Comparative Fatty Acid Profiling of Edible Fishes in Kuala Terengganu, Malaysia
Abstract
:1. Introduction
1.1. Fish as Source of Valuable Fatty Acids
1.2. The Importance of the Taxonomic and Nutritional Characterization of Malaysian Fishes
2. Materials and Methods
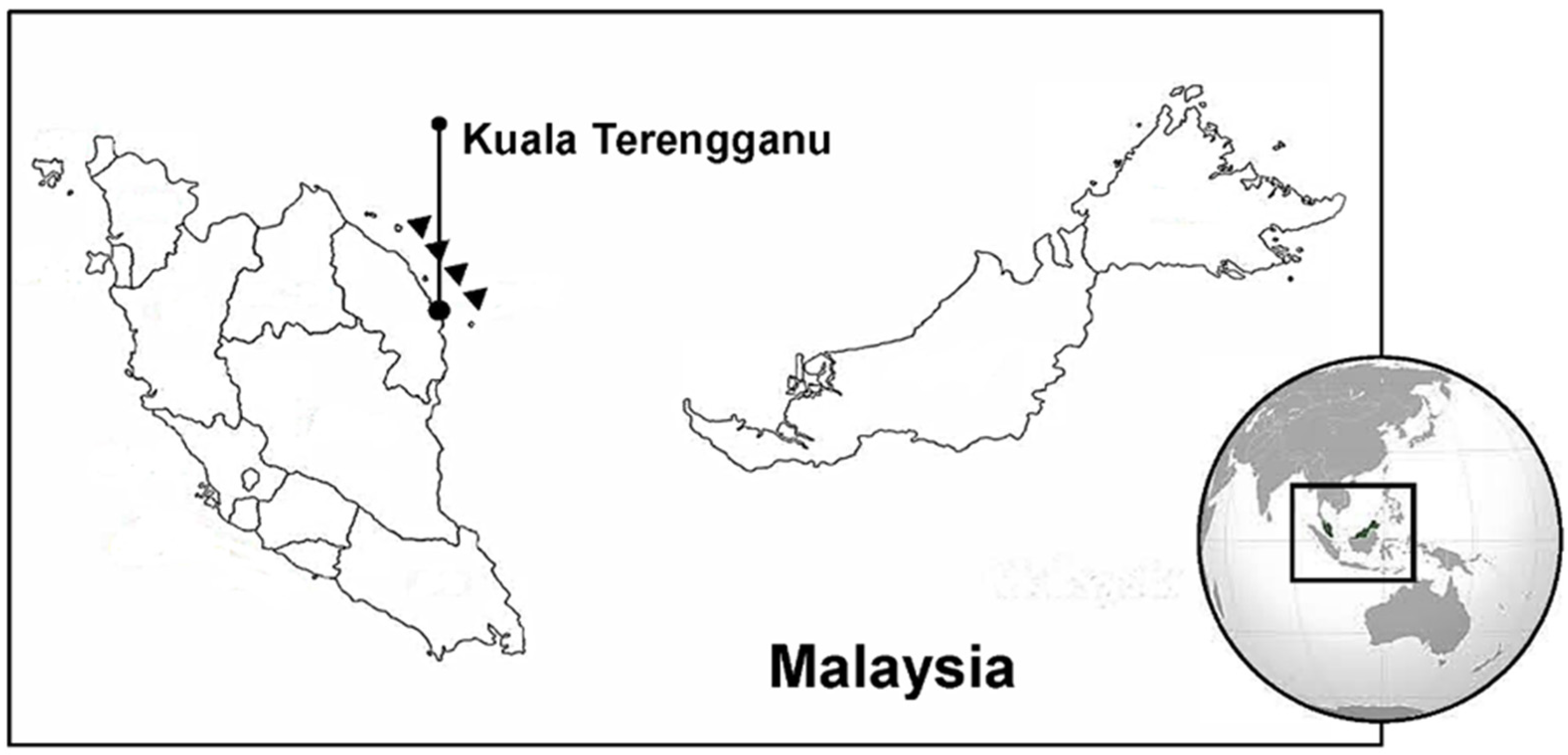
2.1. Site of Fish Collection
2.2. Identification of Malaysian Fish Species
2.3. Metals Quantification
2.4. Lipid Extraction
2.5. Fatty Acids Analysis
2.6. Statistical Analysis
3. Results
3.1. Fish Identification
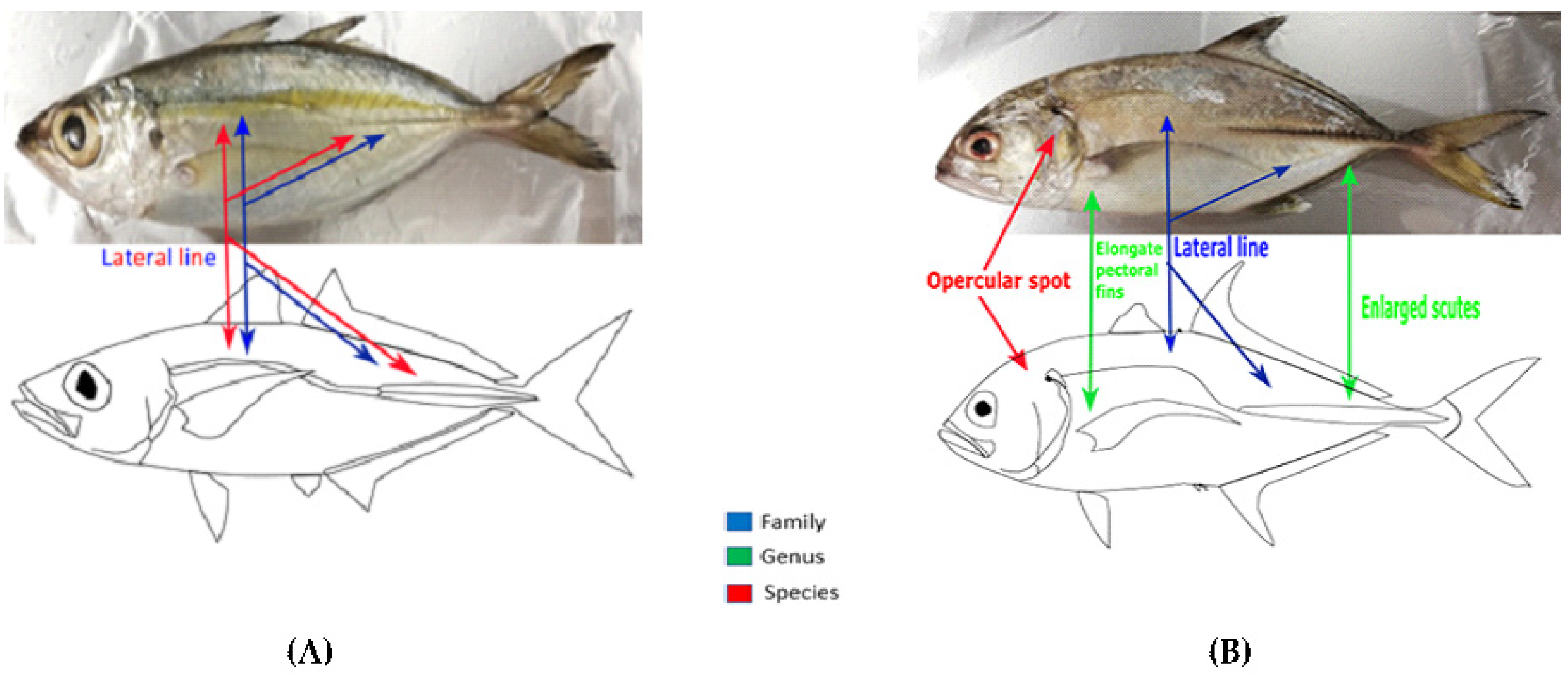
3.1.1. Morphological Identification
3.1.2. Barcoding Sequences
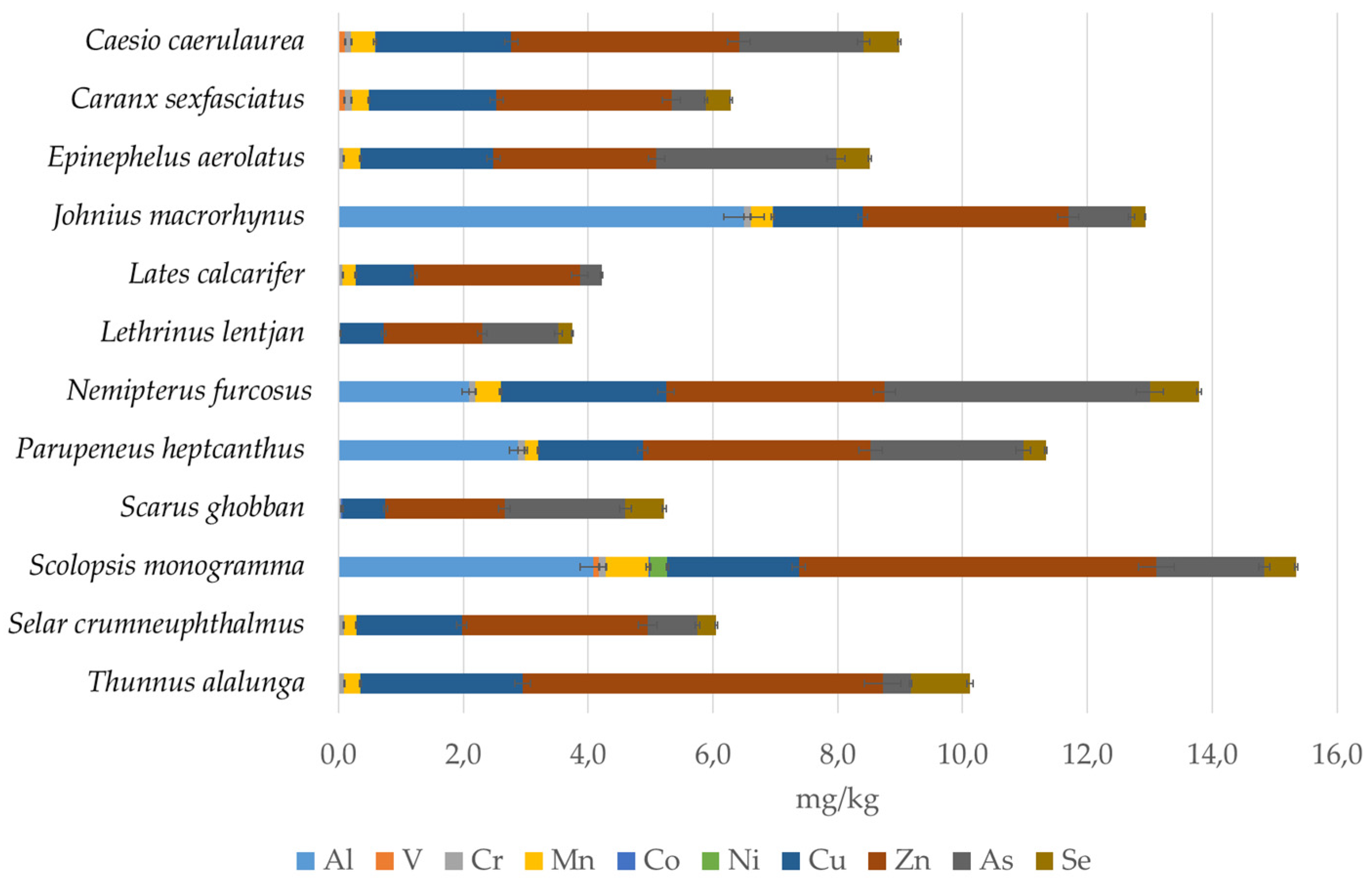
3.2. Metals Accumulation
3.3. Fatty Acids Profile
4. Discussion
4.1. Fish Identification
4.2. Metal Accumulation
4.3. Fatty Acid Profile
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sawe, B.E. Native Fish of Malaysia. Available online: https://www.worldatlas.com/articles/native-fish-of-malaysia.html (accessed on 10 August 2020).
- Malaysian Fisheries Society: MFS. Available online: http://www.mfs.org.my (accessed on 20 May 2020).
- Mazzeo, M.F.; Giulio, B.D.; Guerriero, G.; Ciarcia, G.; Malorni, A.; Russo, G.L.; Siciliano, R.A. Fish Authentication by MALDI-TOF Mass Spectrometry. J. Agric. Food Chem. 2008, 56, 11071–11076. [Google Scholar] [CrossRef]
- Di Finizio, A.; Guerriero, G.; Russo, G.L.; Ciarcia, G. Identification of Gadoid Species (Pisces, Gadidae) by Sequencing and PCR–RFLP Analysis of Mitochondrial 12S and 16S RRNA Gene Fragments. Eur. Food Res. Technol. 2007, 225, 337–344. [Google Scholar] [CrossRef]
- Guerriero, G.; Rabbito, D.; Alwany, M.A.; Madonna, A.; Temraz, T.A.; Sulaiman, O.O.; Bassem, S.M.; Trocchia, S.; Abdel-Gawad, F.K.; Ciarcia, G. Fisheries and Biodiversity along Mediterranean Sea: Italian and Egyptian Coast Overview. Euro-Mediterr. J. Environ. Integr. 2017, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, C.; García-Moreno, P.J.; Yesiltas, B.; Sørensen, A.-D.M. (Eds.) Lipid Oxidation and Traditional Methods for Evaluation. In Omega-3 Delivery Systems; Elsevier: San Diego, CA, USA, 2021; pp. 183–200. [Google Scholar]
- Félix, R.; Valentão, P.; Andrade, P.B.; Félix, C.; Novais, S.C.; Lemos, M.F.L. Evaluating the in vitro Potential of Natural Extracts to Protect Lipids from Oxidative Damage. Antioxidants 2020, 9, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slivinska, L.G.; Shcherbatyy, A.R.; Lukashchuk, B.O.; Gutyj, B.V. The State of Antioxidant Protection System in Cows under the Influence of Heavy Metals. Regul. Mech. Biosyst. 2020, 11, 237–242. [Google Scholar] [CrossRef]
- Leonard, S.S.; Harris, G.K.; Shi, X. Metal-Induced Oxidative Stress and Signal Transduction. Free Radic. Biol. Med. 2004, 37, 1921–1942. [Google Scholar] [CrossRef]
- Guerriero, G.; Trocchia, S.; Abdel Gawad, F.K.; Ciarcia, G. Roles of reactive oxygen species in the spermatogenesis regulation. Front. Endocrinol. 2014, 5, 56. [Google Scholar] [CrossRef] [Green Version]
- Rikans, L.E.; Hornbrook, K.R. Lipid Peroxidation, Antioxidant Protection and Aging. Biochim. Biophys. Acta BBA Mol. Basis Dis. 1997, 1362, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Viarengo, A.; Canesi, L.; Pertica, M.; Poli, G.; Moore, M.N.; Orunesu, M. Heavy Metal Effects on Lipid Peroxidation in the Tissues of Mytilus galloprovincialis Lam. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1990, 97, 37–42. [Google Scholar] [CrossRef]
- Velasco, J.; Dobarganes, C.; Márquez-Ruiz, G. Oxidative Rancidity in Foods and Food Quality. In Chemical Deterioration and Physical Instability of Food and Beverages; Skibsted, L.H., Risbo, J., Andersen, M.L., Eds.; Elsevier: Cambridge, UK, 2010; pp. 3–32. [Google Scholar]
- Guerriero, G.; Bassem, S.M.; Khalil, W.K.B.; Temraz, T.A.; Ciarcia, G.; Abdel-Gawad, F.K. Temperature changes and marine fish species (Epinephelus coioides and Sparus aurata): Role of oxidative stress biomarkers in toxicological food studies. Emir. J. Food Agric. 2018, 30, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Gawad, F.K.; Khalil, W.K.B.; Bassem, S.M.; Kumar, V.; Parisi, C.; Inglese, S.; Temraz, T.A.; Nassar, H.F.; Guerriero, G. The duckweed, Lemna minor modulates heavy metal-induced oxidative stress in the Nile tilapia Oreochromis niloticus. Water 2020, 12, 2983. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Luchtman, D.W.; Song, C. Cognitive Enhancement by Omega-3 Fatty Acids from Childhood to Old Age: Findings from Animal and Clinical Studies. Neuropharmacology 2013, 64, 550–565. [Google Scholar] [CrossRef]
- Lavie, C.J.; Milani, R.V.; Mehra, M.R.; Ventura, H.O. Omega-3 Polyunsaturated Fatty Acids and Cardiovas-Cular Diseases. J. Am. Coll. Cardiol. 2009, 54, 585–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djoussé, L.; Akinkuolie, A.O.; Wu, J.H.Y.; Ding, E.L.; Gaziano, J.M. Fish Consumption, Omega-3 Fatty Acids and Risk of Heart Failure: A Meta-Analysis. Clin. Nutr. 2012, 31, 846–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chattipakorn, N.; Settakorn, J.; Petsophonsakul, P.; Suwannahoi, P.; Mahakranukrauh, P.; Srichairatanakool, S.; Chattipakorn, S.C. Cardiac Mortality Is Associated with Low Levels of Omega-3 and Omega-6 Fatty Acids in the Heart of Cadavers with a History of Coronary Heart Disease. Nutr. Res. 2009, 29, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Oehlenschläger, J. Seafood: Nutritional Benefits and Risk Aspects. Int. J. Vitam. Nutr. Res. 2012, 82, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Łuczyńska, J.; Paszczyk, B. Health Risk Assessment of Heavy Metals and Lipid Quality Indexes in Freshwater Fish from Lakes of Warmia and Mazury Region, Poland. Int. J. Environ. Res. Public Health 2019, 16, 3780. [Google Scholar] [CrossRef] [Green Version]
- Salini, M.J.; Wade, N.M.; Araújo, B.C.; Turchini, G.M.; Glencross, B.D. Eicosapentaenoic Acid, Arachidonic Acid and Eicosanoid Metabolism in Juvenile Barramundi Lates calcarifer. Lipids 2016, 51, 973–988. [Google Scholar] [CrossRef]
- Connor, S.L.; Gustafson, J.R.; Sexton, G.; Becker, N.; Artaud-Wild, S.; Connor, W.E. The Diet Habit Survey: A New Method of Dietary Assessment That Relates to Plasma Cholesterol Changes. J. Am. Diet. Assoc. 1992, 92, 41–47. [Google Scholar] [CrossRef]
- Pal, J.; Shukla, B.N.; Maurya, A.K.; Verma, H.O.; Pandey, G. Amitha A Review on Role of Fish in Human Nutrition with Special Emphasis to Essential Fatty Acid. Int. J. Fish. Aquat. Stud. 2018, 6, 427–430. [Google Scholar]
- Metillo, E.B.; Aspiras-Eya, A.A. Fatty Acids in Six Small Pelagic Fish Species and Their Crustacean Prey from the Mindanao Sea, Southern Philippines. Trop. Life Sci. Res. 2014, 25, 105–115. [Google Scholar] [PubMed]
- Van der Meeren, T.; Olsen, R.E.; Hamre, K.; Fyhn, H.J. Biochemical Composition of Copepods for Evaluation of Feed Quality in Production of Juvenile Marine Fish. Aquaculture 2008, 274, 375–397. [Google Scholar] [CrossRef] [Green Version]
- Jackson, G.D.; Bustamante, P.; Cherel, Y.; Fulton, E.A.; Grist, E.P.M.; Jackson, C.H.; Nichols, P.D.; Pethybridge, H.; Phillips, K.; Ward, R.D.; et al. Applying New Tools to Cephalopod Trophic Dynamics and Ecology: Perspectives from the Southern Ocean Cephalopod Workshop, February 2–3, 2006. Rev. Fish Biol. Fish. 2007, 17, 79–99. [Google Scholar] [CrossRef]
- Boecklen, W.J.; Yarnes, C.T.; Cook, B.A.; James, A.C. On the Use of Stable Isotopes in Trophic Ecology. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 411–440. [Google Scholar] [CrossRef] [Green Version]
- Traugott, M.; Kamenova, S.; Ruess, L.; Seeber, J.; Plantegenest, M. Empirically Characterising Trophic Networks: What Emerging DNA-Based Methods, Stable Isotope and Fatty Acid Analyses Can Offer. Adv. Ecol. Res. 2013, 49, 177–224. [Google Scholar]
- Daly, E.A.; Benkwitt, C.E.; Brodeur, R.D.; Litz, M.N.C.; Copeman, L.A. Fatty Acid Profiles of Juvenile Salmon Indicate Prey Selection Strategies in Coastal Marine Waters. Mar. Biol. 2010, 157, 1975–1987. [Google Scholar] [CrossRef]
- Arai, T.; Amalina, R.; Bachok, Z. Diverse Feeding Ecology and Habitat Use in Coral Reef Fishes in the Malay-Sian South China Sea, as Revealed by Liver Fatty Acid Composition. Oceanol. Hydrobiol. Stud. 2015, 44, 120–126. [Google Scholar] [CrossRef]
- Dhurmeea, Z.; Pethybridge, H.; Appadoo, C.; Bodin, N. Lipid and Fatty Acid Dynamics in Mature Female Al-Bacore Tuna (Thunnus alalunga) in the Western Indian Ocean. PLoS ONE 2018, 13, e0194558. [Google Scholar] [CrossRef]
- Cladis, D.P.; Kleiner, A.C.; Freiser, H.H.; Santerre, C.R. Fatty Acid Profiles of Commercially Available Finfish Fillets in the United States. Lipids 2014, 49, 1005–1018. [Google Scholar] [CrossRef]
- FAO. International Symposium on Fisheries Sustainability: Strengthening the Science-Policy Nexus, 18–21 November 2019. Rome. Available online: http://www.fao.org/3/ca6763en/CA6763EN.pdf (accessed on 5 May 2020).
- Azmi, L.S. Physical and Chemical Characterization of Iron Ore from East Coast of Peninsular Malaysia. 2018. Available online: https://ethesis.usm.my/jspui/bitstream/123456789/12122/1/Physical%20and%20chemical%20characterization%20of%20iron%20ore%20from%20east%20coast%20of%20peninsular%20Malaysia_Latifah%20Suha%20Azmi_B1_2018_MJMS.pdf (accessed on 27 June 2020).
- FAO. Available online: http://www.fao.org/about/en/ (accessed on 5 May 2021).
- WoRMS—World Register of Marine Species. Available online: https://www.marinespecies.org/about.php (accessed on 1 May 2021).
- FishBase: A Global Information System on Fishes. Available online: http://www.fishbase.org/home.htm (accessed on 21 August 2020).
- Inkscape. Available online: https://inkscape.org/it/ (accessed on 2 May 2020).
- Ward, R.D. DNA Barcode Divergence among Species and Genera of Birds and Fishes. Mol. Ecol. Resour. 2009, 9, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Madonna, A.; Alwany, M.A.; Rabbito, R.; Trocchia, S.; Labar, S.; Abdel-Gawad, F.K.; D’Angelo, R.; Gallo, A.; Guerriero, G.; Ciarcia, G. Caves Biodiversity in the Marine Area of Riviera d’Ulisse Regional Park, Italy: Grotta Del Maresciallo Overview. J. Biodivers. Endanger. Species 2015, 3, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Gentilucci, M.; Parisi, C.; Coppola, M.R.; Majdoubi, F.-Z.; Madonna, M.; Guerriero, G. Influence of Mediterranean Sea Temperature Increase on Gaeta Gulf (Tyrrhenian Sea) Biodiversity. Proc. Zool. Soc. 2021, 74, 91–103. [Google Scholar] [CrossRef]
- Available online: http://www.ncbi.nlm.nih.gov (accessed on 5 June 2020).
- Mount, D.W. Using a FASTA Sequence Database Similarity Search. Cold Spring Harb. Protoc. 2007, 7, pdb-top16. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, G.; Notariale, R.; Carusone, N.; Giarra, A.; Trifuoggi, M.; Manna, C.; Piscopo, M. New Insights into Alterations in PL Proteins Affecting Their Binding to DNA after Exposure of Mytilus galloprovincialis to Mercury—A Possible Risk to Sperm Chromatin Structure? Int. J. Mol. Sci. 2021, 22, 5893. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Christie, W.W. Preparation of Ester Derivatives of Fatty Acids for Chromatographic Analysis. Adv. Lipid Methodol. 1993, 2, e111. [Google Scholar]
- Tvrzická, E. Analysis of Fatty Acids in Plasma Lipoproteins by Gas Chromatography–Flame Ionization Detec-Tion: Quantitative Aspects. Anal. Chim. Acta 2002, 465, 337–350. [Google Scholar] [CrossRef]
- Prashanth, L.; Kattapagari, K.K.; Chitturi, R.T.; Baddam, V.R.R.; Prasad, L.K. A Review on Role of Essential Trace Elements in Health and Disease. J. NTR Univ. Health Sci. 2015, 4, 75–85. [Google Scholar] [CrossRef]
- Fischer, J. (Ed.) Fish Identification Tools for Biodiversity and Fisheries Assessments: Review and Guidance for Decision-Makers; FAO Fisheries and Aquaculture Technical Paper 2013, No. 585; FAO: Rome, Italy, 2013; pp. 1–118. Available online: http://www.fao.org/3/i3354e/i3354e.pdf (accessed on 21 May 2020).
- Malaysia-Food-Act-1983. Available online: http://extwprlegs1.fao.org/docs/pdf/mal27309.pdf (accessed on 2 May 2020).
- Arai, T.; Amalina, R.; Bachok, Z. Fatty Acid Composition Indicating Diverse Habitat Use in Coral Reef Fishes in the Malaysian South China Sea. Biol. Res. 2015, 48, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Arai, T.; Amalina, R.; Bachok, Z. Variation in Fatty Acid Composition of the Bigeye Snapper Lutjanus lutjanus Collected in Coral Reef Habitats of the Malaysian South China Sea. J. Biol. Res. 2015, 22, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentilucci, M.; Moustafa, A.A.; Abdel-Gawad, F.K.; Mansour, S.R.; Coppola, M.R.; Caserta, L.; Inglese, S.; Pambianchi, G.; Guerriero, G. Advances in Egyptian Mediterranean Coast Climate Change Monitoring. Water 2021, 13, 1870. [Google Scholar] [CrossRef]
- Kamaruzzaman, B.Y.; Rina, Z.; John, B.A.; Jalal, K.C.A. Heavy Metal Accumulation in Commercially Important Fishes of South West Malaysian Coast. Res. J. Environ. Sci. 2011, 5, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Ong, M.C.; Gan, S.L. Assessment of Metallic Trace Elements in the Muscles and Fins of Four Landed Elasmobranchs from Kuala Terengganu Waters, Malaysia. Mar. Pollut. Bull. 2017, 124, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Ariano, A.; Musco, N.; Severino, L.; De Maio, A.; Tramice, A.; Tommonaro, G.; Damiano, S.; Genovese, A.; Olanrewaju, O.S.; Bovera, F.; et al. Chemistry of Tropical Eucheumatoids: Potential for Food and Feed Applications. Biomolecules 2021, 11, 804. [Google Scholar] [CrossRef]
- Azaman, F.; Juahir, H.; Yunus, K.; Azid, A.; Kamarudin, M.K.A.; Toriman, M.E.; Mustafa, A.D.; Amran, M.A.; Che Hasnam, C.N.; Mohd Saudi, A.S. Heavy Metal in Fish: Analysis and Human Health—A Review. J. Teknol. 2015, 77, 61–69. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization; Food and Agriculture Organization of the United Nations; International Atomic Energy Agency (Eds.) Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996; pp. 1–360. ISBN 978-92-4-156173-0. [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Arsenic; US Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2000. Available online: https://www.atsdr.cdc.gov (accessed on 27 May 2021).
- Pizent, A.; Tariba, B.; Zivkovic, T. Reproductive Toxicity of Metals in Men. Arh. Hig. Rada Toksikol. 2012, 63, 35–46. [Google Scholar] [CrossRef]
- Yunus, S.M.; Hamzah, Z.; Ariffin, N.A.N.; Muslim, M.B. Cadmium, Chromium, Copper, Lead, Ferrum and Zinc Levels in the Cockles (Anadara granosa) from Kuala Selangor, Malaysia. Malays. J. Anal. Sci. 2014, 18, 514–521. [Google Scholar]
- Strobel, C.; Jahreis, G.; Kuhnt, K. Survey of n-3 and n-6 Polyunsaturated Fatty Acids in Fish and Fish Products. Lipids Health Dis. 2012, 11, 144. [Google Scholar] [CrossRef] [Green Version]
- Vlahogianni, T.H.; Valavanidis, A. Heavy-Metal Effects on Lipid Peroxidation and Antioxidant Defence Enzymes in Mussels Mytilus galloprovincialis. Chem. Ecol. 2007, 23, 361–371. [Google Scholar] [CrossRef]
- Majdoubi, F.-Z.; Ouizgane, A.; Farid, S.; Mossetti, L.; Droussi, M.; Guerriero, G.; Hasnaoui, M. Fry Survival Rate as a Predictive Marker of Optimal Production of Silver Carp (Hypophthalmichthys molitrix, Valenciennes 1844): A Biostatistical Study in Deroua Fish Farm, Morocco. Proc. Zool. Soc. 2021. [Google Scholar] [CrossRef]
- Huynh, M.D.; Kitts, D.D. Evaluating Nutritional Quality of Pacific Fish Species from Fatty Acid Signatures. Food Chem. 2009, 114, 912–918. [Google Scholar] [CrossRef]
- Garrido, S.; Rosa, R.; Ben-Hamadou, R.; Cunha, M.E.; Chícharo, M.A.; Lingen, C.D. Spatio-Temporal Variability in Fatty Acid Trophic Biomarkers in Stomach Contents and Muscle of Iberian Sardine (Sardina pilchardus) and Its Relationship with Spawning. Mar. Biol. 2008, 154, 1053–1065. [Google Scholar] [CrossRef]
- Gibson, R.A.; Kneebone, R.; Kneebone, G.M. Comparative Levels of Arachidonic Acid and Eicosapentaenoic Acid in Malaysian Fish. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1984, 78, 325–328. [Google Scholar] [CrossRef]
- Rawdah, T.; Elfaer, M. Fatty-Acid Composition of Three Commercially Important Fish of the Arabian Gulf. Food Chem. 1994, 51, 193–196. [Google Scholar] [CrossRef]
- Manthey-Karl, M.; Lehmann, I.; Ostermeyer, U.; Schröder, U. Natural Chemical Composition of Commercial Fish Species: Characterisation of Pangasius, Wild and Farmed Turbot and Barramundi. Foods 2016, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, R.S.; Morrissey, M.T.; Roblero, J. Fatty Acid Composition of U.S. West Coast Albacore Tuna (Thunnus alalunga) and the Effects of Canning and Short-Term Storage. J. Aquat. Food Prod. Technol. 2008, 17, 441–458. [Google Scholar] [CrossRef]
- Galindo, A.; Garrido, D.; Monroig, Ó.; Pérez, J.A.; Betancor, M.B.; Acosta, N.G.; Kabeya, N.; Marrero, M.A.; Bolaños, A.; Rodríguez, C. Polyunsaturated Fatty Acid Metabolism in Three Fish Species with Different Trophic Level. Aquaculture 2021, 530, 735–761. [Google Scholar] [CrossRef]
- Rodrigues, B.L.; da Cruz Silva Canto, A.C.V.; da Costa, M.P.; da Silva, F.A.; Mársico, E.T.; Conte-Junior, C.A. Fat-Ty Acid Profiles of Five Farmed Brazilian Freshwater Fish Species from Different Families. PLoS ONE 2017, 12, e0178898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, C.E.; da Silva Vasconcelos, M.A.; de Almeida Ribeiro, M.; Sarubbo, L.A.; Andrade, S.A.C.; de Melo Filho, A.B. Nutritional and Lipid Profiles in Marine Fish Species from Brazil. Food Chem. 2014, 160, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Her Majesty’s Stationery Office. Nutritional Aspects of Cardiovascular Disease; Report on Health and Social Subjects 1994. No. 46; HMSO: London, UK, 1994. [Google Scholar]
- Crexi, V.T.; Monte, M.L.; de Souza Soares, L.A.; Pinto, L.A.A. Production and Refinement of Oil from Carp (Cyprinus carpio) Viscera. Food Chem. 2010, 119, 945–950. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Labelling Reference Intake Values for n-3 and n-6 Polyunsaturated Fatty Acids: Labelling Reference Intake Values for n-3 and n-6 Polyunsaturated Fatty Acids. EFSA J. 2009, 1176, 1–11. [Google Scholar]
| English Common Name/Local Fish Common Name | Identified Species Taxonomy (Genus, Species, Family) | COI Genbank Accession Number | |
|---|---|---|---|
| 1 | Blue and gold fusilier/ Delah biru emas | Caesio caerulaurea Caesionidae (Lutjanoidea) | JF492993.1 |
| 2 | Bigeye trevally/ Belukok putih | Caranx sexfasciatus Carangidae | KY371313.1 |
| 3 | Areolate grouper/ Kerapu bintik bulat | Epinephelus areolatus Serranidae | KJ202152.1 |
| 4 | Dwarf whipray/ Pari ketuka tanjung | Himantura walga Dasyatidae | KF604912.1 |
| 5 | Big-snout croaker/ Gelam bangkok | Johnius macrorhynus Sciaenidae | KX777976.1 |
| 6 | Barramundi/ Siakap putih | Lates calcarifer Latidae | JF919828.1 |
| 7 | Redspot emperor/ Landok calit merah | Lethrinus lentjan Lethrinidae | MF123937.1 |
| 8 | Fork-tailed threadfin bream/Kerisi | Nemipterus furcosus Nemipteridae | KY371807.1 |
| 9 | Cinnabar goatfish/ Biji nangka | Parupeneus heptacanthus Mullidae | KY371924.1 |
| 10 | Parrot fish/ Bayan belang biru | Scarus ghobban Scaridae | JF494439.1 |
| 11 | Monogrammed monocle bream/Pasir-pasir jalur jeap | Scolopsis monogramma Nemipterida | KP195008.1 |
| 12 | Bigeye scad/ Lolong jalur | Selar crumenophthalmus Carangidae | KY372157.1 |
| 13 | Longfin tuna/ Tuna sirip kuning | Thunnus alalunga Scombridae | KT074102.1 |
| Name | Be | Cd | Hg | Sb | Pb | |
|---|---|---|---|---|---|---|
| 1 | Caesio caerulaurea | <0.2 | <0.02 | <0.01 | <0.02 | 0.033 ± 0.007 |
| 2 | Caranx sexfasciatus | <0.2 | <0.02 | <0.01 | <0.02 | 0.024 ± 0.005 |
| 3 | Epinephelus aerolatus | <0.2 | <0.02 | 0.016 ± 0.003 | <0.02 | 0.039 ± 0.008 |
| 5 | Johnius macrorhynus | <0.2 | <0.02 | 0.024 ± 0.005 | <0.02 | <0.02 |
| 6 | Lates calcarifer | <0.2 | <0.02 | <0.01 | <0.02 | <0.02 |
| 7 | Lethrinus lentjan | <0.2 | <0.02 | <0.01 | <0.02 | 0.10 ± 0.02 |
| 8 | Nemipterus furcosus | <0.2 | <0.02 | 0.090 ± 0.018 | <0.02 | 0.031 ± 0.006 |
| 9 | Parupeneus heptcanthus | <0.2 | <0.02 | 0.023 ± 0.005 | <0.02 | 0.025 ± 0.005 |
| 10 | Scarus ghobban | <0.2 | <0.02 | <0.01 | <0.02 | 0.021 ± 0.004 |
| 11 | Scolopsis monogramma | <0.2 | <0.02 | 0.015 ± 0.003 | <0.02 | 0.059 ± 0.012 |
| 12 | Selar crumneuphthalmus | <0.2 | <0.02 | <0.01 | <0.02 | <0.02 |
| 13 | Thunnus alalunga | <0.2 | <0.02 | <0.01 | <0.02 | 0.033 ± 0.007 |
| Malaysian MTL 1 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
| European MTL 2 | --- | 0.05 | 0.50 | --- | 0.30 | |
| Name | Li | Al | V | Cr | Mn | Co | Ni | Cu | Zn | As | Se | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Caesio caerulaurea | <0.2 | <2 | 0.10 ± 0.02 | 0.10 ± 0.02 | 0.38 ± 0.08 | 0.020 ± 0.004 | <0.2 | 2.2 ± 0.4 | 3.6 ± 0.7 | 2.0 ± 0.4 | 0.58 ± 0.12 |
| 2 | Caranx sexfasciatus | <0.2 | <2 | 0.10 ± 0.02 | 0.11 ± 0.02 | 0.27 ± 0.05 | <0.02 | <0.2 | 2.1 ± 0.4 | 2.8 ± 0.6 | 0.6 ± 0.1 | 0.40 ± 0.08 |
| 3 | Epinephelus aerolatus | <0.2 | <2 | <0.1 | 0.08 ± 0.02 | 0.27 ± 0.05 | <0.02 | <0.2 | 2.1 ± 0.4 | 2.6 ± 0.5 | 2.9 ± 0.6 | 0.54 ± 0.11 |
| 5 | Johnius macrorhynus | <0.2 | 6.5 ± 1.2 | <0.1 | 0.11 ± 0.02 | 0.35 ± 0.07 | 0.020 ± 0.004 | <0.2 | 1.4 ± 0.3 | 3.3 ± 0.6 | 1.0 ± 0.2 | 0.22 ± 0.04 |
| 6 | Lates calcarifer | <0.2 | <2 | <0.1 | 0.07 ± 0.01 | 0.21 ± 0.04 | <0.02 | <0.2 | 0.9 ± 0.2 | 2.7 ± 0.5 | 0.3 ± 0.1 | <0.2 |
| 7 | Lethrinus lentjan | <0.2 | <2 | <0.1 | 0.03 ± 0.01 | <0.2 | <0.02 | <0.2 | 0.7 ± 0.1 | 1.6 ± 0.3 | 1.2 ± 0.2 | 0.23 ± 0.04 |
| 8 | Nemipterus furcosus | <0.2 | 2.1 ± 0.4 | <0.1 | 0.10 ± 0.02 | 0.41 ± 0.08 | <0.02 | <0.2 | 2.7 ± 0.5 | 3.5 ± 0.7 | 4.3 ± 0.8 | 0.79 ± 0.16 |
| 9 | Parupeneus heptcanthus | <0.2 | 2.9 ± 0.6 | <0.1 | 0.10 ± 0.02 | 0.22 ± 0.04 | <0.02 | <0.2 | 1.7 ± 0.3 | 3.6 ± 0.7 | 2.4 ± 0.5 | 0.36 ± 0.07 |
| 10 | Scarus ghobban | <0.2 | <2 | <0.1 | 0.04 ± 0.01 | <0.2 | 0.020 ± 0.004 | <0.2 | 0.7 ± 0.1 | 1.9 ± 0.4 | 1.9 ± 0.4 | 0.62 ± 0.12 |
| 11 | Scolopsis monogramma | <0.2 | 4.1 ± 0.8 | 0.10 ± 0.02 | 0.11 ± 0.02 | 0.68 ± 0.14 | 0.020 ± 0.004 | 0.28 ± 0.06 | 2.1 ± 0.4 | 5.7 ± 1.1 | 1.7 ± 0.3 | 0.51 ± 0.10 |
| 12 | Selar crumneuphthalmus | <0.2 | <2 | <0.1 | 0.09 ± 0.02 | 0.20 ± 0.04 | <0.02 | <0.2 | 1.7 ± 0.3 | 3.0 ± 0.6 | 0.8 ± 0.2 | 0.31 ± 0.06 |
| 13 | Thunnus alalunga | <0.2 | <2 | <0.1 | 0.09 ± 0.02 | 0.26 ± 0.05 | <0.02 | <0.2 | 2.6 ± 0.5 | 5.8 ± 1.2 | 0.4 ± 0.1 | 0.95 ± 0.19 |
| Malaysian MTL 1 | --- | --- | --- | --- | --- | --- | --- | --- | --- | 1 # | --- | |
| European MTL 2 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| a | b | c | d | e | f | g | h | ||
|---|---|---|---|---|---|---|---|---|---|
| Species | Total Lipids (g/100 g) | Total FA a (mg/100 g) | SFA b (mg/100 g) | MUFA c (mg/100 g) | PUFA d: ω-3 e + ω-6 f FA (mg/100 g) | ω-3 e FA (mg/100 g) | ω-3/ω-6 PUFA Ratio | PUFA/SFA Ratio | |
| 1 | Caesio caerulaurea | 0.67 ± 0.04 | 104.7 ± 7.6 | 61.1 ± 2.3 | 18.2 ± 1.8 | 25.4 ± 3.5 | 19.1 ± 2.5 | 3.1 ± 0.9 | 0.41 ± 0.10 |
| 2 | Caranx sexfasciatus | 0.90 ± 0.05 | 151.6 ± 7.8 | 80.0 ± 2.2 | 43.1 ± 2.5 | 28.4 ± 3.1 | 23.0 ± 2.5 | 4.2 ± 0.9 | 0.36 ± 0.06 |
| 3 | Epinephelus areolatus | 0.84 ± 0.04 | 29.2 ± 3.8 | 14.2 ± 1.7 | 4.5 ± 0.1 | 10.5 ± 2.0 | 7.2 ± 1.6 | 2.2 ± 0.8 | 0.74 ± 0.16 |
| 4 | Himantura walga | 1.12 ± 0.06 | 636.5 ± 47.7 | 360.2 ± 23.8 | 105.6 ± 9.3 | 170.7 ± 14.6 | 127.8 ± 4.4 | 3.0 ± 0.8 | 0.47 ± 0.08 |
| 5 | Johnius macrorhynus | 1.16 ± 0.05 | 474.6 ± 25.7 | 252.2 ± 13.4 | 134.5 ± 2.0 | 88.0 ± 10.2 | 58.9 ± 6.1 | 2.0 ± 0.5 | 0.35 ± 0.05 |
| 6 | Latscalcarifer | 1.56 ± 0.06 | 672.8 ± 46.3 | 221.8 ± 9.5 | 241.4 ± 23.6 | 209.6 ± 13.2 | 112.0 ± 4.3 | 1.2 ± 0.2 | 0.95 ± 0.15 |
| 7 | Lethrinus lentjan | 1.14 ± 0.05 | 384.4 ± 20.7 | 197.5 ± 11.5 | 97.3 ± 2,7 | 89.6 ± 6.4 | 60.6 ± 4.0 | 2.1 ± 0.3 | 0.45 ± 0.05 |
| 8 | Nemipterus furcosus | 1.06 ± 0.05 | 64.0 ± 1.9 | 30.1 ± 0.6 | 7.9 ± 0.4 | 26.0 ± 0.6 | 22.9 ± 0.5 | 7.4 ± 0.4 | 0.87 ± 0.07 |
| 9 | Parupeneus heptacanthus | 0.57 ± 0.03 | 125.8 ± 10.7 | 72.3 ± 4.9 | 16.0 ± 1.4 | 37.5 ± 4.4 | 23.9 ± 2.4 | 1.8 ± 0.44 | 0.52 ± 0.11 |
| 10 | Scaurus ghobban | 0.85 ± 0.04 | 239.7 ± 27.9 | 110.0 ± 4.6 | 56.3 ± 9.4 | 73.4 ± 13.9 | 44.1 ± 10.5 | 1.5 ± 0.5 | 0.67 ± 0.24 |
| 11 | Scolopsis monogramma | 0.64 ± 0.04 | 197.5 ± 10.7 | 63.9 ± 3.0 | 31.4 ± 1.2 | 102.22 ± 6.46 | 70.47 ± 5.29 | 2.2 ± 0.2 | 1.60 ± 0.16 |
| 12 | Selar crumenophthalmus | 1.84 ± 0.07 | 400.30 ± 15.82 | 226,.2 ± 5.2 | 78.0 ± 4.4 | 96.0 ± 6.2 | 76.3 ± 3.6 | 3.9 ± 0.7 | 0.42 ± 0.05 |
| 13 | Thunnus alalunga | 1.14 ± 0.05 | 233.80 ± 9.03 | 85.1 ± 2.1 | 40.2 ± 1.6 | 108.5 ± 5.4 | 95.4 ± 4.5 | 7.3 ± 0.8 | 1.28 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tramice, A.; Trifuoggi, M.; Ahmad, M.F.; Lam, S.S.; Iodice, C.; Velotto, G.; Giarra, A.; Inglese, S.; Cupo, A.; Guerriero, G.; et al. Comparative Fatty Acid Profiling of Edible Fishes in Kuala Terengganu, Malaysia. Foods 2021, 10, 2456. https://doi.org/10.3390/foods10102456
Tramice A, Trifuoggi M, Ahmad MF, Lam SS, Iodice C, Velotto G, Giarra A, Inglese S, Cupo A, Guerriero G, et al. Comparative Fatty Acid Profiling of Edible Fishes in Kuala Terengganu, Malaysia. Foods. 2021; 10(10):2456. https://doi.org/10.3390/foods10102456
Chicago/Turabian StyleTramice, Annabella, Marco Trifuoggi, Mohammad Fadhli Ahmad, Su Shiung Lam, Carmine Iodice, Gennaro Velotto, Antonella Giarra, Sara Inglese, Adelaide Cupo, Giulia Guerriero, and et al. 2021. "Comparative Fatty Acid Profiling of Edible Fishes in Kuala Terengganu, Malaysia" Foods 10, no. 10: 2456. https://doi.org/10.3390/foods10102456
APA StyleTramice, A., Trifuoggi, M., Ahmad, M. F., Lam, S. S., Iodice, C., Velotto, G., Giarra, A., Inglese, S., Cupo, A., Guerriero, G., & Tommonaro, G. (2021). Comparative Fatty Acid Profiling of Edible Fishes in Kuala Terengganu, Malaysia. Foods, 10(10), 2456. https://doi.org/10.3390/foods10102456









