Technol. 2017, 131, 16–30. [Google Scholar] [CrossRef]
- Cui, M.; Zhou, R.; Wang, Y.; Zhang, M.; Liu, K.; Ma, C. Beneficial effects of sulfated polysaccharides from the red seaweed Gelidium pacificum Okamura on mice with antibiotic-associated diarrhea. Food Funct. 2020, 11, 4625–4637. [Google Scholar] [CrossRef]
- Seedevi, P.; Moovendhan, M.; Viramani, S.; Shanmugam, A. Bioactive potential and structural chracterization of sulfated polysaccharide from seaweed (Gracilaria corticata). Carb. Polym. 2017, 155, 516–524. [Google Scholar] [CrossRef]
- Lebbar, S.; Fanuel, M.; Gall, S.L.; Falourd, X.; Ropartz, D.; Bressollier, P.; Gloaguen, V.; Faugeron-Girard, C. Agar extraction by-products from Gelidium sesquipedale as a source of glycerol-galactosides. Molecules 2018, 23, 3364. [Google Scholar] [CrossRef] [Green Version]
- Pei, R.; Zhai, H.; Qi, B.; Hao, S.; Huang, H.; Yang, X. Isolation, purification and monosaccharide composition analysis of polysaccharide from Gelidium amansii. Food Ferment. Indust. 2020, 7, 57–62. [Google Scholar]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macrom. 2020, 159, 1165–1176. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, Q.; Wang, Y.; Yang, Q.; Yu, H.; Li, H.; Chen, J.; Fu, L. Sulfated polysaccharides from red seaweed Gelidium amansii: Structural characteristics, antioxidant and anti-glycation properties, and development of bioactive films. Food Hydroc. 2021, 119, 106820. [Google Scholar] [CrossRef]
- Lim, G.O.; Hong, Y.H.; Song, K.B. Incorporating grapefruit seed extract into Gelidium corneum-whey protein isolate blend packaging film increases the shelf life of fish paste. J. Food Sci. Nutr. 2008, 13, 370–374. [Google Scholar] [CrossRef]
- Barbosa, R.G.; Trigo, M.; Dovale, G.; Rodríguez, A.; Aubourg, S.P. Antioxidant and antimicrobial behavior of alga Gracilaria gracilis extracts during hake (Merluccius merluccius) chilled storage. Bulg. Chem. Comm. 2018, 50, 118–124. [Google Scholar]
- Arulkumar, A.; Satheeshkumar, K.; Paramasivam, S.; Rameshthangam, P.; Miranda, J.M. Chemical biopreservative effects of red seaweed on the shelf life of black tiger shrimp (Penaeus monodon). Foods 2020, 9, 634. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, O.; Losada, V.; Aubourg, S.P.; Barros-Velázquez, J. Sensory, microbial and chemical effects of a slurry ice system on horse mackerel (Trachurus trachurus). J. Sci. Food Agric. 2005, 85, 235–242. [Google Scholar] [CrossRef] [Green Version]
- García-Soto, B.; Aubourg, S.P.; Calo-Mata, P.; Barros-Velázquez, J. Extension of the shelf life of chilled hake (Merluccius merluccius) by a novel icing medium containing natural organic acids. Food Cont. 2013, 34, 356–363. [Google Scholar] [CrossRef] [Green Version]
- Ben-Gigirey, B.; Sousa, J.V.B.d.; Villa, T.; Barros-Velázquez, J. Characterization of biogenic amine-producing Stenotrophomonas maltophilia strains isolated from white muscle of fresh and frozen albacore tuna. Int. J. Food Microb. 2000, 57, 19–31. [Google Scholar] [CrossRef]
- Antonacopoulos, N. Verbesserte Apparatus zur quantitativen Destillation wasserdampfflüchtiger Stoffe. Zeitschrift für Lebensmittel-Untersuchung und Forschung. 1960, 13, 113–160. [Google Scholar] [CrossRef]
- Tozawa, H.; Erokibara, K.; Amano, K. Proposed modification of Dyer’s method for trimethylamine determination in codfish. In Fish Inspection and Quality Control; Kreuzer, R., Ed.; Fishing News Books Ltd.: London, UK, 1971; pp. 187–190. [Google Scholar]
- El-Baroty, G.S.; El-Baz, F.K.; Abd-Elmoein, A.; El-Baky, H.H.A.; Ali, M.M.; Ibrahim, A.E. Evaluation of glycolipids of some Egyptian marine algae as a source of bioactive substances. Electr. J. Environm. Agric. Food Chem. 2011, 10, 2114–2128. [Google Scholar]
- Zeid, A.H.A.; Aboutabl, E.A.; Sleem, A.A.; El-Rafie, H.M. Water soluble polysaccharides extracted from Pterocladia capillacea and Dictyopteris membranacea and their biological activities. Carb. Polym. 2014, 113, 62–66. [Google Scholar] [CrossRef]
- Miranda, J.M.; Zhang, B.; Barros-Velázquez, J.; Aubourg, S.P. Preservative effect of aqueous and ethanolic extracts of the macroalga Bifurcaria bifurcata on the quality of chilled hake (Merluccius merluccius). Molecules 2021, 26, 3774. [Google Scholar] [CrossRef] [PubMed]

| Salmonella enterica CECT 4396 |
| Pseudomonas fluorescens ATCC 17397 |
| Pseudomonas putida ATCC 12633 |
| Escherichia coli CECT 4387 |
| Bacillus subtilis ATCC 6051 |
| Bacillus cereus CECT 148 |
| Bacillus licheniformis ATCC 27811 |
| Vibrio alginolyticus ATCC 17749 |
| Vibrio parahaemolyticus ATCC 17802 |
| Proteus mirabilis ATCC 29906 |
| Enterobacter aerogenes ATCC 13048 |
| Listeria monocytogenes CECT 4032 |
| Klebsiella pneumoniae ATCC 9997 |
| Diameter of Inhibition Zone (mm) | |||
|---|---|---|---|
| Volume of Alga Extract | |||
| Bacterial Species | 10 µL | 20 µL | 25 µL |
| Salmonella enterica CECT 4396 | 10 | 11 | 12 |
| Pseudomonas fluorescens ATCC 17397 | ND * | ND | ND |
| Pseudomonas putida ATCC 12633 | ND | ND | ND |
| Escherichia coli CECT 4387 | ND | 13 | 14 |
| Bacillus subtilis ATCC 6051 | 10 | 12 | 13 |
| Bacillus cereus CECT 148 | ND | 11 | 14 |
| Bacillus licheniformis ATCC 27811 | ND | ND | 11 |
| Vibrio alginolyticus ATCC 17749 | ND | ND | 13 |
| Vibrio parahaemolyticus ATCC17802 | ND | 11 | 12 |
| Proteus mirabilis ATCC 29906 | ND | ND | 11 |
| Enterobacter aerogenes ATCC 13048 | ND | 11 | 13 |
| Listeria monocytogenes CECT 4032 | ND | ND | 11 |
| Klebsiella pneumoniae ATCC 9997 | ND | 11 | 13 |
| Bacterial Species | Breakpoint * |
|---|---|
| Salmonella enterica CECT 4396 | 12.5 mg·mL−1 |
| Escherichia coli CECT 4387 | 50 mg·mL−1 |
| Bacillus subtilis ATCC 6051 | 3.125 mg·mL−1 |
| Bacillus cereus CECT 148 | 0.625 mg·mL−1 |
| Bacillus licheniformis ATCC 27811 | 25 mg·mL−1 |
| Proteus mirabilis ATCC 29906 | 50 mg·mL−1 |
| Enterobacter aerogenes ATCC 13048 | 25 mg·mL−1 |
| Listeria monocytogenes CECT 4032 | 50 mg·mL−1 |
| Klebsiella pneumoniae ATCC 9997 | 50 mg·mL−1 |
| Microbial Group | Icing Condition | Chilling Time (Days) | ||
|---|---|---|---|---|
| 0 | 4 | 11 | ||
| Psychrotrophs | CT | 2.99 A (0.53) | 3.89 aB (0.15) | 7.65 bC (0.28) |
| AFE-1 | 3.53 aA (0.66) | 7.65 bB (0.37) | ||
| AFE-2 | 3.58 aA (0.48) | 6.29 aB (0.04) | ||
| AFE-3 | 3.80 aB (0.21) | 6.36 aC (0.07) | ||
| Enterobacteriaceae | CT | 1.00 A (0.00) | 1.10 aA (0.17) | 3.30 bB (0.30) |
| AFE-1 | 1.10 aA (0.17) | 2.51 abB (0.57) | ||
| AFE-2 | 1.10 aA (0.17) | 3.11 bB (0.64) | ||
| AFE-3 | 1.20 aA (0.35) | 2.19 aB (0.61) | ||
| Lipolytics | CT | 2.00 A (0.00) | 3.47 bB (0.16) | 5.65 aC (0.30) |
| AFE-1 | 2.75 aB (0.12) | 5.34 aC (0.44) | ||
| AFE-2 | 2.40 aA (0.46) | 5.28 aB (0.43) | ||
| AFE-3 | 2.79 aB (0.31) | 5.50 aC (0.61) | ||
| Chemical Parameter | Icing Condition | Chilling Time (Days) | ||
|---|---|---|---|---|
| 0 | 4 | 11 | ||
| pH | CT | 5.70 A (0.02) | 5.89 aB (0.09) | 6.14 bC (0.05) |
| AFE-1 | 5.80 aA (0.08) | 5.99 aB (0.06) | ||
| AFE-2 | 5.91 aAB (0.17) | 6.04 abB (0.12) | ||
| AFE-3 | 5.97 aB (0.11) | 6.04 abB (0.14) | ||
| Total volatile base-nitrogen (TVB-N; mg·kg−1 muscle) | CT | 257.15 A (5.14) | 257.10 aA (23. 50) | 315.77 bB (5.62) |
| AFE-1 | 260.66 aA (6.31) | 311.23 bC (4.52) | ||
| AFE-2 | 260.60 aA (6.77) | 300.21 abB (21.60) | ||
| AFE-3 | 269.18 aAB (19.01) | 283.48 aB (14.28) | ||
| Trimethylamine-nitrogen (TMA-N; mg·kg−1 muscle) | CT | 0.55 A (0.13) | 3.43 bB (0.71) | 23.33 bC (0.43) |
| AFE-1 | 1.67 aB (0.48) | 13.61 aC (1.73) | ||
| AFE-2 | 1.41 aB (0.43) | 12.95 aC (4.13) | ||
| AFE-3 | 1.83 aB (0.58) | 11.59 aC (3.85) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, J.M.; Trigo, M.; Barros-Velázquez, J.; Aubourg, S.P. Antimicrobial Activity of Red Alga Flour (Gelidium sp.) and Its Effect on Quality Retention of Scomber scombrus during Refrigerated Storage. Foods 2022, 11, 904. https://doi.org/10.3390/foods11070904
Miranda JM, Trigo M, Barros-Velázquez J, Aubourg SP. Antimicrobial Activity of Red Alga Flour (Gelidium sp.) and Its Effect on Quality Retention of Scomber scombrus during Refrigerated Storage. Foods. 2022; 11(7):904. https://doi.org/10.3390/foods11070904
Chicago/Turabian StyleMiranda, José M., Marcos Trigo, Jorge Barros-Velázquez, and Santiago P. Aubourg. 2022. "Antimicrobial Activity of Red Alga Flour (Gelidium sp.) and Its Effect on Quality Retention of Scomber scombrus during Refrigerated Storage" Foods 11, no. 7: 904. https://doi.org/10.3390/foods11070904
APA StyleMiranda, J. M., Trigo, M., Barros-Velázquez, J., & Aubourg, S. P. (2022). Antimicrobial Activity of Red Alga Flour (Gelidium sp.) and Its Effect on Quality Retention of Scomber scombrus during Refrigerated Storage. Foods, 11(7), 904. https://doi.org/10.3390/foods11070904








