Postharvest Microwave Drying of Basil (Ocimum basilicum L.): The Influence of Treatments on the Quality of Dried Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Basil Plants
2.2. Basil Samples Characterization
2.3. Drying Treatments
2.4. Quality Parameters
2.4.1. Tissue Characterization
2.4.2. Essential Oils
3. Results and Discussion
3.1. Drying Treatment Performances
3.2. Tissue Integrity Evaluation
3.3. Chemical Composition of the Essential Oils
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- UNESCO. Available online: https://ich.unesco.org/en/RL/mediterranean-diet-00884 (accessed on 22 March 2022).
- Borges, C.V.; Minatel, I.O.; Gomez-Gomez, H.A.; Lima, G.P.P. Medicinal Plants: Influence of Environmental Factors on the Content of Secondary Metabolites. In Medicinal Plants and Environmental Challenges; Ghorbanpour, M., Varma, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 259–277. [Google Scholar] [CrossRef]
- Calin-Sanchez, A.; Lech, K.; Szumny, A.; Figiel, A.; Carbonell-Barrachina, A. Volatile composition of sweet basil essential oil (Ocimum basilicum L.) as affected by drying method. Food Res. Int. 2012, 48, 217–225. [Google Scholar] [CrossRef]
- Thamkaew, G.; Sjöholm, I.; Gómez Galindo, F. A review of drying methods for improving the quality of dried herbs, Critical Reviews. Food Sci. Nutr. 2021, 61, 1763–1786. [Google Scholar] [CrossRef]
- Khan, M.K.I.; Ghauri, Y.M.; Alvi, T.; Amin, U.; Khan, M.I.; Nazir, A.; Farhan Saeed, F.; Aadil, R.M.; Nadeem, M.T.; Babu, I.; et al. Microwave assisted drying and extraction technique; kinetic modelling, energy consumption and influence on antioxidant compounds of fenugreek leaves. Food Sci. Technol. 2022, 42, e56020. [Google Scholar] [CrossRef]
- Barba, A.A.; Dalmoro, A.; d’Amore, M. Microwave assisted drying of cellulose derivative (HPMC) granular solids. Powd. Technol. 2013, 237, 581–585. [Google Scholar] [CrossRef]
- Barba, A.A.; D’Amore, M. Relevance of dielectric properties in microwave assisted processes. In Microwave Materials Characterization; Costanzo, S., Ed.; InTechOpen: Rijeka, Croatia, 2012; pp. 91–118. [Google Scholar] [CrossRef] [Green Version]
- Metaxas, A.C.; Meredith, R.J. Industrial Microwave Heating; Peter Peregrinus Ltd.: London, UK, 1993; ISBN 0906048893. [Google Scholar]
- Feng, H.; Yin, Y.; Tang, J. Microwave drying of food and agricultural materials: Basics and heat and mass transfer modeling. Food Eng. Rev. 2012, 4, 89–106. [Google Scholar] [CrossRef]
- Li, Q.X.; Chang, C.L. Basil (Ocimum basilicum L.) oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: Salt Lake City, UT, USA, 2015; pp. 231–238. [Google Scholar]
- Joshi, R.K. Chemical composition and antimicrobial activity of the essential oil of Ocimum basilicum L. (sweet basil) from Western Ghats of North West Karnataka, India. Anc. Sci. Life 2014, 33, 151156. [Google Scholar] [CrossRef]
- Sledz, M.; Wiktor, A.; Nowacka, M.; Witrowa-Rajchert, D. Drying kinetics, microstructure and antioxidant properties of basil treated by ultrasound. J. Food Proc. Eng. 2017, 40, e12271. [Google Scholar] [CrossRef] [Green Version]
- Makri, O.; Kintzios, S. Ocimum sp. (basil): Botany, cultivation, pharmaceutical properties, and biotechnology. J. Herbs Spices Med. Plants 2008, 13, 123–150. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial activity of basil, oregano, and thyme essential oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Telfser, A.; Gomez Galindo, F. Effect of reversible permeabilization in combination with different drying methods on the structure and sensorial quality of dried basil (Ocimum basilicum L.) leaves. LWT 2019, 99, 148–155. [Google Scholar] [CrossRef]
- Altay, K.; Hayaloglu, A.A.; Dirim, S.N. Determination of the drying kinetics and energy efficiency of purple basil (Ocimum basilicum L.) leaves using different drying methods. Heat Mass Transf. 2019, 55, 2173–2184. [Google Scholar] [CrossRef]
- Özcan, M.; Arslan, D.; Ünver, A. Effect of drying methods on the mineral content of basil (Ocimum basilicum L.). J. Food Eng. 2005, 69, 375–379. [Google Scholar] [CrossRef]
- Oladele, S.; Jimoh, K. Microwave-drying of Scent Leaf (Ocimum gratissimum). J. Sustain. Technol. 2017, 8, 38–52. [Google Scholar]
- Altay, K.; Dirim, S.N.; Hayaloglu, A.A. The effect of gamma irradiation on microbial load of purple basil (Ocimum basilicum L.) leaves dried in different methods. J. Food Saf. 2019, 39, e12610. [Google Scholar] [CrossRef]
- Agenda ONU. Available online: https://sdgs.un.org/2030agenda (accessed on 22 March 2022).
- European Spice Association. Available online: www.esa-spices.org (accessed on 22 March 2022).
- Council of Europe. European Pharmacopeia, 5th ed.; Council of Europe: Strasbourg Cedex, France, 2004; Volume I, pp. 217–218. [Google Scholar]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavour and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicone and Carbowax 20M phases. J. Chromatogr. 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Goodner, K.L. Practical retention index models of OV-101, DB-1, DB-5, and DB-Wax for flavor and fragrance compounds. LWT 2008, 41, 951–958. [Google Scholar] [CrossRef]
- Wiley Registry of Mass Spectral Data: WITH NIST Spectral Data CD-ROM: Inc. John Wiley & Sons: 9780470047866. Available online: https://www.bookdepository.com/Wiley-Registry-Mass-Spectral-Data-WITH-NIST-Spectral-Data-CD-ROM-Inc-JohnWiley-Sons/9780470047866 (accessed on 21 September 2021).
- Moses, J.A.; Norton, T.; Alagusundaram, K.; Tiwari, B.K. Novel drying techniques for the food industry. Food Eng. Rev. 2014, 6, 43–55. [Google Scholar] [CrossRef]
- Guiné, R. The drying of foods and its effect on the physical-chemical, sensorial and nutritional properties. Int. J. Food Eng. 2018, 2, 93–100. [Google Scholar] [CrossRef]
- Parizotto, C.A.; Dall’Oglio, E.L.; de Vasconcelosa, L.G.; de Sousa, P.T.; Taques Filhoa, E.G.R., Jr.; Kuhnenb, C.A. Measuring dielectric properties for microwave-assisted extraction of essential oils using singlemode and multimode reactors. R. Soc. Chem. 2019, 9, 5259–5269. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Campos, R.; Fayos-Fernández, J.; Lozano-Guerrero, A.J.; Martínez-González, A.; Monzó-Cabrera, J.; Mediavilla, I.; Peña-Carro, D.; Esteban-Pascual, L.S. Permittivity Measurements for Cypress and Rockrose Biomass Versus Temperature, Density, and Moisture Content. Sensors 2020, 20, 4684. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Tudela, J.A.; Luna, C.; Allende, A.; Gil, M.I. Low oxygen levels and light exposure affect quality of fresh-cut Romaine lettuce. Postharvest Biol. Technol. 2011, 59, 34–42. [Google Scholar] [CrossRef]
- Mihailova, G.; Kocheva, K.; Hazem, V.; Kalaji, M.; Georgieva, K. Application of a diffusion model to measure ion leakage of resurrection plant leaves undergoing desiccation. Plant Phys. Biochem. 2018, 125, 185–192. [Google Scholar] [CrossRef]
- Dalmoro, A.; Naddeo, C.; Caputo, S.; Lamberti, G.; Guadagno, L.; D’Amore, M.; Barba, A.A. On the relevance of thermophysic characterizations in microwave treatments of legumes. Food Funct. 2018, 9, 1816–1828. [Google Scholar] [CrossRef]
- Barba, A.A.; Naddeo, C.; Caputo, S.; Lamberti, G.; D’Amore, M.; Dalmoro, A. Microwave treatments of cereals: Effects on thermophysical and parenchymal-related properties. Foods 2019, 9, 14. [Google Scholar] [CrossRef]
- Jiang, A.Y.; Shiina, T.; Nakamura, N.; Nakahara, A. Electrical conductivity evaluation of postharvest strawberry damage. J. Food Sci. 2001, 66, 1392–1395. [Google Scholar] [CrossRef]
- Cozzolino, R.; Pace, B.; Cefola, M.; Martignetti, A.; Stocchero, M.; Fratianni, F.; Nazzaro, F.; De Giulio, B. Assessment of volatile profile as potential marker of chilling injury of basil leaves during postharvest storage. Food Chem. 2016, 213, 361–368. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, E.; Lightfoot, I.N. Transport Phenomena, 2nd ed.; John Wiley & Sons: Malden, MA, USA, 2002. [Google Scholar]
- Routray, W.; Orsat, V.; Gariepy, Y. Effect of different drying methods on the microwave extraction of phenolic components and antioxidant activity of highbush blueberry leaves. Dry. Technol. 2014, 32, 1888–1904. [Google Scholar] [CrossRef]
- Nacar, S.; Tansı, S. Chemical components of different basil (Ocimum basilicum L.) cultivars grown in Mediterranean regions in Turkey. Isr. J. Plant Sci. 2000, 48, 109–112. [Google Scholar] [CrossRef]
- Dris, D.; Tine-Djebbar, F.; Bouabida, H.; Soltani, N. Chemical composition and activity of an Ocimum basilicum essential oil on Culex pipiens larvae: Toxicological, biometrical and biochemical aspects. S. Afr. J. Bot. 2017, 113, 362–369. [Google Scholar] [CrossRef]
- Piras, A.; Gonçalves, M.J.; Alves, J.; Falconieri, D.; Porcedda, S.; Maxia, A.; Salgueiro, L. Ocimum tenuiflorum L. and Ocimum basilicum L., two spices of Lamiaceae family with bioactive essential oils. Ind. Crops Prod. 2018, 113, 89–97. [Google Scholar] [CrossRef]
- Amor, G.; Sabbah, M.; Caputo, L.; Idbella, M.; De Feo, V.; Porta, R.; Feqtali, T.; Mauriello, G. Basil essential oil: Composition, antimicrobial properties, and microencapsulation to produce active chitosan films for food packaging. Foods 2021, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- De Martino, L.; Amato, G.; Caputo, L.; Nazzaro, F.; Scognamiglio, M.R.; De Feo, V. Variations in composition and bioactivity of Ocimum basilicum cv ‘Aroma 2’ essential oils. Ind. Crops. Prod. 2021, 172, 114068. [Google Scholar] [CrossRef]
- Sárosi, S.; Sipos, L.; Kókai, Z.; Pluhár, Z.; Szilvássy, B.; Novák, I. Effect of different drying techniques on the aroma profile of Thymus vulgaris analyzed by GC–MS and sensory profile methods. Ind. Crops Prod. 2013, 46, 210–216. [Google Scholar] [CrossRef]
- Drinić, Z.; Pljevljakušić, D.; Živković, J.; Bigović, D.; Šavikin, K. Microwave-assisted extraction of Origanum vulgare L. spp. hirtum essential oil: Comparison with conventional hydro-distillation. Food Bioprod. Proc. 2020, 120, 158–165. [Google Scholar] [CrossRef]
- Di Cesare, L.F.; Forni, E.; Viscardi, D.; Nani, R.C. Changes in the chemical composition of basil caused by different drying procedures. J. Agri. Chem. 2003, 51, 3575–3581. [Google Scholar] [CrossRef]
- Stewart, D. The Chemistry of Essential Oils Made Simple: God’s Love Manifest in Molecules; Care Publications: Marble Hill, MO, USA, 2005. [Google Scholar]
- Pirbalouti, A.G.; Mahdad, E.; Craker, L. Effects of drying methods on qualitative and quantitative properties of essential oil of two basil landraces. Food Chem. 2013, 141, 2440–2449. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Sánchez Palomo, E.; Castro, L.; González Viñas, M.A.; Pérez-Coello, M.S. Changes produced in the aroma compounds and structural integrity of basil (Ocimum basilicum L) during drying. J. Sci. of Food Agric. 2004, 84, 2070–2076. [Google Scholar] [CrossRef]
- Venskutonis, R. Effect of drying on the volatile constituents of thyme (Thymus vulgaris L.) and sage (Salvia officinalis L.). Food Chem. 1997, 59, 219–227. [Google Scholar] [CrossRef]
- Jerkovic, I.; Mastellic, J.; Milos, M. The impact of both the season of collection and drying on the volatile constituents of Origanum vulgare L. ssp hirtum grown wild in Croatia. Int. J. Food Sci. Technol. 2001, 36, 649–654. [Google Scholar]



| Samples Code 1 | Drying Method/Operative Parameters |
|---|---|
| CD1 | Shade drying/shady room conditions for 6 days |
| CD2 | Hot–air drying/static oven at 50 °C for 24 h |
| MWD1 | Assisted microwave heating/2300 W for 8 min |
| MWD2 | Assisted microwave heating/1150 W for 30 min |
| Samples Code | Residual Moisture Content % Wet Basis | Treatment Times |
|---|---|---|
| Fresh | 85.09 ± 2.64 | -- |
| CD1 | 55.46 ± 8.27 | 6 days |
| CD2 | 10.52 ± 1.80 | 24 h |
| MWD1 | 10.53 ± 1.72 | 8 min |
| MWD2 | 10.48 ± 3.71 | 30 min |
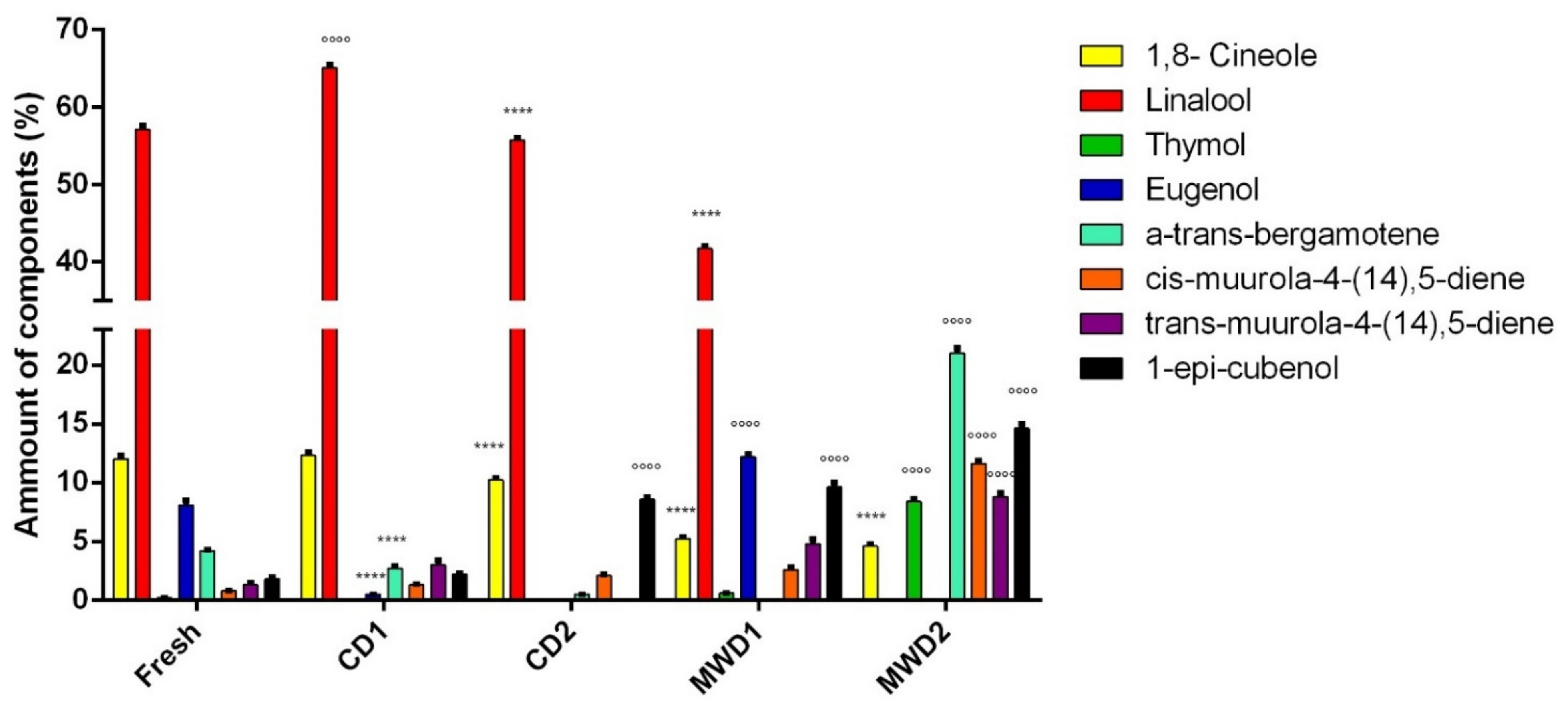
| n | Compound Name | % | KI a | KI b | Identif. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fresh | CD1 | CD2 | MWD1 | MWD2 | |||||
| 1 | 2-Methyl butanal | 0.2 ± 0.01 | 0.1 ± 0.01 | 0.5 ± 0.02 ° | 0.3 ± 0.01 | - | 745 | 920 | 1,2 |
| 2 | α-Pinene | 0.3 ± 0.02 | 0.2 ± 0.01 | 0.1 ± 0.01 | - | - | 861 | 1036 | 1,2,3 |
| 3 | β- Pinene | t | t | - | - | - | 873 | 1110 | 1,2,3 |
| 4 | δ-3-Carene | 1.1 ± 0.05 | 1.0 ± 0.1 | 0.8 ± 0.02 * | 0.1 ± 0.01 **** | - | 897 | 1153 | 1,2,3 |
| 5 | Myrcene | - | 0.1 ± 0.03 | - | - | - | 917 | 1173 | 1,2,3 |
| 6 | 1,8- Cineole | 12.0 ± 0.3 | 12.3 ± 0.3 ° | 10.2 ± 0.2 **** | 5.2 ± 0.19 **** | 4.6 ± 0.2 **** | 949 | 1213 | 1,2,3 |
| 7 | β- Ocimene <Z> | 0.4 ± 0.01 | 1.7 ± 0.1 °°°° | - | 0.1 ± 0.01 * | - | 970 | 1246 | 1,2,3 |
| 8 | α-Terpinene | t | t | - | - | - | 1001 | 1166 | 1,2,3 |
| 9 | Linalool | 57.1 ± 0.5 | 65.1 ± 0.4 °°°° | 55.7 ± 0.3 **** | 41.7 ± 0.35 **** | - | 1020 | 1553 | 1,2,3 |
| 10 | Terpinolene | 3.2 ± 0.1 | 1.4 ± 0.1 **** | 2.5 ± 0.09 **** | 1.2 ± 0.1 **** | 6.8 ± 0.4 °°°° | 1021 | 1291 | 1,2,3 |
| 11 | Isoborneol | 0.2 ± 0.01 | 0.3 ± 0.02 | 0.2 ± 0.01 | 0.2 ± 0.01 | - | 1052 | 1633 | 1,2,3 |
| 12 | cis-Dihydrocarvone | 0.2 ± 0.01 | 0.2 ± 0.01 | 0.2 ± 0.01 | 0.3 ± 0.02 | - | 1075 | - | 1,2,3 |
| 13 | trans-Pulegol | t | t | - | - | - | 1088 | 1614 | 1,2 |
| 14 | cis-Sabinene hydrate | 0.2 ± 0.02 | 0.3 ± 0.02 | - | t | - | 1096 | 1470 | 1,2 |
| 15 | Isobornyl acetate | 0.3 ± 0.03 | 0.4 ± 0.03 | 0.6 ± 0.02 * | 0.3 ± 0.02 | 0.1 ± 0.01 | 1185 | 1582 | 1,2 |
| 16 | Thymol | 0.2 ± 0.01 | t | - | 0.6 ± 0.03 °°° | 8.4 ± 2.1 °°°° | 1218 | 2172 | 1,2,3 |
| 18 | δ-Elemene | 0.4 ± 0.04 | 0.6 ± 0.04 | 0.8 ± 0.04 °°° | 1.1 ± 0.1 °°°° | - | 1228 | 1460 | 1,2,3 |
| 19 | α-Ylangene | t | t | - | t | - | 1241 | 1491 | 1,2 |
| 20 | β-Cubebene | - | - | 0.4 ± 0.01 °°°° | - | - | 1264 | 1445 | 1,2 |
| 21 | Eugenol | 8.1 ± 0.4 | 0.5 ± 0.01 **** | - | 12.2 ± 0.2 °°°° | - | 1256 | 2186 | 1,2,3 |
| 22 | Z-Isoeugenol acetate | 0.8 ± 0.01 | t | - | 0.9 ± 0.04 | - | 1275 | 2395 | 1,2 |
| 23 | β- Elemene | - | - | 5.0 ± 0.1 °°°° | - | - | 1281 | 1598 | 1,2 |
| 24 | 1-Ethenyl-1-methyl-2,4-bis(1-methylethenyl)-cyclohexane | 2.4 ± 0.09 | 2.8 ± 0.1 °°° | - | 6.0 ± 0.2 °°°° | 6.5 ± 0.21 °°°° | 1282 | 1593 | 1,2 |
| 25 | (E)-Caryophyllene | 0.4 ± 0.03 | 0.1 ± 0.01 * | - | 0.6 ± 0.02 | - | 1285 | 1575 | 1,2,3 |
| 26 | β-Ylangene | 1.1 ± 0.2 | 1.4 ± 0.1 ° | 2.5 ± 0.11 °°°° | 3.3 ± 0.16 °°°° | 3.7 ± 0.3 °°°° | 1299 | 1589 | 1,2,3 |
| 27 | β-Copaene | 0.6 ± 0.03 | 0.7 ± 0.09 | 1.1 ± 0.5 °°°° | 1.6 ± 0.08 °°°° | 0.4 ± 0.06 | 1312 | 1628 | 1,2 |
| 28 | α-trans-Bergamotene | 4.2 ± 0.1 | 2.7 ± 0.2 **** | 0.5 ± 0.01 **** | - | 21.0 ± 0.43 °°°° | 1320 | 1573 | 1,2 |
| 29 | Aromadendrene | 0.3 ± 0.05 | 0.4 ± 0.02 | - | 1.0 ± 0.1 °°°° | 0.7 ± 0.03 °°° | 1325 | 1628 | 1,2,3 |
| 30 | α-Humulene | 0.5 ± 0.06 | 0.8 ± 0.07 ° | 1.3 ± 0.1 °°°° | 1.6 ± 0.14 °°°° | 1.5 ± 0.1 °°°° | 1333 | 1671 | 1,2,3 |
| 31 | allo-Aromadendrene | 0.4 ± 0.03 | 0.8 ± 0.06 °°° | 0.9 ± 0.03 °°°° | 1.2 ± 0.11 °°°° | 1.3 ± 0.09 °°°° | 1343 | 1638 | 1,2,3 |
| 32 | cis-Muurola-4-(14),5-diene | 0.8 ± 0.04 | 1.3 ± 0.1 °°°° | 2.1 ± 0.09 °°°° | 2.6 ± 0.19 °°°° | 11.6 ± 0,25 °°°° | 1361 | 1675 | 1,2 |
| 33 | γ-Gurjunene | 0.3 ± 0.02 | 0.4 ± 0.03 | 0.5 ± 0.01 | 0.8 ± 0.04 °°°° | 1.2 ± 0.1 °°°° | 1367 | 1687 | 1,2 |
| 34 | γ- Muurolene | 0.3 ± 0.02 | 0.3 ± 0.02 | 0.4 ± 0.03 | 0.9 ± 0.04 °°°° | 1.6 ± 0.13 °°°° | 1376 | 1684 | 1,2 |
| 35 | Aristolochene | 0.6 ± 0.05 | 1.2 ± 0.1 °°°° | 1.7 ± 0.08 °°°° | 2.4 ± 0.4 °°°° | 2.8 ± 0.21 °°°° | 1387 | - | 1,2 |
| 36 | γ-Himalachene | t | - | 4.9 ± 0.15 °°°° | - | - | 1391 | - | 1,2 |
| 37 | trans-Muurola-4-(14),5-diene | 1.3 ± 0.2 | 3.0 ± 0.4 °°°° | - | 4.8 ± 0.4 °°°° | 8.8 ± 0.3 °°°° | 1395 | 1711 | 1,2 |
| 38 | δ-Cadinene | 0.2 ± 0.01 | 0.3 ± 0.01 | - | 0.6 ± 0.02 °°°° | - | 1402 | 1751 | 1,2 |
| 39 | cis-β-Elemenone | t | 0.1 ± 0.01 | - | 0.1 ± 0.01 | - | 1484 | 2091 | 1,2 |
| 40 | 1,10-di-epi-Cubenol | 0.1 ± 0.03 | 0.1 ± 0.02 | 0.3 ± 0.06 | 0.5 ± 0.01 °°° | 0.7 ± 0.02 °°°° | 1490 | 2054 | 1,2 |
| 41 | 1-epi-Cubenol | 1.8 ± 0.2 | 2.2 ± 0.09 °°°° | 8.6 ± 0.2 °°°° | 9.6 ± 0.4 °°°° | 14.6 ± 0.4 °°°° | 1509 | 2025 | 1,2 |
| Total | 99.0 | 99.2 | 98.0 | 98.1 | 98.0 | ||||
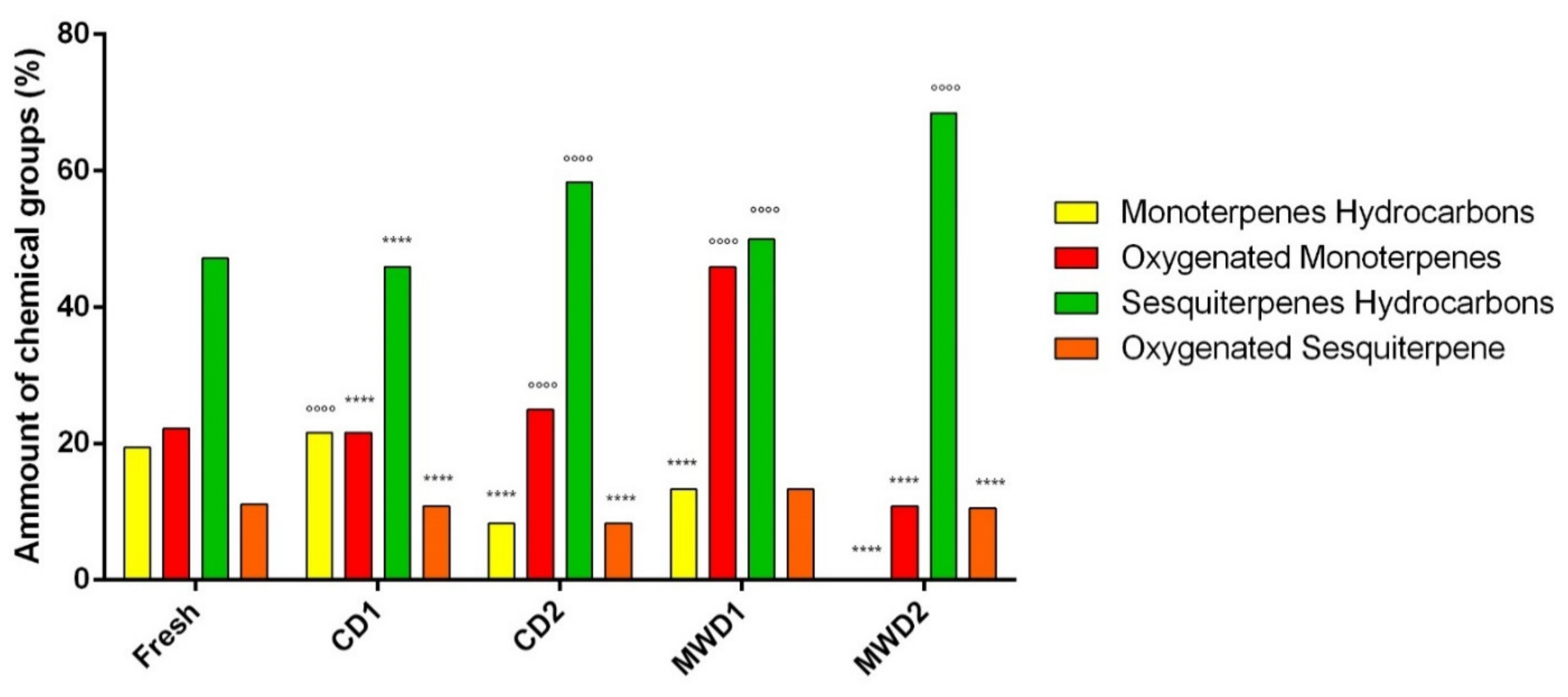
| Monoterpenes hydrocarbons | 19.4 | 21.6 | 8.3 | 13.3 | - | ||||
| Oxygenated monoterpenes | 22.2 | 21.6 | 25.0 | 23.3 | 21.1 | ||||
| Sesquiterpenes hydrocarbons | 47.2 | 45.7 | 58.3 | 50.0 | 68.4 | ||||
| Oxygenated sesquiterpenes | 11.1 | 10.8 | 8.3 | 13.3 | 10.5 | ||||
| Yield (w/v, %) | 0.1 | 0.4 | 0.2 | 0.3 | 1.1 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Martino, L.; Caputo, L.; Amato, G.; Iannone, M.; Barba, A.A.; De Feo, V. Postharvest Microwave Drying of Basil (Ocimum basilicum L.): The Influence of Treatments on the Quality of Dried Products. Foods 2022, 11, 1029. https://doi.org/10.3390/foods11071029
De Martino L, Caputo L, Amato G, Iannone M, Barba AA, De Feo V. Postharvest Microwave Drying of Basil (Ocimum basilicum L.): The Influence of Treatments on the Quality of Dried Products. Foods. 2022; 11(7):1029. https://doi.org/10.3390/foods11071029
Chicago/Turabian StyleDe Martino, Laura, Lucia Caputo, Giuseppe Amato, Marco Iannone, Anna Angela Barba, and Vincenzo De Feo. 2022. "Postharvest Microwave Drying of Basil (Ocimum basilicum L.): The Influence of Treatments on the Quality of Dried Products" Foods 11, no. 7: 1029. https://doi.org/10.3390/foods11071029
APA StyleDe Martino, L., Caputo, L., Amato, G., Iannone, M., Barba, A. A., & De Feo, V. (2022). Postharvest Microwave Drying of Basil (Ocimum basilicum L.): The Influence of Treatments on the Quality of Dried Products. Foods, 11(7), 1029. https://doi.org/10.3390/foods11071029









