Nutritional Profile and Health Benefits of Ganoderma lucidum “Lingzhi, Reishi, or Mannentake” as Functional Foods: Current Scenario and Future Perspectives
Abstract
:1. Introduction
1.1. What Does History Say about Ganoderma lucidum?
1.2. Ganoderma lucidum through the Glasses of Botanists, Taxonomists, Economists, and Scientometric Analysis
1.2.1. Through Botanists’ Glasses
1.2.2. Through Taxonomists’ Glasses
- Kingdom: Fungi
- Division: Basidiomycota
- Class: Agaricomycetes
- Order: Polyporales
- Family: Ganodermataceae
- Genus: Ganoderma
- Species: G. lucidum
1.2.3. Through Economists’ Glasses
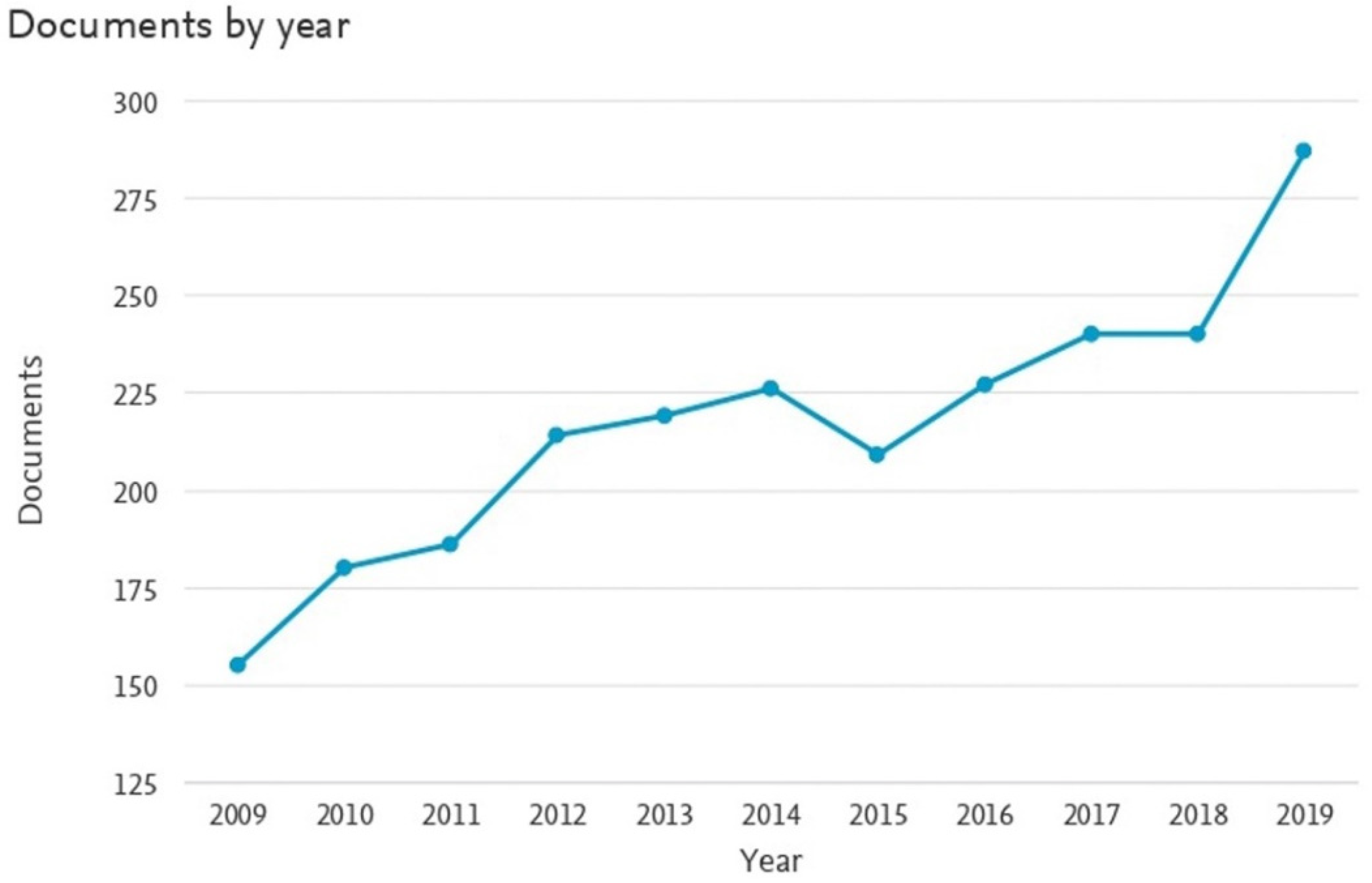
1.2.4. Scientometric Analysis
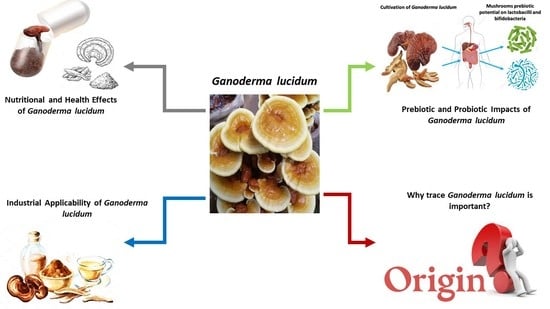
1.3. Why Should Mushrooms, including Ganoderma lucidum, Be Considered Functional Foods?
1.3.1. How to Define Functional Food?
1.3.2. What Do the Definitions of Functional Foods Conclude?
1.3.3. Functional Foods and Their Relation with Gut Health
- It converts food to nutrients;
- The human gut, via epithelial cell walls, assists in the absorption process of nutrients into the blood;
- The human gut inhibits toxic and strange particles from entering the bloodstream.
1.3.4. Ganoderma lucidum as a Functional Food: How?
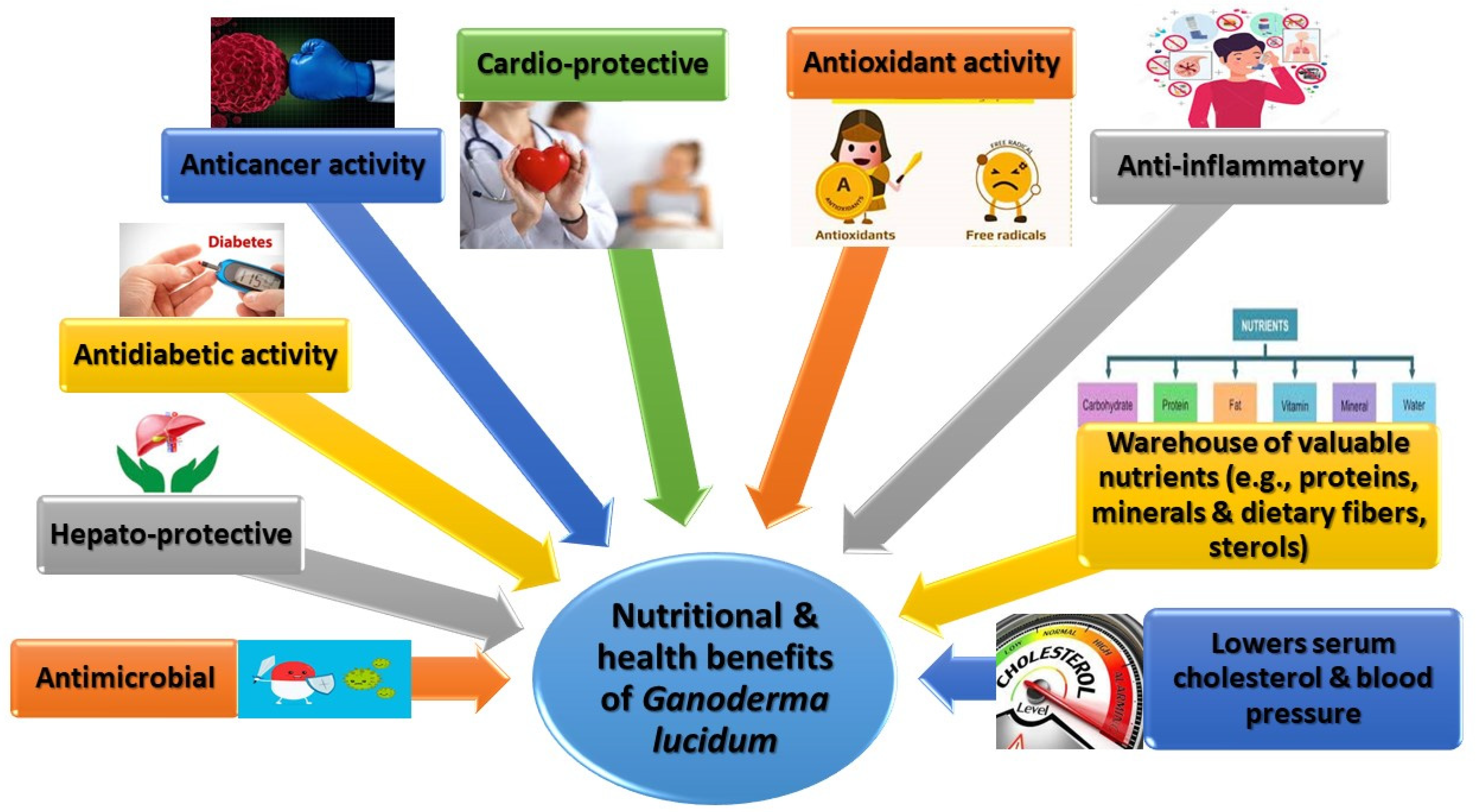
2. The Nutritional Profile of Ganoderma lucidum
- G. lucidum contains 3.5 g of dietary fiber per 100 g of mushroom (d/w).
- G. lucidum contains significant amounts of major minerals (e.g., phosphorus, sulfur) and other trace mineral contents; i.e., Cu, Mg, and Fe.
- Based on the nutritional profile of G. lucidum, this mushroom possesses a high nutrient potential that reflects positively on its health benefits.
2.1. Ganoderma lucidum Is a Factory of Biologically Active Useful Compounds
2.2. Polysaccharides and Peptidoglycans
2.3. Triterpenes
2.4. Other Bioactive Compounds
2.4.1. Germanium
2.4.2. Proteins
- Enzymes; e.g., a metalloprotease that delays clotting time [6].
3. Ganoderma lucidum as a Functional Food
3.1. Antimicrobial Activity
3.2. Antiviral Potential
3.2.1. Ganoderma lucidum against Enterovirus 71 (EV71)
3.2.2. Ganoderma lucidum against Dengue Virus (DENV)
3.2.3. Ganoderma lucidum against the 2019 Novel Coronavirus (SARS-CoV-2)
3.3. Antioxidant and Antiaging Activity
3.4. Anticancer Activity
3.5. Antidiabetic Activity
3.6. Cardioprotective Effects
3.7. Hepatoprotection
3.8. Anti-Inflammatory Effects
3.9. Prebiotic Potential
3.10. The Health Risks of Ganoderma lucidum and Its Products
4. Future Trends
4.1. Do the Beneficial Medical Properties of G. lucidum Need More Scientific Evidence?
4.2. Future of the G. lucidum Mushroom in the Food Industry
4.3. Is Tracing the Species and Geo-Origin of G. lucidum Essential?
5. Infographic for Ganoderma lucidum: Current Scenario and Future Perspectives
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, S.T. The world mushroom industry: Trends and technological development. Int. J. Med. Mushrooms 2006, 8, 297–314. [Google Scholar] [CrossRef]
- Wasser, S.P. Reishi (Ganoderma lucidum). In Encyclopedia of Dietary Supplements, 2nd ed.; Coates, P.M., Betz, J.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D., Eds.; Informa Healthcare: London, UK, 2010; pp. 680–690. [Google Scholar]
- Zhao, X.-R.; Huo, X.-K.; Dong, P.-P.; Wang, C.; Huang, S.-S.; Zhang, B.-J.; Zhang, H.-L.; Deng, S.; Liu, K.-X.; Ma, X.-C. Inhibitory effects of highly oxygenated lanostane derivatives from the fungus Ganoderma lucidum on p-glycoprotein and α-glucosidase. J. Nat. Prod. 2015, 78, 1868–1876. [Google Scholar] [CrossRef]
- Money, N.P. Are mushrooms medicinal? Fungal Biol. 2016, 120, 449–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahata, A. Ganoderma lucidum: A potent medicinal mushroom with numerous health benefits. Pharm. Anal. Acta 2013, 4, e159. [Google Scholar] [CrossRef] [Green Version]
- Wachtel-Galor, S.; Yuen, J.; Buswell, J.A.; Benzie, I.F.F. Ganoderma lucidum (Lingzhi or Reishi). In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2011. Available online: https://www.ncbi.nlm.nih.gov/books/NBK92757/ (accessed on 16 February 2022).
- Lindequist, U.; Niedermeyer, T.H.; Jülich, W.D. The pharmacological potential of mushrooms. Evid. Based Complement. Altern. Med. 2005, 2, 285–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Sheikha, A.F.; Hu, D.M. How to trace the geographic origin of mushrooms? Trends Food Sci. Technol. 2018, 78, 292–303. [Google Scholar] [CrossRef]
- Radwan, F.F.; Perez, J.M.; Haque, A. Apoptotic and immune restoration effects of ganoderic acids define a new prospective for complementary treatment of cancer. J. Clin. Cell Immunol. 2011, S3, 4. [Google Scholar] [CrossRef] [Green Version]
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011, 89, 1323–1332. [Google Scholar] [CrossRef]
- Lee, K.-H.; Morris-Natschke, S.L.; Yang, X.; Huang, R.; Zhou, T.; Wu, S.-F.; Shi, Q.; Itokawa, H. Recent progress of research on medicinal mushrooms, foods, and other herbal products used in traditional Chinese medicine. J. Tradit. Complement. Med. 2012, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.-S.; Lu, J.-J.; Guo, J.-J.; Li, Y.-B.; Tan, W.; Dang, Y.-Y.; Zhong, Z.-F.; Xu, Z.-T.; Chen, X.-P.; Wang, Y.-T. Ganoderic acid DM, a natural triterpenoid, induces DNA damage, G1 cell cycle arrest and apoptosis in human breast cancer cells. Fitoterapia 2012, 83, 408–414. [Google Scholar] [CrossRef]
- Bishop, K.S.; Kao, C.H.; Xu, Y.; Glucina, M.P.; Paterson, R.R.; Ferguson, L.R. From 2000 years of Ganoderma lucidum to recent developments in nutraceuticals. Phytochemistry 2015, 114, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Loyd, A.L.; Richter, B.S.; Jusino, M.A.; Truong, C.; Smith, M.E.; Blanchette, R.A.; Smith, J.A. Identifying the “mushroom of immortality”: Assessing the Ganoderma species composition in commercial Reishi products. Front. Microbiol. 2018, 9, 1557. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Shen Nong Materia Medica; People’s Hygiene Press: Beijing, China, 1955. (In Chinese) [Google Scholar]
- Jong, S.C.; Birmingham, J.M. Medicinal benefits of the mushroom Ganoderma. Adv. Appl. Microbiol. 1992, 37, 101–134, (Translated). [Google Scholar] [CrossRef]
- Zhou, S.; Gao, Y. The immunomodulating effects of Ganoderma lucidum (Curt.: Fr.) P. Karst. (Ling Zhi, reishi mushroom) (Aphyllophoromycetideae). Int. J. Med. Mushrooms 2002, 4, 1–11. [Google Scholar] [CrossRef]
- Sone, Y.; Okuda, R.; Wada, N.; Kishida, E.; Misaki, A. Structure and antitumor activities of the polysaccharide isolated from fruiting body and the growing culture of mycelium of Ganoderma lucidum. Agric. Biol. Chem. 1985, 49, 2641–2653. [Google Scholar] [CrossRef]
- Mizuno, T.; Wang, G.; Zhang, J.; Kawagishi, H.; Nishitoba, T.; Li, J. Reishi, Ganoderma lucidum and Ganoderma tsugae: Bioactive substances and medicinal effects. Food Rev. Int. 1995, 11, 151–166. [Google Scholar] [CrossRef]
- Arora, D. Mushroom Demystified: A Comprehensive Guide to the Fleshy Fungi, 2nd ed.; Ten Speed Press: Berekely, CA, USA, 1986. [Google Scholar]
- Chen, A.W. Cultivation of the medicinal mushroom Ganoderma lucidum (Curtis: Fr), P.karst. (Reishi) in North America. Int. J. Med. Mushrooms 1999, 1, 263–282. [Google Scholar] [CrossRef]
- Curtis, W. Flora Londinensis: Or Plates and Descriptions of Such Plants as Grow Wild in the Environs of London; Printed by the Author: London, UK, 1781. [Google Scholar]
- Fries, E.M. Systema Mycologicum, Sistens Fungorum Ordines, Genera et Species; Gryphiswaldiae, Sumtibus Ernesti Mauritti; The Horticultural Society of New York Inc.: New York, NY, USA, 1821; Volume 1. [Google Scholar]
- Teng, S.C. Notes on polyporaceae from China. Sinensia 1934, 5, 198–200. [Google Scholar]
- Cao, Y.; Wu, S.-H.; Dai, Y.-C. Species clarification of the prize medicinal Ganoderma mushroom “Lingzhi”. Fungal Divers. 2012, 56, 49–62. [Google Scholar] [CrossRef]
- Chang, S.-T.; Miles, P.G. Ganoderma lucidum—A leader of edible and medicinal mushrooms. In Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact, 2nd ed.; Chang, S.-T., Miles, P.G., Eds.; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2004; pp. 357–372. [Google Scholar]
- Leung, P.C.; Xue, C.; Cheng, Y.C. A Comprehensive Guide to Chinese Medicine; Toh Tuck Link: World Scientific Publisher Co. Pte. Ltd.: Singapore, 2003. [Google Scholar]
- Zhao, S.; Ye, G.; Fu, G.; Cheng, J.-X.; Yang, B.B.; Peng, C. Ganoderma lucidum exerts anti-tumor effects on ovarian cancer cells and enhances their sensitivity to cisplatin. Int. J. Oncol. 2011, 38, 1319–1327. [Google Scholar] [CrossRef]
- Li, S.; Dong, C.; Wen, H.; Liu, X. Development of Ling-zhi industry in China—Emanated from the artificial cultivation in the Institute of Microbiology, Chinese Academy of Sciences (IMCAS). Mycology 2016, 7, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Badalyan, S.M. The main groups of therapeutic compounds of medicinal mushrooms. Med. Mycol. 2001, 3, 16–23. [Google Scholar]
- Wu, Y.; Choi, M.-H.; Li, J.; Yang, H.; Shin, H.-J. Mushroom cosmetics: The present and future. Cosmetics 2016, 3, 22. [Google Scholar] [CrossRef]
- Lai, T.; Gao, Y.; Zhou, S.F. Global marketing of medicinal Ling Zhi mushroom Ganoderma lucidum (W.Curt.: Fr.)Lloyd (Aphyllophoromycetideae) products and safety concerns. Int. J. Med. Mushrooms 2004, 6, 189–194. [Google Scholar] [CrossRef]
- Perumal, K. Indigenous Technology on Organic Cultivation of Reishi; AMM Murugappa Chettiar Research Centre: Chennai, TN, India, 2009; pp. 1–12. [Google Scholar]
- Taofiq, O.; González-Paramás, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—A review. Ind. Crops Prod. 2016, 90, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Hapuarachchi, K.K.; Elkhateeb, W.A.; Karunarathna, S.C.; Cheng, C.R.; Bandara, A.R.; Kakumyan, P.; Hyde, K.D.; Daba, G.M.; Wen, T.C. Current status of global Ganoderma cultivation, products, industry and market. Mycosphere 2018, 9, 1025–1052. [Google Scholar] [CrossRef]
- Shimizu, T. Health claims on functional foods: The Japanese regulations and an international comparison. Nutr. Res. Rev. 2003, 16, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization (FAO). Authors Report on Functional Foods, Food Quality and Standards Service (AGNS). 2007. Available online: http://www.fao.org/ag/agn/agns/files/Functional_Foods_Report_Nov2007.pdf (accessed on 25 February 2010).
- Martirosyan, D.M.; Singharaj, B. Health Claims and Functional Food: The Future of Functional Foods under FDA and EFSA Regulation. In Functional Foods for Chronic Diseases; Food Science Publisher: Dallas, TX, USA, 2016; pp. 410–424. [Google Scholar]
- Martirosyan, D.; Pisarski, K. Bioactive Compounds: Their Role in Functional Food and Human Health, Classifications, and Definitions. In Bioactive Compounds and Cancer; Martirosyan, D., Zhou, J.-R., Eds.; Food Science Publisher: San Diego, CA, USA, 2017; pp. 238–277. [Google Scholar]
- MacAulay, J.; Petersen, B.; Shank, F. Functional Foods: Opportunities and Challenges; Institute of Food Technologists (IFT) Expert Report; Institute of Food Technologists: Chicago, IL, USA, 2005. [Google Scholar]
- Crowe, K.M.; Francis, C. Position of the academy of nutrition and dietetics: Functional foods. J. Acad. Nutr. Diet. 2013, 113, 1096–1103. [Google Scholar] [CrossRef]
- Link, R. What Are Functional Foods? All You Need to Know. 17 January 2020. Available online: https://www.healthline.com/nutrition/functional-foods?c=560339028350 (accessed on 16 February 2022).
- Arshad, M.S.; Khalid, W.; Ahmad, R.S.; Khan, M.K.; Ahmad, M.H.; Safdar, S.; Kousar, S.; Munir, H.; Shabbir, U.; Zafarullah, M.; et al. Functional Foods and Human Health: An Overview. In Functional Foods—Phytochemicals and Health Promoting Potential; Arshad, M.S., Ahmad, M.H., Eds.; IntechOpen Limited: London, UK, 2021; pp. 1–14. [Google Scholar] [CrossRef]
- Doyon, M. Functional foods: A conceptual definition. Br. Food J. 2008, 110, 1133–1149. [Google Scholar] [CrossRef]
- Cencic, A.; Chingwaru, W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef]
- Chang, S.-T. Overview of Mushroom Cultivation and Utilization as Functional Foods (Chapter 1). In Mushrooms as Functional Foods; Cheung, P.C.K., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 1–33. [Google Scholar]
- Cheung, P.C.K. Mushrooms as Functional Foods; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kumar, K. Role of edible mushrooms as functional foods—A review. South Asian J. Food Technol. Environ. 2015, 1, 211–218. [Google Scholar] [CrossRef]
- Cash, E.J. Mushrooms Need to Be Further Explored as Functional Foods, Say Researchers. Nutraingredients Newsletter, 20 October 2017. Available online: https://www.nutraingredients.com/Article/2017/10/20/Mushrooms-need-to-be-further-explored-as-functional-foods-say-researchers (accessed on 29 June 2021).
- Raghavendra, V.B.; Venkitasamy, C.; Pan, Z.; Nayak, C. Functional Foods from Mushroom. In Microbial Functional Foods and Nutraceuticals, 1st ed.; Gupta, V.K., Treichel, H., Shapaval, V., de Oliveira, L.A., Tuohy, M.G., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 65–91. [Google Scholar]
- Reis, F.S.; Martins, A.; Vasconcelos, M.H.; Morales, P.; Ferreira, I.C.F.R. Functional foods based on extracts or compounds derived from mushrooms. Trends Food Sci. Technol. 2017, 66, 48–62. [Google Scholar] [CrossRef]
- Bryant, J.M.; Bouchard, M.; Haque, A. Anticancer activity of ganoderic acid DM: Current status and future perspective. J. Clin. Cell Immunol. 2017, 8, 535. [Google Scholar] [CrossRef] [PubMed]
- Kaul, T.N. Biology and Conservation of Mushrooms; Oxford and IBH Publishing Co. Pvt. Ltd.: New Delhi, India, 2001; pp. 117–145. [Google Scholar]
- Species Fungorum. Species FungorumInitiative. Coordinated by the Royal Botanic Gardens, Kew. 2020. Available online: http://www.speciesfungorum.org/Names/Names.asp (accessed on 16 February 2022).
- Hawksworth, D.L.; Lücking, R. Fungal Diversity Revisited: 2.2 to 3.8 Million Species. In The Fungal Kingdom; Heitman, J., Howlett, B.J., Crous, P.W., Stukenbrock, E.H., James, T.Y., Gow, N.A.R., Eds.; ASM Press: Washington, DC, USA, 2017; pp. 79–95. [Google Scholar] [CrossRef]
- Kamal, S.; Pandey, J.; Ghignone, S.; Varma, A. Mushroom biology and biotechnology an overview. In A Textbook of Molecular Biotechnology, 3rd ed.; Chauhan, A.K., Varma, A., Eds.; I. K. International Publishing House Pvt. Ltd.: New Delhi, India, 2009; pp. 573–628. [Google Scholar]
- Sadler, M. Nutritional properties of edible fungi. Nutr. Bull. 2003, 28, 305–308. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, J.; Yin, Y.; Zhao, J.; Sun, X.; Tang, K. Ganodermataceae: Natural products and their related pharmacological functions. Am. J. Chin. Med. 2007, 35, 559–574. [Google Scholar] [CrossRef]
- Roy, D.N.; Azad, A.K.; Sultana, F.; Anisuzzaman, A.S.M.; Khondkar, P. Nutritional profile and mineral composition of two edible mushroom varieties consumed and cultivated in Bangladesh. J. Phytopharmacol. 2015, 4, 217–220. [Google Scholar] [CrossRef]
- Dietary Reference Intakes (DRIs). Dietary Reference Intakes of Nutrients-Based Reference Values. These Are Established by Nutrition Board of National Academy of Sciences; National Academy Press: Washington, DC, USA, 2004; Available online: http://www.nap.edu (accessed on 29 June 2021).
- Dietary Reference Intakes (DRIs). The Essential Guide to Nutrient Requirements; National Academy Press: Washington, DC, USA, 2006; Available online: https://www.nap.edu/read/11537/chapter/45 (accessed on 29 June 2021).
- Manzi, P.; Marconi, S.; Aguzzi, A.; Pizzoferrato, L. Commercial mushrooms: Nutritional quality and effect of cooking. Food Chem. 2004, 84, 201–206. [Google Scholar] [CrossRef]
- Casselbury, K. Recommended Daily Fat Intakes for Females. 2018. Available online: https://healthyeating.sfgate.com/recommended-daily-fat-intakes-females-6305.html (accessed on 29 June 2021).
- Duthie, G.G.; Susan, J.; Janet, A.; Kyle, M. Plant polyphenols in cancer and heart disease: Implications as nutritional antioxidants. Nutr. Res. Rev. 2000, 13, 79–106. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Al Masud, A.; Lira, N.Y.; Shakil, S. Proximate analysis, phtochemical screening and antioxidant activity of different strains of Ganoderma lucidum (Reishi Mushroom). Open J. Biol. Sci. 2020, 5, 24–27. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, H.; Zhang, X.; Yan, J. Amino acids from Ganoderma lucidum: Extraction optimization, composition analysis, hypoglycemic and antioxidant activities. Curr. Pharm. Anal. 2018, 14, 562–570. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Zhao, X.; Tang, Q.; Dernedde, J.; Zhang, J.; Fan, H. Anti-inflammatory properties of GLPss58, a sulfated polysaccharide from Ganoderma lucidum. Int. J. Biol. Macromol. 2018, 107, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.F. Ganoderma lucidum: A rational pharmacological approach to surmount cancer. J. Ethnopharmacol. 2020, 260, 113047. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.T.; Wasser, S.P. The Cultivation and Environmental Impact of Mushrooms. Oxford Research Encyclopedia Environmental Science. 2017. Available online: https://oxfordre.com/environmentalscience/view/10.1093/acrefore/9780199389414.001.0001/acrefore-9780199389414-e-231 (accessed on 16 February 2022).
- Cör, D.; Knez, Z.; Hrnčič, M.K. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: A review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Xu, J.; Liu, C.; Zhu, Y.; Nelson, D.R.; Zhou, S.; Li, C.; Wang, L.; Guo, X.; Sun, Y.; et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat. Commun. 2012, 3, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Montemayor, M.M.; Ling, T.; Suárez-Arroyo, I.J.; Ortiz-Soto, G.; Santiago-Negrón, C.L.; Lacourt-Ventura, M.Y.; Valentín-Acevedo, A.; Lang, W.H.; Rivas, F. Identification of biologically active Ganoderma lucidum compounds and synthesis of improved derivatives that confer anti-cancer activities in vitro. Front. Pharmacol. 2019, 10, 115. [Google Scholar] [CrossRef] [Green Version]
- Sudheer, S.; Alzorqi, I.; Manickam, S.; Ali, A. Bioactive Compounds of the Wonder Medicinal Mushroom “Ganoderma lucidum”. In Bioactive Molecules in Food; Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2019; pp. 1863–1893. [Google Scholar] [CrossRef]
- Parepalli, Y.; Chavali, M.; Sami, R.; Khojah, E.; Elhakem, A.; El Askary, A.; Singh, M.; Sinha, S.; El-Chaghaby, G. Evaluation of some active nutrients, biological compounds and health benefits of reishi mushroom (Ganoderma lucidum) Int. J. Pharmacol. 2021, 17, 243–250. [Google Scholar] [CrossRef]
- El Mansy, S.M. Ganoderma: The mushroom of immortality. Microb. Biosyst. 2019, 4, 45–57. [Google Scholar]
- Zhang, Y.; Wang, D.; Chen, Y.; Liu, T.; Zhang, S.; Fan, H.; Liu, H.; Li, Y. Healthy function and high valued utilization of edible fungi. Food Sci. Hum. Wellness 2021, 10, 408–420. [Google Scholar] [CrossRef]
- Gong, P.; Wang, S.; Liu, M.; Chen, F.; Yang, W.; Chang, X.; Liu, N.; Zhao, Y.; Wang, J.; Chen, X. Extraction methods, chemical characterizations and biological activities of mushroom polysaccharides: A mini-review. Carbohydr. Res. 2020, 494, 108037. [Google Scholar] [CrossRef]
- Ma, H.T.; Hsieh, J.F.; Chen, S.T. Anti-diabetic effects of Ganoderma lucidum. Phytochemistry 2015, 114, 109–113. [Google Scholar] [CrossRef]
- Zhang, W.; Tao, J.; Yang, X.; Yang, Z.; Zhang, L.; Liu, H.; Wu, K.; Jianguo Wu, J. Antiviral effects of two Ganoderma lucidum triterpenoids against enterovirus 71 infection. Biochem. Biophys. Res. Commun. 2014, 449, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Bang, T.H.; Ohnuki, K.; Sawai, T.; Sawai, K.; Shimizu, K. Inhibition of neuraminidase by Ganoderma triterpenoids and implications for neuraminidase inhibitor design. Sci. Rep. 2015, 5, 13194. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.F.R.; Heleno, S.A.; Reis, F.S.; Stojkovic, D.; Queiroz, M.J.R.P.; Vasconcelos, M.H.; Sokovic, M. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochemistry 2015, 114, 38–55. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.W.; Tomlinson, B.; Chan, P.; Lam, C.W.K. The beneficial effects of Ganoderma lucidum on cardiovascular and metabolic disease risk. Pharm. Biol. 2021, 59, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S. Studies on Genetic Variability and Bioactive Molecules Production by Ganoderma Species. Ph.D. Thesis, Shoolini University of Biotechnology and Management Sciences, Bajhol, Solan, India, 2014. [Google Scholar]
- Lee, B.; Park, J.; Park, J.; Shin, H.-J.; Kwon, S.; Yeom, M.; Sur, B.; Kim, S.; Kim, M.; Lee, H.; et al. Cordyceps militaris improves neurite outgrowth in Neuro2A cells and reverses memory impairment in rats. Food Sci. Biotechnol. 2011, 20, 1599–1608. [Google Scholar] [CrossRef]
- Gao, P.; Hirano, T.; Chen, Z.; Yasuhara, T.; Nakata, Y.; Sugimoto, A. Isolation and identification of C-19 fatty acids with anti-tumor activity from the spores of Ganoderma lucidum (reishi mushroom). Fitoterapia 2012, 83, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Zhang, H.; Li, W.; Xie, M. Current development of polysaccharides from Ganoderma: Isolation, structure and bioactivities. Bioact. Carbohydr. Diet. Fibre 2013, 1, 10–20. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, X.; Hu, Y.-S.; Wu, Y.; Wang, Q.-Z.; Li, N.-N.; Guo, Q.-C.; Dong, X.-C. Evaluation of in vivo antioxidant activities of Ganoderma lucidum polysaccharides in STZ-diabetic rats. Food Chem. 2009, 115, 32–36. [Google Scholar] [CrossRef]
- XiaoPing, C.; Yan, C.; Shuibing, L.; YouGou, C.; JianYun, L.; LanPing, L. Free radical scavenging of Ganoderma lucidum polysaccharides and its effect on antioxidant enzymes and immunity activities in cervical carcinoma rats. Carbohydr. Polym. 2009, 77, 389–393. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, Z.; Yang, Y. Antioxidant and immunoregulatory activity of Ganoderma lucidum polysaccharide. Carbohydr. Polym. 2013, 95, 200–206. [Google Scholar] [CrossRef]
- Miyazaki, T.; Nishijima, M. Studies on fungal polysaccharides. XXVII. Structural examination of a water-soluble, antitumor polysaccharide of Ganoderma lucidum. Chem. Pharm. Bull. 1981, 29, 3611–3616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hikino, H.; Konno, C.; Mirin, Y.; Hayashi, T. Isolation and hypoglycemic activity of ganoderans A and B, glycans of Ganoderma lucidum fruit bodies. Planta Med. 1985, 4, 339–340. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, M.; Gonda, R.; Kasahara, Y.; Hikino, H. Glycan structures of ganoderans B and C, hypoglycemic glycans of Ganoderma lucidum fruit bodies. Phytochemistry 1986, 25, 2817–2820. [Google Scholar] [CrossRef]
- Bao, X.; Liu, C.; Fang, J.; Li, X. Structural and immunological studies of a major polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Carbohydr. Res. 2001, 332, 67–74. [Google Scholar] [CrossRef]
- Wachtel-Galor, S.; Buswell, J.A.; Tomlinson, B.; Benzie, I.F.F. Lingzhi polyphorous fungus. In Herbal and Traditional Medicine: Molecular Aspects of Health, 1st ed.; Wachtel-Galor, S., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 179–228. [Google Scholar]
- Wang, P.-Y.; Zhu, X.-L.; Lin, Z.-B. Antitumor and immunomodulatory effects of polysaccharides from broken-spore of Ganoderma lucidum. Front. Pharmacol. 2012, 3, 135. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Wang, H.; Pang, X.; Yao, W.; Gao, X. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2010, 46, 451–457. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Niksic, M.; Jakovljevic, D.; Helsper, J.P.F.G.; Van Griensven, L.J.L.D. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem. 2011, 129, 1667–1675. [Google Scholar] [CrossRef]
- Ospina, N.M.; Alvarez, S.P.O.; Sierra, D.M.E.; Vahos, D.F.R.; Ocampo, P.A.Z.; Orozco, C.P.O. Isolation of chitosan from Ganoderma lucidum mushroom for biomedical applications. J. Mater. Sci. Mater. Med. 2015, 26, 135. [Google Scholar] [CrossRef]
- Ji, Z.; Tang, Q.; Zhang, J.; Yang, Y.; Jia, W.; Pan, Y. Immunomodulation of RAW264.7 macrophages by GLIS, a proteopolysaccharide from Ganoderma lucidum. J. Ethnopharmacol. 2007, 112, 445–450. [Google Scholar] [CrossRef]
- Nishitoba, T.; Sato, H.; Kasai, T.; Kawagishi, H.; Sakamura, S. New bitter C27 and C30 terpenoids from fungus Ganoderma lucidum (Reishi). Agric. Biol. Chem. 1984, 48, 2905–2907. [Google Scholar] [CrossRef]
- Sato, H.; Nishitoba, T.; Shirasu, S.; Oda, K.; Sakamura, S. Ganoderiol A and B, new triterpenoids from the fungus Ganoderma lucidum (Reishi). Agric. Biol. Chem. 1986, 50, 2887–2890. [Google Scholar] [CrossRef]
- Budavari, S. The Merck Index; Merck & Co., Inc.: Whitehouse Station, NJ, USA, 1989. [Google Scholar]
- Gonzalez, A.G.; Leon, F.; Rivera, A.; Munoz, C.M.; Bermejo, J. Lanostanoid triterpenes from Ganoderma lucidum. J. Nat. Prod. 1999, 62, 1700–1701. [Google Scholar] [CrossRef]
- Ma, J.; Ye, Q.; Hua, Y.; Zhang, D.; Cooper, R.; Chang, M.N.; Chang, J.Y.; Sun, H.H. New lanostanoids from the mushroom Ganoderma lucidum. J. Nat. Prod. 2002, 65, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Nakamura, Y.; Tagata, M.; Tokuda, H.; Yasukawa, K.; Uchiyama, E.; Suzuki, T.; Kimura, Y. Anti-inflammatory and anti-tumor-promoting effects of triterpene acids and sterols from the fungus Ganoderma lucidum. Chem. Biodivers. 2007, 4, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Grieb, B.; Thyagarajan, A.; Sliva, D. Ganoderic acids suppress growth and invasive behavior of breast cancer cells by modulating AP-1 and NF-kappaB signaling. Int. J. Mol. Med. 2008, 21, 577–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Bicker, W.; Wu, J.; Xie, M.Y.; Lindner, W. Ganoderma species discrimination by dual-mode chromatographic fingerprinting: A study on stationary phase effects in hydrophilic interaction chromatography and reduction of sample misclassification rate by additional use of reversed-phase chromatography. J. Chromatogr. A 2010, 1217, 1255–1265. [Google Scholar] [CrossRef]
- Chiu, S.W.; Wang, Z.M.; Leung, T.M.; Moore, D. Nutritional value of Ganoderma extract and assessment of its genotoxicity and antigenotoxicity using comet assays of mouse lymphocytes. Food Chem. Toxicol. 2000, 38, 173–178. [Google Scholar] [CrossRef]
- Kolesnikova, O.P.; Tuzova, M.N.; Kozlov, V.A. Screening of immunoactive properties of alkanecarbonic acid derivatives and germanium-organic compounds in vivo. Immunologiya 1997, 10, 36–38. [Google Scholar]
- Van Der Hem, L.; Van Der Vliet, A.; Bocken, C.F.M.; Kino, K.; Hoitsma, A.J.; Tax, W.J.M. Lingzhi-8: Studies of a new immunomodulating agent. Transplantation 1995, 60, 438–443. [Google Scholar] [CrossRef]
- Sun, J.; He, H.; Xie, B.J. Novel antioxidant peptides from fermented mushroom Ganoderma lucidum. J. Agric. Food Chem. 2004, 52, 6646–6652. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, J.; He, H.; Guo, H.; Zhang, S. Hepatoprotective effects of Ganoderma lucidum peptides against D-galactosamine-induced liver injury in mice. J. Ethnopharmacol. 2008, 117, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ng, T.B. Ganodermin, an antifungal protein from fruiting bodies of the medicinal mushroom Ganoderma lucidum. Peptides 2006, 27, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Deepalakshmi, K.; Mirunalini, S. Therapeutic properties and current medical usage of medicinal mushroom: Ganoderma lucidum. Int. J. Pharm. Sci. Res. 2011, 2, 1922–1929. [Google Scholar] [CrossRef]
- Zhao, R.-L.; He, Y.-M. Network pharmacology analysis of the anti-cancer pharmacological mechanisms of Ganoderma lucidum extract with experimental support using Hepa1-6-bearing C57 BL/6 mice. J. Ethnopharmacol. 2018, 210, 287–295. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, J.P.; Chung, C.K.; Chen, X.J. Antitumor activity of the sporoderm-broken germinating spores of Ganoderma lucidum. Cancer Lett. 2002, 182, 155–161. [Google Scholar] [CrossRef]
- Tang, W.; Gao, Y.; Chen, G.; Gao, H.; Dai, X.; Ye, J.; Chan, E.; Huang, M.; Zhou, S. A randomized, double-blind and placebo-controlled study of a Ganoderma lucidum polysaccharide extract in neurasthenia. J. Med. Food 2005, 8, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Paterson, R.R. Ganoderma—A therapeutic fungal biofactory. Phytochemistry 2006, 67, 1985–2001. [Google Scholar] [CrossRef] [Green Version]
- Mir, M.A.; Sharma, T.; Sharma, K.; Anjum, S.; Mir, B.A. Anti-urolithiatic and anti-arthritis activity of various extracts of Ganoderma lucidum. Nat. Prod. Chem. Res. 2017, 5, 297. [Google Scholar] [CrossRef]
- Yen, G.C.; Wu, J.Y. Antioxidant and radical scavenging properties of extracts from Ganoderma tsugae. Food Chem. 1999, 65, 375–379. [Google Scholar] [CrossRef]
- Mau, J.L.; Lin, H.C.; Chen, C.C. Antioxidant properties of several medicinal mushrooms. J. Agric. Food Chem. 2002, 50, 6072–6077. [Google Scholar] [CrossRef]
- Sudheesh, N.A.; Ajith, T.A.; Janardhanan, K.K. Ganoderma lucidum ameliorate mitochondrial damage in isoproterenol-induced myocardial infarction in rats by enhancing the activities of TCA cycle enzymes and respiratory chain complexes. Int. J. Cardiol. 2013, 165, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lan, J.; Dai, X.; Ye, J.; Zhou, S. A phase I/II study of Lingzhi mushroom Ganoderma lucidum (W. Curt.: Fr.) Lloyd (Aphyllophoromycetideae) extract in patients with type II diabetes mellitus. Int. J. Med. Mushrooms 2004, 6, 33–40. [Google Scholar] [CrossRef]
- Teng, B.-S.; Wang, C.-D.; Yang, H.-J.; Wu, J.-S.; Zhang, D.; Zheng, M.; Fan, Z.-H.; Pan, D.; Zhou, P. A protein tyrosine phosphatase 1B activity inhibitor from the fruiting bodies of Ganoderma lucidum (Fr.) Karst and its hypoglycemic potency on streptozotocin-induced type 2 diabetic mice. J. Agric. Food Chem. 2011, 59, 6492–6500. [Google Scholar] [CrossRef] [PubMed]
- Basnet, B.B.; Liu, L.; Bao, L.; Liu, H. Current and future perspective on antimicrobial and anti-parasitic activities of Ganoderma sp.: An update. Mycology 2017, 8, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zhou, S.; Huang, M.; Xu, A. Antibacterial and antiviral value of the genus Ganoderma P. Karst. species (Aphyllophoromycetideae): A review. Int. J. Med. Mushrooms 2003, 5, 235–246. [Google Scholar] [CrossRef]
- Keypour, S.; Riahi, H.; Moradali, M.F.; Rafati, H. Investigation of the antibacterial activity of a chloroform extract of Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (W. Curt.: Fr.) P. Karst. (Aphyllophoromycetideae). Int. J. Med. Mushrooms 2008, 10, 345–349. [Google Scholar] [CrossRef]
- Jonathan, S.G.; Awotona, F.E. Studies on antimicrobial potentials of three Ganoderma species. Afr. J. Biomed. Res. 2010, 13, 133–139. [Google Scholar]
- Hernández-Márquez, E.; Lagunas-Martínez, A.; Bermudez-Morales, V.H.; Burgete-García, A.I.; León-Rivera, I.; Montiel-Arcos, E.; García-Villa, E.; Gariglio, P.; Madrid-Marina, V.V.; Ondarza-Vidaurreta, R.N. Inhibitory activity of Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (Higher Basidiomycetes) on transformed cells by Human Papillomavirus. Int. J. Med. Mushrooms 2014, 16, 179–187. [Google Scholar] [CrossRef]
- Shah, P.; Modi, H.A.; Shukla, M.D.; Lahiri, S.K. Preliminary phytochemical analysis and antibacterial activity of Ganoderma lucidum collected from Dang District of Gujarat, India. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 246–255. [Google Scholar]
- Liu, D.Z.; Zhu, Y.Q.; Li, X.F.; Shan, W.G.; Gao, P.F. New triterpenoids from the fruiting bodies of Ganoderma lucidum and their bioactivities. Chem. Biodivers. 2014, 11, 982–986. [Google Scholar] [CrossRef]
- Shang, X.; Tan, Q.; Liu, R.; Yu, K.; Li, P.; Zhao, G.-P. In vitro anti-Helicobacter pylori effects of medicinal mushroom extracts, with special emphasis on the Lion’s Mane mushroom, Hericium erinaceus (higher Basidiomycetes). Int. J. Med. Mushrooms 2013, 15, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Ćilerdžić, J.; Stajić, M.; Vukojević, J. Potential of submergedly cultivated mycelia of Ganoderma spp. as antioxidant and antimicrobial agents. Curr. Pharm. Biotechnol. 2016, 17, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Karwa, A.; Rai, M. Naturally occurring medicinal mushroom-derived antimicrobials: A case-study using lingzhi or reishi Ganoderma lucidum (W. Curt.: Fr.) P. Karst. (Higher Basidiomycetes). Int. J. Med. Mushrooms 2012, 14, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Ćilerdžić, J.; Vukojević, J.; Stajić, M.; Stanojković, T.; Glamočlija, J. Biological activity of Ganoderma lucidum basidiocarps cultivated on alternative and commercial substrate. J. Ethnopharmacol. 2014, 155, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Eo, S.K.; Kim, Y.S.; Lee, C.K.; Han, S.S. Antimicrobial activity of Ganoderma lucidum extract alone and in combination with some antibiotics. Arch. Pharm. Res. 1994, 17, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Vazirian, M.; Faramarzi, M.A.; Ebrahimi, S.E.S.; Esfahani, H.R.M.; Samadi, N.; Hosseini, S.A.; Asghari, A.; Manayi, A.; Mousazadeh, A.; Asef, M.R.; et al. Antimicrobial effect of the Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (higher Basidiomycetes) and its main compounds. Int. J. Med. Mushrooms 2014, 16, 77–84. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, T.; Hu, Y.; Wang, X.; Du, J.; Li, Y.; Sun, S.; Sun, X.; Li, Z.; Jin, Q. Survey of enterovirus infections from hand, foot and mouth disease outbreak in China, 2009. Virol. J. 2011, 8, 508. [Google Scholar] [CrossRef] [Green Version]
- Sa-Ard, P.; Sarnthima, R.; Khammuang, S.; Kanchanarach, W. Antioxidant, antibacterial and DNA protective activities of protein extracts from Ganoderma lucidum. J. Food Sci. Technol. 2015, 52, 2966–2973. [Google Scholar] [CrossRef] [Green Version]
- Heleno, S.A.; Ferreira, I.C.F.R.; Esteves, A.P.; Ćirić, A.; Glamočlija, J.; Martins, A.; Soković, M.; Queiroz, M.J.R.P. Antimicrobial and demelanizing activity of Ganoderma lucidum extract, p-hydroxybenzoic and cinnamic acids and their synthetic acetylated glucuronide methyl esters. Food Chem. Toxicol. 2013, 58, 95–100. [Google Scholar] [CrossRef]
- Sun, X.; Jin, X.; Pan, W.; Wang, J. Syntheses of new rare earth complexes with carboxymethylated polysaccharides and evaluation of their in vitro antifungal activities. Carbohydr. Polym. 2014, 113, 194–199. [Google Scholar] [CrossRef]
- El-Mekkawy, S.; Meselhy, M.R.; Nakamura, N.; Tezuka, Y.; Hattori, M.; Kakiuchi, N.; Shimotohno, K.; Kawahata, T.; Otake, T. Anti- HIV-1 and anti-HIV-1-protease substances from Ganoderma lucidum. Phytochemistry 1998, 49, 1651–1657. [Google Scholar] [CrossRef]
- Eo, S.K.; Kim, Y.S.; Lee, C.K.; Han, S.S. Possible mode of antiviral activity of acidic protein bound polysaccharide isolated from Ganoderma lucidum on herpes simplex viruses. J. Ethnopharmacol. 2000, 72, 475–481. [Google Scholar] [CrossRef]
- Donatini, B. Control of oral Human Papillomavirus (HPV) by medicinal mushrooms, trametes versicolor and Ganoderma lucidum: A preliminary clinical trial. Int. J. Med. Mushrooms 2014, 16, 497–498. [Google Scholar] [CrossRef] [PubMed]
- Shamaki, B.U.; Sandabe, U.K.; Ogbe, A.O.; Abdulrahman, F.I.; El-Yuguda, A.-D. Methanolic soluble fractions of lingzhi or reishi medicinal mushroom, Ganoderma lucidum (Higher basidiomycetes) Extract inhibit neuraminidase activity in newcastle disease virus (LaSota). Int. J. Med. Mushrooms 2014, 16, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, K.; Akihisa, T.; Tokuda, H.; Ukiya, M.; Oshikubo, M.; Kimura, Y.; Asano, T.; Nomura, A.; Nishino, H. Lucidenic acids P and Q, methyl lucidenate P, and other triterpenoids from the fungus Ganoderma lucidum and their inhibitory effects on epstein−barr virus activation. J. Nat. Prod. 2003, 66, 1582–1585. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Fang, L.; Zhang, Z.; Jin, J.; Zhang, K. Antihepatitis activities in the broth of Ganoderma lucidum supplemented with a Chinese herbal medicine. Am. J. Chin. Med. 2006, 34, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Q.; Wang, S.F. Anti-hepatitis B activities of ganoderic acid from Ganoderma lucidum. Biotechnol. Lett. 2006, 28, 837–841. [Google Scholar] [CrossRef]
- Zhu, Q.; Amen, Y.M.; Ohnuki, K.; Shimizu, K. Anti-influenza effects of Ganoderma lingzhi: An animal study. J. Funct. Foods 2017, 34, 224–228. [Google Scholar] [CrossRef]
- Blomberg, J.; Lycke, E.; Ahlfors, K.; Johnsson, T.; Wolontis, S.; von Zeipel, G. New enterovirus type associate with epidemic of aseptic meningitis and-or hand, foot, and mouth disease. Lancet 1974, 2, 112. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, J.; Lu, J. Enterovirus 71 vaccine: Close but still far. Int. J. Infect. Dis. 2010, 14, e739–e743. [Google Scholar] [CrossRef] [Green Version]
- Shang, L.; Xu, M.; Yin, Z. Antiviral drug discovery for the treatment of enterovirus 71 infections. Antivir. Res. 2013, 97, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Fowlkes, A.L.; Honarmand, S.; Glaser, C.; Yagi, S.; Schnurr, D.; Oberste, M.S.; Anderson, L.; Pallansch, M.A.; Khetsuriani, N. Enterovirus-associated encephalitis in the California encephalitis project, 1998–2005. J. Infect. Dis. 2008, 198, 1685–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, J.P., Jr.; Baden, L.; Pallansch, M.A.; Anderson, L.J. Enterovirus 71 infections and neurologic disease—United States, 1977–1991. J. Infect. Dis. 1994, 169, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.Y.; Huang, Y.C.; Lin, T.Y. Fulminant neurogenic pulmonary oedema with hand, foot, and mouth disease. Lancet 1998, 352, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Liu, C.C.; Chang, Y.C.; Chen, C.Y.; Wang, S.T.; Yeh, T.F. Neurologic complications in children with enterovirus 71 infection. N. Engl. J. Med. 1999, 341, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ren, L.; Xiong, Z.; Li, J.; Xiao, Y.; Zhao, R.; He, Y.; Bu, G.; Zhou, S.; Wang, J.; et al. Enterovirus 71 outbreak in the People’s Republic of China in 2008. J. Clin. Microbiol. 2009, 47, 2351–2352. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.Y.; Liu, W.; Luo, J.; Liu, Y.; Zhu, Y.; Berman, H.; Wu, J. Characterization of an outbreak of hand, foot, and mouth disease in Nanchang, China in 2010. PLoS ONE 2011, 6, e25287. [Google Scholar] [CrossRef] [Green Version]
- Kok, C.C. Therapeutic and prevention strategies against human enterovirus 71 infection. World J. Virol. 2015, 4, 78–95. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Woerdenbag, H.J. Traditional Chinese herbal medicine. Pharm. World Sci. 1995, 17, 103–112. [Google Scholar] [CrossRef]
- Li, T.; Peng, T. Traditional Chinese herbal medicine as a source of molecules with antiviral activity. Antivir. Res. 2013, 97, 1–9. [Google Scholar] [CrossRef]
- Ma, B.; Ren, W.; Zhou, Y.; Ma, J.; Ruan, Y.; Wen, C.N. Triterpenoids from the spores of Ganoderma lucidum. N. Am. J. Med. Sci. 2011, 3, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.E.P.; Alencar, C.H.; Kamimura, M.T.; de Carvalho Araújo, F.M.; De Simone, S.G.; Dutra, R.F.; Guedes, M.I.F. Occurrence of natural vertical transmission of dengue-2 and dengue-3 viruses in Aedes aegypti and Aedes albopictus in Fortaleza, Ceará, Brazil. PLoS ONE 2012, 7, e41386. [Google Scholar] [CrossRef]
- Simmons, C.P.; Farrar, J.J.; van Vinh Chau, N.; Wills, B. Dengue. N. Engl. J. Med. 2012, 366, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Akiner, M.M.; Demirci, B.; Babuadze, G.; Robert, V.; Schaffner, F. Spread of the invasive mosquitoes Aedes aegypti and Aedes albopictus in the Black Sea region increases risk of chikungunya, dengue, and Zika outbreaks in Europe. PLoS Negl. Trop. Dis. 2016, 10, e0004664. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, Y. Principal components analysis based unsupervised feature extraction applied to gene expression analysis of blood from dengue haemorrhagic fever patients. Sci. Rep. 2017, 7, 44016. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.H.-C.; Alonso, S.; Ng, L.F.-P.; Thein, T.-L.; Pang, V.J.-X.; Leo, Y.-S.; Lye, D.C.-B.; Yeob, T.-W. Increased serum hyaluronic acid and heparan sulfate in dengue fever: Association with plasma leakage and disease severity. Sci. Rep. 2017, 7, 46191. [Google Scholar] [CrossRef] [Green Version]
- Le Duyen, H.T.; Cerny, D.; Trung, D.T.; Pang, J.; Velumani, S.; Toh, Y.X.; Qui, P.T.; Hao, N.V.; Simmons, C.; Haniffa, M.; et al. Skin dendritic cell and T cell activation associated with dengue shock syndrome. Sci. Rep. 2017, 7, 14224. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.; Lert-Itthiporn, W.; Cavadas, B.; Fernandes, V.; Chuansumrit, A.; Anunciação, O.; Casademont, I.; Koeth, F.; Penova, M.; Tangnararatchakit, K.; et al. Joint ancestry and association test indicate two distinct pathogenic pathways involved in classical dengue fever and dengue shock syndrome. PLoS Negl. Trop. Dis. 2018, 12, e0006202. [Google Scholar] [CrossRef]
- Mustafa, M.; Rasotgi, V.; Jain, S.; Gupta, V. Discovery of fifth serotype of dengue virus (DENV-5): A new public health dilemma in dengue control. Med. J. Armed Forces India 2015, 71, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, V.D.; Tripathi, I.P.; Tripathi, R.C.; Bharadwaj, S.; Mishra, S.K. Genomics, proteomics and evolution of dengue virus. Brief Funct. Genom. 2017, 16, 217–227. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Vasudevan, S.G.; Lescar, J. The flavivirus NS2B–NS3 protease–helicase as a target for antiviral drug development. Antivir. Res. 2015, 118, 148–158. [Google Scholar] [CrossRef]
- Constant, D.A.; Mateo, R.; Nagamine, C.M.; Kirkegaard, K. Targeting intramolecular proteinase NS2B/3 cleavages for transdominant inhibition of dengue virus. Proc. Natl. Acad. Sci. USA 2018, 115, 10136–11014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, A.C.; Steele, R.; Liu, G.; Tounge, B.A.; Montelione, G.T. Inhibitor bound dengue NS2B-NS3pro reveals multiple dynamic binding modes. Biochemistry 2018, 57, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Lee, K.E.; Dwivedi, V.D.; Yadava, U.; Panwar, A.; Lucas, S.J.; Pandey, A.; Kang, S.G. Discovery of Ganoderma lucidum triterpenoids as potential inhibitors against Dengue virus NS2B-NS3 protease. Sci. Rep. 2019, 9, 19059. [Google Scholar] [CrossRef] [Green Version]
- Gralinski, L.E.; Menachery, V.D. Return of the coronavirus: 2019-nCoV. Viruses 2020, 12, 135. [Google Scholar] [CrossRef] [Green Version]
- Burki, T.K. Coronavirus in China. Lancet Respir. Med. 2020, 8, P238. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Director-General’s Remarks at the Media Briefing on 2019-nCoV on 11 February 2020. 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 (accessed on 29 June 2021).
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical characteristics of 2019 novel coronavirus infection in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Coronavirus Disease (COVID-19) Outbreak Situation. 2021. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 29 June 2021).
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Xia, Z. Traditional Chinese Medicine (TCM)—Does its contemporary business booming and globalization really reconfirm its medical efficacy & safety? Med. Drug Discov. 2019, 1, 100003. [Google Scholar] [CrossRef]
- Gao, R.-R.; Hu, Y.-T.; Dan, Y.; Hao, L.-J.; Liu, X.; Song, J.-Y. Chinese herbal medicine resources: Where we stand. Chin. Herb. Med. 2020, 12, 3–13. [Google Scholar] [CrossRef]
- Yang, Y.; Islam, M.S.; Wang, J.; Li, Y.; Chen, X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): A review and perspective. Int. J. Biol. Sci. 2020, 16, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.S.; Lee, J.; Lee, J.M.; Kim, Y.; Chin, Y.-W.; Jee, J.-G.; Keum, Y.-S.; Jeong, Y.-J. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg. Med. Chem. Lett. 2012, 22, 4049–4054. [Google Scholar] [CrossRef]
- Fung, K.P.; Leung, P.C.; Tsui, K.W.S.; Wan, C.C.D.; Wong, K.B.; Waye, M.Y.M.; Au, W.N.S.; Wong, C.K.; Lam, W.K.C.; Lau, B.S.C. Immunomodulatory activities of the herbal formula Kwan Du Bu Fei Dang in healthy subjects: A randomised, double-blind, placebo-controlled study. Hong Kong Med. J. 2011, 17, 41–43. [Google Scholar]
- Abdullah, N.; Ismail, S.M.; Aminudin, N.; Shuib, A.S.; Lau, B.F. Evaluation of selected culinary-medicinal mushrooms for antioxidant and ACE inhibitory activities. Evid. Based Complement. Altern. Med. 2012, 2012, 464238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kana, Y.; Chen, T.; Wu, Y.; Wu, J. Antioxidant activity of polysaccharide extracted from Ganoderma lucidum using response surface methodology. Int. J. Biol. Macromol. 2015, 72, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, H.; Zhang, Y.; Xing, L.; Li, S.; Wang, X.; Sun, Z. Chemical composition and antioxidant properties of five edible Hymenomycetes mushrooms. Int. J. Food Sci. Technol. 2015, 50, 465–471. [Google Scholar] [CrossRef]
- Collins, A.R. Antioxidant intervention as a route to cancer prevention. Eur. J. Cancer 2005, 41, 1923–1930. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Wachtel-Galor, S. Biomarkers of long-term vegetarian diets. Adv. Clin. Chem. 2009, 47, 169–220. [Google Scholar]
- Mohan, K.; Padmanaban, M.; Uthayakumar, V. Isolation, structural characterization and antioxidant activities of polysaccharide from Ganoderma lucidum (Higher Basidiomycetes). Am. J. Biol. Life Sci. 2015, 3, 168–175. [Google Scholar]
- Zeng, Q.; Zhou, F.; Lei, L.; Chen, J.; Lu, J.; Zhou, J.; Cao, K.; Gao, L.; Xia, F.; Ding, S.; et al. Ganoderma lucidum polysaccharides protect fibroblasts against UVB-induced photoaging. Mol. Med. Rep. 2017, 15, 111–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). Mortality Statistics. World Health Report. 2008. Available online: http://www.who.int/gho/publications/world_health_statistics/EN_WHS08_Full.pdf (accessed on 29 June 2021).
- El Sheikha, A.F. Medicinal plants: Ethno-uses to biotechnology era. In Biotechnology and Production of Anti-Cancer Compounds; Malik, S., Ed.; Part of Springer Nature; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 1–38. [Google Scholar]
- Wasser, S.P.; Weis, A.L. Medicinal properties of substances occurring in higher basidiomycetes mushrooms: Current perspectives. Int. J. Med. Mushrooms 1999, 1, 31–62. [Google Scholar] [CrossRef] [Green Version]
- Borchers, A.T.; Krishnamurthy, A.; Keen, C.L.; Meyers, F.J.; Gershwin, M.E. The immunobiology of mushrooms. Exp. Biol. Med. 2008, 233, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, S.; Lohezic-Le, D.F.; Sauleau, P.; Bezivin, C.; Boustie, J. Cytotoxic activity of methanol extracts from Basidiomycete mushrooms on murine cancer cell lines. Pharmazie 2004, 59, 290–293. [Google Scholar] [PubMed]
- Zaidman, B.Z.; Yassin, M.; Mahajna, J.; Wasser, S.P. Medicinal mushroom modulators of molecular targets as cancer therapeutics. Appl. Microbiol. Biotechnol. 2005, 67, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Yuen, J.W.; Gohel, M.D. The dual roles of Ganoderma antioxidants on urothelial cell DNA under carcinogenic attack. J. Ethnopharmacol. 2008, 118, 324–330. [Google Scholar] [CrossRef]
- Trajković, L.M.H.; Mijatović, S.A.; Maksimović-Ivanić, D.D.; Stojanović, I.D.; Momčilović, M.B.; Tufegdžić, S.J.; Maksimović, V.M.; Marjanovi, Z.S.; Stošić-Grujičić, S.D. Anticancer properties of Ganoderma lucidum methanol extracts in vitro and in vivo. Nutr. Cancer 2009, 61, 696–707. [Google Scholar] [CrossRef]
- Calviño, E.; Manjón, J.L.; Sancho, P.; Tejedor, M.C.; Herráez, A.; Diez, J.C. Ganoderma lucidum induced apoptosis in NB4 human leukemia cells: Involvement of Akt and Erk. J. Ethnopharmacol. 2010, 128, 71–78. [Google Scholar] [CrossRef]
- Thyagarajan, A.; Jedinak, A.; Nguyen, H.; Terry, C.; Baldridge, L.A.; Jiang, J.; Sliva, D. Triterpenes from Ganoderma lucidum induce autophagy in colon cancer through the inhibition of p38 mitogen-activated kinase (p38 MAPK). Nutr. Cancer 2010, 62, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Radwan, F.F.Y.; Doonan, B.P.; God, J.M.; Zhang, L.; Bell, P.D.; Haque, A. A possible cross-talk between autophagy and apoptosis in generating an immune response in melanoma. Apoptosis 2012, 17, 1066–1078. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Li, H.; Zhou, H.; Zhang, S.; Liu, Z.; Zhou, Q.; Sun, F. Recombinant Lz-8 from Ganoderma lucidum induces endoplasmic reticulum stress-mediated autophagic cell death in SGC-7901 human gastric cancer cells. Oncol. Rep. 2012, 27, 1079–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, M.; Reis, F.S.; Sousa, D.; Tavares, C.; Lima, R.T.; Ferreira, I.C.F.R.; dos Santos, T.; Vasconcelos, M.H. A methanolic extract of Ganoderma lucidum fruiting body inhibits the growth of a gastric cancer cell line and affects cellular autophagy and cell cycle. Food Funct. 2014, 5, 1389–1394. [Google Scholar] [CrossRef]
- Reis, F.S.; Lima, R.T.; Morales, P.; Ferreira, I.C.F.R.; Vasconcelos, M.H. Methanolic extract of Ganoderma lucidum induces autophagy of AGS human gastric tumor cells. Molecules 2015, 20, 17872–17882. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhou, Z.; Ren, X.; Wang, Y.; Yang, R.; Luo, J.; Strappe, P. Effect of Ganoderma lucidum spores intervention on glucose and lipid metabolism gene expression profiles in type 2 diabetic rats. Lipids Health Dis. 2015, 14, 49. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Chan, E.; Zhou, F. Immunomodulating activities of Ganoderma, a mushroom with medicinal properties. Food Rev. Int. 2004, 20, 123–161. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Zuo, J.; Gong, X.; Yi, F.; Zhu, W.; Li, L. Advances in research on the active constituents and physiological effects of Ganoderma lucidum. Biomed. Dermatol. 2019, 3, 6. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Du, J.-L.; Cao, L.-P.; Jia, R.; Shen, Y.-J.; Zhao, C.-Y.; Xu, P.; Yin, G.-J. Anti-inflammatory and hepatoprotective effects of Ganoderma lucidum polysaccharides on carbon tetrachloride-induced hepatocyte damage in common carp (Cyprinus carpio L.). Int. Immunopharmacol. 2015, 25, 112–120. [Google Scholar] [CrossRef]
- Wu, J.-G.; Kan, Y.-J.; Wu, Y.-B.; Yi, J.; Chen, T.-Q.; Wu, J.-Z. Hepatoprotective effect of ganoderma triterpenoids against oxidative damage induced by tert-butyl hydroperoxide in human hepatic HepG2 cells. Pharm. Biol. 2016, 54, 919–929. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Fan, J.; Liu, Y.; Guo, W.; Cao, H.; Xiao, J.; Wang, Y.; Liu, B. Hepatoprotective activity of Ganoderma lucidum triterpenoids in alcohol-induced liver injury in mice, an iTRAQ-based proteomic analysis. Food Chem. 2019, 271, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Song, C.-H.; Yang, B.-K.; Ra, K.-S.; Shon, D.-H.; Park, E.-J.; Go, G.-I.; Kim, Y.-H. Hepatoprotective effect of extracellular polymer produced by submerged culture of Ganoderma lucidum WK-003. J. Microbiol. Biotechnol. 1998, 8, 277–279. [Google Scholar]
- Lee, C.-H.; Choi, E.Y. Macrophages and inflammation. J. Rheum. Dis. 2018, 25, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Wei, B.; Zhang, R.; Zhai, J.; Zhu, J.; Yang, F.; Yue, D.; Liu, X.; Lu, C.; Sun, X. Suppression of Th17 cell response in the alleviation of dextran sulfate sodium-induced colitis by Ganoderma lucidum polysaccharides. J. Immunol. Res. 2018, 2018, 2906494. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- De Sousa, V.M.C.; Dos Santos, E.F.; Sgarbieri, V.C. The importance of prebiotics in functional foods and clinical practice. Food Nutr. Sci. 2011, 2, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Bhakta, M.; Kumar, P. Mushroom polysaccharides as a potential prebiotics. Int. J. Health Sci. Res. 2013, 3, 77–84. [Google Scholar]
- Cani, P.D.; Delzenne, N.M. The role of the gut microbiota in energy metabolism and metabolic disease. Curr. Pharm. Des. 2009, 15, 1546–1558. [Google Scholar] [CrossRef] [Green Version]
- Jayachandran, M.; Xiao, J.; Xu, B. A critical review on health promoting benefits of edible mushrooms through gut microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef] [Green Version]
- Meneses, M.E.; Martínez-Carrera, D.; Torres, N.; Sánchez-Tapia, M.; Aguilar-López, M.; Morales, P.; Sobal, M.; Bernabé, T.; Escudero, H.; Granados-Portillo, O.; et al. Hypocholesterolemic properties and prebiotic effects of Mexican Ganoderma lucidum in C57BL/6 Mice. PLoS ONE 2016, 11, e0159631. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.-C.; Guoa, W.-L.; Li, L.; Yu, X.-D.; Liu, B. Polysaccharide peptides from Ganoderma lucidum ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet-fed rats. J. Funct. Foods 2019, 57, 48–58. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, H.; Wang, P.G.; Chen, M. Evaluation of the efficacy and safety of Ganoderma lucidum mycelium-fermented liquid on gut microbiota and its impact on cardiovascular risk factors in human. RSC Adv. 2017, 7, 45093. [Google Scholar] [CrossRef] [Green Version]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Cardiovascular Diseases (CVDs). 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 29 June 2021).
- Chang, C.-J.; Lin, C.-S.; Lu, C.-C.; Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Tseng, S.-F.; Wu, T.-R.; Chen, Y.-Y.M.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.K.; Jeong, S.C.; Song, C.H. Hypolipidemic effect of exo- and endo-biopolymers produced from submerged mycelial culture of Ganoderma lucidum in rats. J. Microbiol. Biotechnol. 2002, 12, 872–877. [Google Scholar]
- Wanmuang, H.; Leopaircut, J.; Kositchaiwat, C. Fatal fulminant hepatitis associated with Ganoderma lucidum (Lingzhi) mushroom powder. J. Med. Assoc. Thai 2007, 90, 179–181. [Google Scholar]
- Ulbricht, C.; Isaac, R.; Milkin, T.; Poole, E.P.; Rusie, E.; Serrano, J.M.G.; Weissner, W.; Windsor, R.C.; Woods, J. An evidence-based systematic review of stevia by the Natural Standard Research Collaboration. Cardiovasc. Hematol. Agents Med. Chem. 2010, 8, 113–127. [Google Scholar] [CrossRef]
- Wang, P.-A.; Xiao, H.; Zhong, J.-J. CRISPR-Cas9 assisted functional gene editing in the mushroom Ganoderma lucidum. Appl. Microbiol. Biotechnol. 2020, 104, 1661–1671. [Google Scholar] [CrossRef]
- Wu, D.-T.; Deng, Y.; Chen, L.-X.; Zhao, J.; Bzhelyansky, A.; Li, S.-P. Evaluation on quality consistency of Ganoderma lucidum dietary supplements collected in the United States. Sci. Rep. 2017, 7, 7792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, L.; Liu, H.; Li, J.; Li, T.; Wang, Y. Feature fusion of ICP-AES, UV-Vis and FTMIR for origin traceability of Boletus edulis mushrooms in combination with chemometrics. Sensors 2018, 18, 241. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Qin, J.-Z.; Chen, P.; Chen, X.; Zhang, Y.-Z.; Zhao, S.-J. Quality difference study of six varieties of Ganoderma lucidum with different origins. Front. Pharmacol. 2012, 3, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, Â.; Petrović, J.; Stojković, D.; Barros, L.; Glamočlija, J.; Soković, M.; Martins, A.; Ferreira, I.C.F.R. Polyporus squamosus (Huds.) Fr from different origins: Chemical characterization, screening of the bioactive properties and specific antimicrobial effects against Pseudomonas aeruginosa. LWT-Food Sci. Technol. 2016, 69, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Tešanović, K.; Pejin, B.; Šibul, F.; Matavulj, M.; Rašeta, M.; Janjušević, L.; Karaman, M. A comparative overview of antioxidative properties and phenolic profiles of different fungal origins: Fruiting bodies and submerged cultures of Coprinus comatus and Coprinellus truncorum. J. Food Sci. Technol. 2017, 54, 430–438. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Li, T.; Liu, H.; Li, J.; Wang, Y. Traceability of Boletaceae mushrooms using data fusion of UV–visible and FTIR combined with chemometrics methods. J. Sci. Food Agric. 2017, 98, 2215–2222. [Google Scholar] [CrossRef] [PubMed]



| Commercial Product Name/Producing Country | Uses |
|---|---|
| CV Skinlabs Body Repair Lotion, USA | Wound healing and anti-inflammatory |
| Dr. Andrew Weil for Origins Mega-Mushroom Skin Relief Face Mask, USA | Anti-inflammatory properties |
| Moon Juice Spirit Dust, USA | Immune system |
| Estée Lauder Re-Nutriv Sun Supreme Rescue Serum sun care product, USA | Triple-action repair technology to enhance the skin’s own natural defenses against the visible effects of sun exposure and sun-stressed skin |
| Four Sigma Foods Instant Reishi Herbal Mushroom Tea, UK | Immunity boost |
| Kat Burki Form Control Marine Collagen Gel, UK | Boosting collagen, improving elasticity, and providing hydration |
| Tela Beauty Organics Encore Styling Cream, UK | Providing hair with sun protection and preventing color fading |
| Menard Embellir Refresh Massage, France | Skin antiaging |
| Yves Saint Laurent Temps Majeur Elixir De Nuit, France | Antiaging |
| Pureology NanoWorks Shineluxe, France | Antiaging and antifading |
| Hankook Sansim Firming Cream (Tan Ryuk SANG), Korea | Making skin tight and vitalized |
| La Bella Figura Gentle Enzyme Cleanser, Italy | Antioxidants and vitamin D |
| DXNGanozhi Moisturizing Micro Emulsion, Malaysia | Hydrating and nourishing the skin |
| Guangzhou Bocaly Bio-Tec. Ganoderma Cell-Repairing Antiaging Face Mask, China | Antiwrinkle, firming, lightening, moisturizer, and nourishing, pigmentation corrector; pore cleaning and whitening |
| Nanjing Zhongke Pharmaceuticals Ganoderma Face Cream Set (day/night cream and eye gel set), China | Immunity boost and antifatigue |
| Shenzhen Hai Li Xuan Technology HailiCare Skin Whitening Cream, China | Removing freckles and whitening |
| Menard Embellir Night Cream, Japan | Eliminating toxins and helping repair skin damage associated with overexposure to UV radiation and free radicals |
| MAVEX Rejuvenating Treatment, Hong Kong | Antioxidant action and deep cellular renewal; fight degenerative processes and the negative action of free radicals |
| Constitute | Content | DRIs * (g/day) | Value in 100 g Mushroom/DRIs × 100 | ||
|---|---|---|---|---|---|
| Value | g/100 g Mushroom (Wet-Weight Basis) | g/100 g Mushroom (Dry-Weight Basis) | |||
| Moisture % | 47 | ||||
| Total solids (TS) % | 53 | ||||
| pH value | 5.6 | ||||
| Energy (kcal) | 238.98 ** | Men: 2215 *** | 10.79 | ||
| Women: 2025 | 11.80 | ||||
| Water-soluble proteins % | 19.5 | 36.80 | Men (total proteins) ****: 56 | 34.82 | |
| Women (total proteins): 46 | 42.39 | ||||
| Total lipids % | 3.00 | 5.66 | 44–77 ***** | 3.90–6.82 | |
| Total ash % | 6.3 | ||||
| Reducing sugars % | 4.39 | 8.28 | |||
| Nonreducing sugars % | 1.02 | 1.92 | |||
| Total sugars % | 5.41 | 10.21 | 130 | 4.16 | |
| Crude fibers % | 3.5 | Men: 38 | 9.21 | ||
| Women: 25 | 14.00 | ||||
| Polyphenols “as gallic acid” | 0.04 | 0.08 | 1 ****** | 7.5 | |
| Mineral | Mineral content (mg/100 g mushroom) | DRIs (mg/day) | Value in 100 g mushroom/DRIs × 100 | ||
| Major minerals | |||||
| Potassium | 432 | 4700 | 9.19 | ||
| Phosphorus | 225 | 700 | 32.14 | ||
| Sulfur | 129 | 200–1500 | 8.60–64.50 | ||
| Magnesium | 7.95 | Men: 400 | 2.00 | ||
| Women: 310 | 2.60 | ||||
| Sodium | 2.82 | 1500 | 0.20 | ||
| Calcium | 1.88 | 1000 | 0.20 | ||
| Trace minerals | |||||
| Copper | 26 | 0.9 | 2889 | ||
| Manganese | 22 | Men: 2.3 | 956.52 | ||
| Women: 1.8 | 1222.22 | ||||
| Iron | 2.22 | Men: 8 | 27.75 | ||
| Women: 18 | 12.33 | ||||
| Zinc | 0.7 | Men: 11 | 6.40 | ||
| Women: 8 | 8.75 | ||||
| Vitamin | Vitamin content (mg/100 g mushroom) | DRIs (mg/day) | Value in 100 g mushroom/DRIs × 100 | ||
| Thiamine (B1) | 3.49 | Men: 1.2 | 290.83 | ||
| Women: 1.1 | 317.27 | ||||
| Riboflavin (B2) | 17.10 | Men: 1.3 | 1315.38 | ||
| Women: 1.1 | 1554.54 | ||||
| Niacin (B3) | 61.9 | Men: 16 | 386.87 | ||
| Women: 14 | 442.14 | ||||
| Pyridoxine (B6) | 0.71 | Men: 1.4 | 50.71 | ||
| Women: 1.2 | 59.16 | ||||
| Ascorbic acid | 32.2 | Men: 90 | 35.77 | ||
| Women: 75 | 42.93 | ||||
| Bioactive Compounds | Biological Effects | References |
|---|---|---|
| Triterpenoids | ||
| Ganoderic acids, lucidumol, lucialdehyde, lucidenic acids, ganodermic, ganolucidic acids, ganoderals, ganoderiols | Anticancer | Wachtel-Galor et al. [6], El Mansy [75] |
| Triterpenoids | Antidiabetic | Ahmad [68], Ma et al. [78] |
| Ganoderic acids T-Q and lucideinic acids A, D2, E2, and P | Anti-inflammatory | El Mansy [75] |
| Triterpenes | Antioxidant | El Mansy [75] |
| Ganoderic acids, ganodermin, ganoderic acid A, ganodermadiol, ganodermanondiol, lucidumol B, ganodermanontriol, ganoderic acid B, ganolucidic acid B | Antimicrobial | Cör et al. [70], Sudheer et al. [73] |
| Triterpenoids, ganoderic acid, ganoderiol F, ganodermanontriol | Antiviral | Bishop et al. [13], Zhang et al. [79], Zhu et al. [80] |
| Polysaccharides | ||
| 1→3, 1→4, and 1→6-linked β and α-D (or L)-glucans, GLP-2B | Anticancer | Wachtel-Galor et al. [6], Ferreira et al. [81] |
| Polysaccharides | Antidiabetic | Ahmad [68], Ma et al. [78] |
| Polysaccharides | Antioxidant | El Mansy [75] |
| Polysaccharides | Antimicrobial | Cör et al. [70] |
| Polysaccharides (ganopoly) | Cardiovascular problems | Chan et al. [82] |
| Proteins, Glycoproteins, and Peptidoglycans | ||
| Glycopeptides and peptidoglycans | Anticancer | Wachtel-Galor et al. [6], Sudheer et al. [73], Ferreira et al. [81], |
| Protein Ling Zhi-8 (LZ-8), lectin, ribosome-inactivating proteins, antimicrobial proteins, glycopeptides/glycoproteins, peptidoglycans/proteoglycans, ganodermin A, ribonucleases, proteinases, metalloproteases, laccases | Immunomodulatory, anticancer, and antitumor | Wachtel-Galor et al. [6], El Mansy [75] |
| Proteoglycans, proteins (LZ-8) | Antidiabetic | Ahmad [68], Ma et al. [78] |
| Polysaccharide–peptide complex | Antioxidant | Mehta [83] |
| Phenolic compounds | ||
| Phenolic components, phenolic extracts | Antioxidant | Mehta [83] |
| Saponins | Anticancer and antioxidant | Lee et al. [84] |
| Sterols; e.g., ergosterol | Provitamin D2 | Wachtel-Galor et al. [6] |
| Long-chain fatty acids | Antitumor | Gao et al. [85] |
| Parts/Products/Compounds | Tested Microorganism | References |
|---|---|---|
| Antibacterial activity | ||
| Fruiting bodies | Helicobacter pylori ATCC 43504, Staphylococcus aureus ATCC 26003 | Liu et al. [131], Shang et al. [132] |
| Mycelia extract | Bacillus cereus (clinical isolate), Micrococcus flavus ATCC 10240, S. aureus ATCC 6538, Listeria monocytogenes NCTC 7973, Escherichia coli ATCC 35218, Enterobacter cloacae (human isolate), Pseudomonas aeruginosa ATCC 27853, Salmonella typhimurium ATCC 13311 | Ćilerdžić et al. [133] |
| Fruiting bodies | S. aureus (MTCC 96), B. cereus (MTCC 430), P. aeruginosa (MTCC 424) | Karwa and Rai [134] |
| Fruiting bodies | S. aureus (ATCC 6538), Bacillus subtilis (ATCC 6633) | Ćilerdžić et al. [135] |
| Ergosta-5,7,22-trien-3β-yl acetate, ergosta-7,22-dien-3β-yl acetate, ergosta-7,22-dien-3-one, ergosta-7,22-dien-3β-ol, ergosta-5,7,22-trien-3β-ol, ganodermadiol | S. aureus (ATCC 6538), B. subtilis (ATCC 6633) | Ćilerdžić et al. [135] |
| Carpophores | Bacillus anthracis ATCC 6603, B. cereus ATCC 27348, B. subtilis ATCC 6633, Micrococcus luteus ATCC 9341, S. aureus ATCC 25923, E. coil ATCC 259 22, Klebsiella oxytoca ATCC 8724, Klebsiella pneumonia ATCC 10031, Proteus vulgaris ATCC 27853, S. typhi ATCC 6229 | Yoon et al. [136] |
| Basidiocarps | B. cereus (clinical isolate), M. flavus ATCC 10240, S. aureus ATCC 6538, L. monocytogenes NCTC 7973, E. coli ATCC 35218, E. cloacae (human isolate), P. aeruginosa ATCC 27853, S. typhimurium ATCC 13311 | Vazirian et al. [137] |
| 12b-acetoxy-3β,7 β -dihydroxy- 11,15,23-trioxolanost-8-en-26-oic acid butyl ester | S. aureus (ATCC 6538), B. subtilis (ATCC 6633) | Yang et al. [138] |
| Mycelia (Protein extract) | Staphylococcus epidermidis, B. subtilis, B. cereus, E. coli, P. aeruginosa | Sa-Ard et al. [139] |
| Fruiting bodies (Protein extract) | S. epidermidis, S. aureus, B. subtilis, B. cereus, E. coli, P. aeruginosa | Sa-Ard et al. [139] |
| NG * | S. aureus (ATCC 6538), B. cereus (clinical isolate), L. monocytogenes (NCTC 7973), M. flavus (ATCC 10240), P. aeruginosa (ATCC 27853), E. coli (ATCC 35210), S. typhimurium (ATCC 13311), E. cloacae (human isolate) | Heleno et al. [140] |
| Antifungal activity | ||
| Fruiting bodies | Acremonium strictum BEOFB10m, Aspergillus glaucus BEOFB21m, Aspergillus flavus BEOFB22m, Aspergillus fumigatus BEOFB23m, Aspergillus nidulans BEOFB24m, Aspergillus niger BEOFB25m, Aspergillus terreus BEOFB26m, Trichoderma viride BEOFB61m | Vazirian et al. [137] |
| Fruiting bodies | A. fumigatus (human isolate), Aspergillus versicolor (ATCC 11730), Aspergillus ochraceus (ATCC 12066), A. niger (ATCC 6275), T. viride (IAMz5061), Penicillium funiculosum (ATCC 36839), Penicillium ochrochloron (ATCC 9112), Penicillium verrucosum var. cyclopium (food isolate) | Heleno et al. [140] |
| Rare Earth-Carboxymethylated Ganoderma applanatum Polysaccharide | Valsa mali, Fusarium oxysporum, Gaeumannomyces graminis, Colletotrichum gloeosporioides, Alternaria brassicae | Sun et al. [141] |
| Ganodermin | Botrytis cinerea, F. oxysporum, Physalo sporapiricola | Wang and Ng [113] |
| Mycelia | Acremonium strictum, A. glaucus, A. flavus, A. fumigatus, A. nidulans, A. niger, A. terreus, T. viride | Ćilerdžić et al. [133] |
| Antiviral activity | ||
| Ganoderiol F & Ganodermanontriol | HIV 1(HIV-1 protease) | El-Mekkawy et al. [142] |
| Carpophores | Herpes simplex virus types 1 (HSV-1) and 2 (HSV-2), influenza A virus (Flu A), and vesicular stomatitis virus (VSV) Indiana and New Jersey strains | El-Mekkawy et al. [142] |
| Acidic protein-bound polysaccharide | HSV-1 and HSV-2 | Eo et al. [143] |
| Fruiting bodies | Oral human papillomavirus (HPV) | Donatini [144] |
| NG | Newcastle disease virus (anti-neuraminidase) | Zhu et al. [80], Shamaki et al. [145] |
| Fruiting bodies | Epstein-Barr Virus | Iwatsuki et al. [146] |
| Mycelia | Hepatitis B virus | Li et al. [147] |
| Mycelia (Ganoderic acid) | Hepatitis B | Li and Wang [148] |
| Lanosta-7,9(11),24-trien-3-one,15;26-dihydroxy (GLTA), Ganoderic acid Y | Enterovirus 71 | Zhang et al. [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Sheikha, A.F. Nutritional Profile and Health Benefits of Ganoderma lucidum “Lingzhi, Reishi, or Mannentake” as Functional Foods: Current Scenario and Future Perspectives. Foods 2022, 11, 1030. https://doi.org/10.3390/foods11071030
El Sheikha AF. Nutritional Profile and Health Benefits of Ganoderma lucidum “Lingzhi, Reishi, or Mannentake” as Functional Foods: Current Scenario and Future Perspectives. Foods. 2022; 11(7):1030. https://doi.org/10.3390/foods11071030
Chicago/Turabian StyleEl Sheikha, Aly Farag. 2022. "Nutritional Profile and Health Benefits of Ganoderma lucidum “Lingzhi, Reishi, or Mannentake” as Functional Foods: Current Scenario and Future Perspectives" Foods 11, no. 7: 1030. https://doi.org/10.3390/foods11071030
APA StyleEl Sheikha, A. F. (2022). Nutritional Profile and Health Benefits of Ganoderma lucidum “Lingzhi, Reishi, or Mannentake” as Functional Foods: Current Scenario and Future Perspectives. Foods, 11(7), 1030. https://doi.org/10.3390/foods11071030