Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry
Abstract
:1. Introduction
2. Chemical Composition of Fruit Peel Essential Oils
3. Antimicrobial Properties of Fruit Peel Essential Oils
3.1. Citrus Essential Oils
3.2. Orange Essential Oils
3.3. Grapefruit Essential Oils
3.4. Essential Oils from Other Fruit Peels
| Source of Peel EO | Target Organism | Method Used | Solvent Used | Test Concentration | Remarks | References |
|---|---|---|---|---|---|---|
| Tamarillo | E. coli, P. aeruginosa, S. pyogenes, S. aureus | Disk diffusion | MilliQ, n-hexane, ethanol, methanol | 115 μL of 100 mg/mL on 13 mm disk | E. coli was most sensitive to aqueous extract from the peel (inhibition zone of 24 mm), P. aeruginosa was most sensitive to methanol extract. | [60] |
| Grapefruit | B. subtilis, E. coli, S. aureus, S. enterica serovar Typhimurium, P. aeruginosa | Disk diffusion, MIC determination | - | 20 μL of 100, 50, 25, 12.5, 6.25, 3.125, 1.56, 0.78, 0.39 and 0.195 mg/mL of EO placed on each disk | B. subtilis represented a maximum inhibitory zone of 35.59 mm and MIC value of 0.78 μL/mL. P. aeruginosa was least sensitive representing an inhibition zone of 8.57 mm and MIC value of 25.0 μL/mL | [23] |
| Sweet orange, Lemon, Banana | P. aeruginosa, K. pneumoniae, Serratia marcescens, E. coli, P. vulgaris, S. enterica serovar Typhi, S. aureus, E. faecalis, L. monocytogenes, Aeromonas hydrophila, Streptococcus pyogenes, Lactobacillus casei | Agar well diffusion, MIC determination | Distilled water, Methanol, Ethanol, Ethyl acetate | 5 mg/mL | K. pneumoniae was most susceptible to lemon peel extract (inhibition zone and MIC, 35 mm, and 130 μg/mL, respectively). | [13] |
| Kumquat | E. coli, S. enterica serovar Typhimurium, S. aureus, P. aeruginosa | Disk diffusion and MIC determination by broth microdilution method | Methanol 80%, Ethanol 70%, Acetone, Ethyl acetate, n- Hexane, Chloroform | From 10 mg/mL, 25 μL of extract was placed on each disk. | For all extracts, E. coli was most resistant (inhibition zone 11.3 mm and MIC of 679 μg/mL) while S. aureus was the most susceptible (inhibition zone 16.7 mm and MIC of 496 μg/mL) strain. | [64] |
| Sweet orange | enterotoxigenic E. coli, Lactobacillus sp | Disk diffusion and MIC determination | EO solutions prepared at 90% (v/v), using acetone | 7 μL of EO solution placed on each disk | EO showed higher antimicrobial activity against ETEC, no activity shown against beneficial Lactobacillus sp. | [22] |
| Lemon | B. subtilis, E. coli, S. enterica serovar Typhimurium, S. aureus | Disk diffusion method | - | 0.1 mL of EO solution placed on each disk | Ripened lemon peel EO was more effective against all four strains than the unripe lemon peel EO. | [71] |
| Sweet orange | Bacillus sp., E. coli, S. aureus | Agar well diffusion method | Hot ethanol, Cold ethanol, Hot aqueous, Cold aqueous | 50 and 100 μL of each extract placed on disk | Hot ethanolic extract (100 μL) most effective, showing inhibitory zone of 16, 15 and 16 mm against Bacillus sp., E. coli and S. aureus, respectively. | [56] |
| Feijoa | E. coli, S. aureus | Agar well diffusion method | Water and Methanol extracts | 100 μL of each extract placed on disk | Methanol extract was more effective (Inhibition zone for E. coli and S. aureus was 14.7 and 26.5 mm, respectively). | [65] |
| Sweet orange | S. aureus, B. subtilis, E. coli | MIC determination by tube dilution method | Light phase and cold-pressed EO | - | The MIC of light phase EO for S. aureus, B. subtilis and E. coli was 3.13, 1.56 and 0.78 μL/mL, respectively. | [37] |
| Sweet orange | S. aureus, L. monocytogenes, P. aeruginosa | MIC determination by agar dilution method | EO and hexane extracts | 100 to 2.5 mg/mL | EO was effective against L. monocytogenes (MIC value of 15 mg/mL) but less active against S. aureus and P. aeruginosa. Hexane extract at 10 mg/mL concentration was most effective. | [24] |
| Sweet orange, Lime, Mandarin, Grapefruit | B. subtilis, S. aureus, E. faecalis, E. coli, P. aeruginosa, N. gonorrhoeae | Disk diffusion and MIC determination by agar dilution method | - | 10 μL of EO solution placed on each disk | Lime peel was most effective. MIC of 14 and 11 μL/mL was recorded for S. aureus and E. coli, respectively. | [51] |
| Sour orange, Sweet orange, Grapefruit, Lemon | S. aureus, E. coli, E. faecalis, B. cereus | Agar well diffusion method | Aqueous extract | 50 μL of 100 mg/mL of extract was dispensed in each well | The inhibition zones for S. aureus, E. faecalis, B. cereus and E. coli ranged from 10 to 18 mm, 9 to 17 mm, 11 to 18 mm, and 14 to 21 mm, respectively. | [55] |
| Bitter orange | L. monocytogenes, S. aureus, E. coli DH5α, Citrobacter freundii | Disk diffusion | Hexane extract | - | S. aureus was moderately sensitive to bitter orange extract (inhibition zone of 10mm). The extract did not inhibit Gram-negative organisms. | [52] |
| Grapefruit, Pummelo | E. coli, P. aeruginosa, S. enterica subsp., S. aureus, E. faecalis | Disk diffusion method | Cold-pressed and water-distilled extracted EO | 100 μL of 10 and 20 mg/mL of EO was suspended in each well | 20mg/mL of pummelo peel EO presented antimicrobial activity against Gram negative Salmonella enterica subsp. followed by E. faecalis > E. coli > S. aureus > P. aeruginosa. | [39] |
| Sweet orange, Sweet lemon, Lemon | S. enterica serovar Typhimurium, E. coli | Disk diffusion method | Hexane extract | - | The inhibitory zone for S. enterica serovar Typhimurium and E. coli ranged from 4 mm to 10 mm. | [53] |
| Pomegranate | S. aureus, E. aerogenes, S. enterica serovar Typhimurium and K. pneumoniae | Agar well diffusion method | Methanol, Ethanol (100, 70, 50, 30%), Water | 10 μL of extract: water (1:6) was dispensed in each well | S. aureus was the most sensitive strain, followed by E. aerogenes, S. enterica serovar Typhimurium, K. pneumoniae. The inhibition zone for S. aureus ranged from 24.5 to 20.3 mm. | [69] |
| Banana | S. aureus, S. pyogenes, Enterobacter aerogenes, K. pneumoniae, E. coli, Moraxella catarrhalis | Agar well diffusion | Aqueous extract | - | S. aureus showed an inhibition zone of 30 mm, but E. coli was resistant to the extract. | [67] |
| C. deliciosa | S. aureus, Micrococcus luteus, E. coli, P. vulgaris | Agar diffusion method | - | 15 μL of EO was dispensed on the agar surface | The inhibition zone for all tested organisms ranged from 8 mm to 30 mm. | [63] |
| Lemon, Sweet lemon | S. aureus, S. epidermidis, S. agalactiae, E. faecalis, Streptococcus pneumoniae, S. pyogenes, E. coli, E. aerogenes, K. pneumoniae, Proteus sp., S. enterica serovar Typhimurium, Acinetobacter sp., Moraxella catarrhalis, P. aeruginosa | Agar well diffusion method | Aqueous extract | 20 μL of extract was dispensed in each well | The effect of lemon and sweet lemon peel on microbial isolates was not significantly different. The inhibition zone for lemon and sweet lemon ranged from 20–30 mm and 10–35 mm, respectively. | [62] |
| Grapefruit | B. cereus, S. faecalis, E. coli, K. pneumoniae, Pseudo- coccus sp., S. enterica serovar Typhimurium, Shigella flexneri, S. aureus | Agar well diffusion method | Methanol, Ethanol | 100 μL of 8, 40 and 80 μg/mL concentrations of EO solutions were dispensed in each well | Methanol extract was more effective against all tested strains. B. cereus was the most sensitive bacteria (inhibition zone from 30.33 to 32.67 mm), while E. faecalis was the most resistant one (inhibition zone from 6.0 to 12.0 mm) | [72] |
| Pomegranate | B. subtilis, S. aureus E. coli, K. pneumoniae | Microdilution method | Methanolic and aqueous extracts | 0.097–12.5 mg/mL | The MIC value for the tested strains ranged from 0.2 to 0.78 mg/mL. | [73] |
| Pummelo | S. aureus, B. subtilis, E. coli | Disk diffusion and MIC determination by broth microdilution method | - | 10 μL of 50% (v/v) EO was placed on each disk. MIC concentration ranged from 1.17 to 750 μL/mL (v/v). | The inhibition zones for B. subtilis, S. aureus and E. coli were 17.08, 11.25 and 8.27 mm, respectively. The MIC values for B. subtilis, S. aureus and E. coli were 9.38, 9.38 and 37.50 μL/mL, respectively. | [40] |
| Pomegranate | 16 strains of Salmonella sp. | Disk diffusion and MIC determination | Ethanol | 20 μL of 100, 200 and 500 μg/mL concentration of EO solution was placed on each disk. MIC concentration ranged from 3.9 to 2000 μg/mL | The inhibition zone and the MIC values for Salmonella sp. ranged from 13.3 to 18.8 mm and 62.5 to 1000 μg/mL, respectively. | [74] |
| Lemon | P. aeruginosa, S. enterica serovar Typhimurium, and Micrococcus aureus | Agar well diffusion method and MIC determination | Methanol, Ethanol, Acetone | Dilutions from crude extract were prepared as follows: 1:20, 1:40, 1:60, 1:80, 1:100 | All concentrations of lemon peel extracts effectively inhibited all the three strains tested. | [75] |
| Mandarin, Tangerine, Sweet orange, Lime, Grapefruit | E. coli, S. enterica serovar Typhi, K. pneumoniae, E. cloacae, P. fluorescence, Proteus myxofaciens, S. epidermidis, Streptococcus sp. | Disk diffusion method | - | From 500 μg/mL of stock solution 5 and 10 μL of EO was placed on each disk. | S. enterica serovar Typhi and P. myxofaciens were susceptible to all citrus EO tested. | [15] |
| Grapefruit | S. aureus, E. faecalis, S. epidermidis, E. coli, S. enterica serovar Typhimurium, S. marcescens and P. vulgaris | Disk diffusion method | - | 20 μL of extract was dispensed in each well | S. enterica serovar Typhimurium was the most resistant (15 mm) strain followed by E. faecalis (16 mm), S. epidermis (17 mm), S. marcescens (19 mm), P. vulgaris (21 mm) and S. aureus (53 mm). | [38] |
| Pomegranate | E. coli, Pseudomonas fluorescens, S. enterica serovar Typhimurium, S. aureus, B. cereus | MIC determination by tube dilution method | Water | Final concentration of 0.01, 0.05, 0.1% was prepared in saline | S. aureus and B. cereus got inhibited at a concentration of 0.01%, P. fluorescens at 0.1%, E. coli and S. enterica serovar Typhimurium were not inhibited. | [76] |
| Pomegranate | L. monocytogenes, S. aureus B. subtilis, E. coli, P. aeruginosa, K. pneumoniae, Yersinia enterocolitica | Agar well diffusion and MIC determination by agar dilution method | Methanolic (80%) and water extracts | 800 μg/100 μL of extract was suspended in each well. MIC concentration ranged from 0 to 4 mg/mL | The inhibition zone for methanolic extract ranged from 13–20 mm. MIC determination showed that Y. enterocolitica was the most sensitive strain representing MIC of 0.25 mg/mL. | [77] |
| Sour lime | B. subtilis, B. cereus, S. aureus, E. coli, E. aerogenes S. enterica serovar Typhimurium | Disk diffusion method | - | - | B. subtilis, B. cereus, S. aureus, S. enterica serovar Typhimurium, E. coli and E. aerogenes showed inhibition zones of 22, 19.8, 18, 17, 16 and 10.5 mm, respectively. | [68] |
| Sweet orange | S. aureus, B. subtilis, E. coli | Disk diffusion and MIC determination by broth microdilution method | - | 10 μL of 50% (v/v) EO was placed on each disk. MIC concentration ranged from 1.17 to 750 μL/mL (v/v). | The inhibition zones for S. aureus, B. subtilis and E. coli were 23.37, 18.89 and 17.21 mm, respectively. The MIC values for S. aureus, B. subtilis and E. coli were 4.66, 9.33 and 18.75 μL/mL, respectively. | [54] |
| Lemon, Grapefruit, Bitter orange, Sweet orange, Mandarin, Bergamot | S. aureus, B. cereus, Mycobacterium smegmatis, L. monocytogenes, M. luteus, E. coli, K. pneumoniae, P. aeruginosa, P. vulgaris | Disk diffusion method | - | 20 μL of EO solution was placed on each disk | Lemon peel EO exhibited better antimicrobial activity towards all bacteria with inhibition zone ranging from 10 to 16 mm. | [57] |
| Bergamot | E. coli, P. putida, S. enterica, L. innocua, B. subtilis, S. aureus, Lactococcus lactis | MIC determined using Bioscreen C | Ethanol (70, 100%) | 200–1000 μg/mL | The MIC values for E. coli, S. enterica, P. putida were 200, 400, 500 μg/mL, respectively. Gram-positive bacteria showed no effect. | [61] |
4. Effect of Chemical Components of Essential Oils on Food Spoilage and Pathogenic Microbes
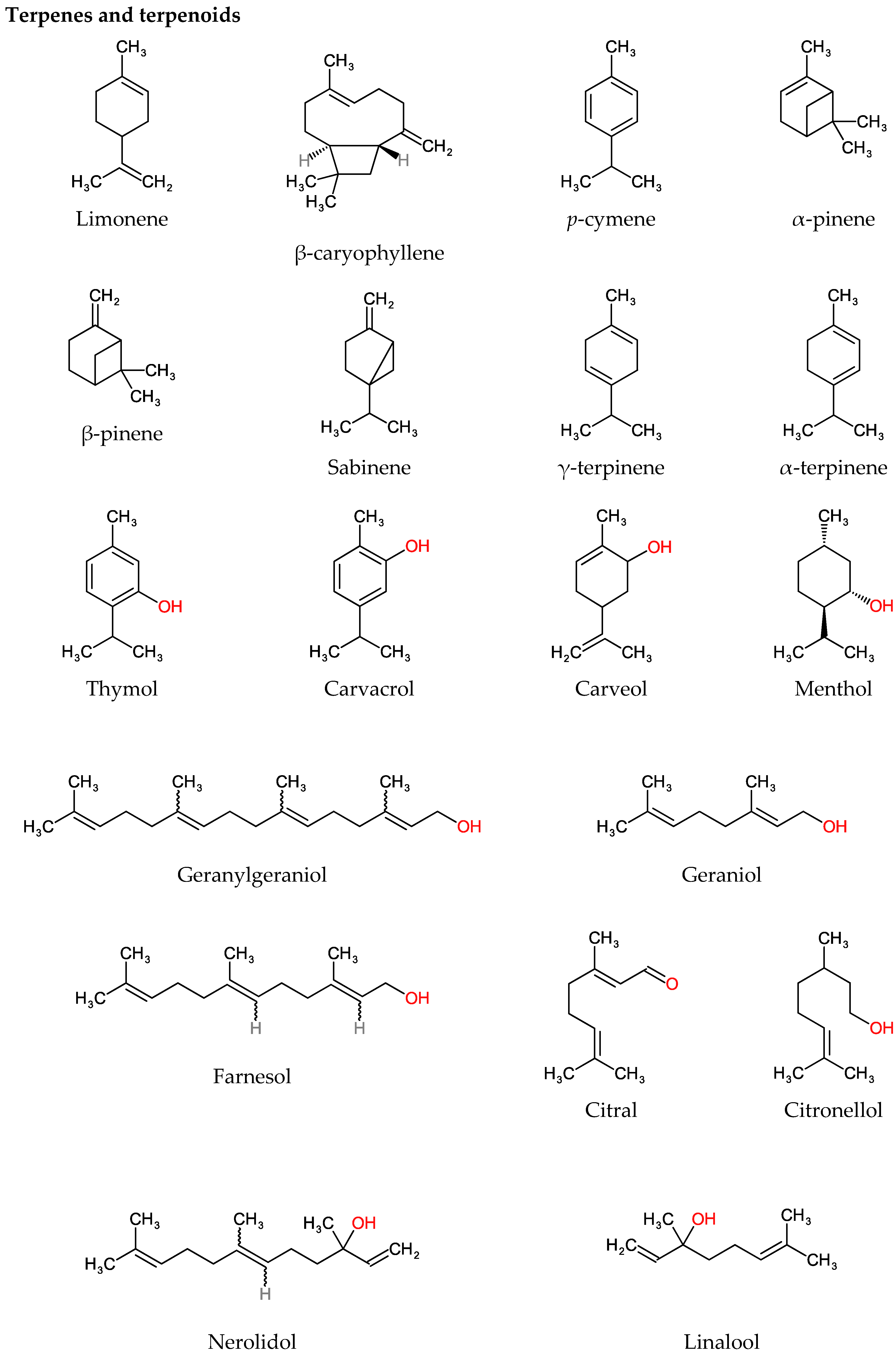
4.1. Terpenes and Terpenoids
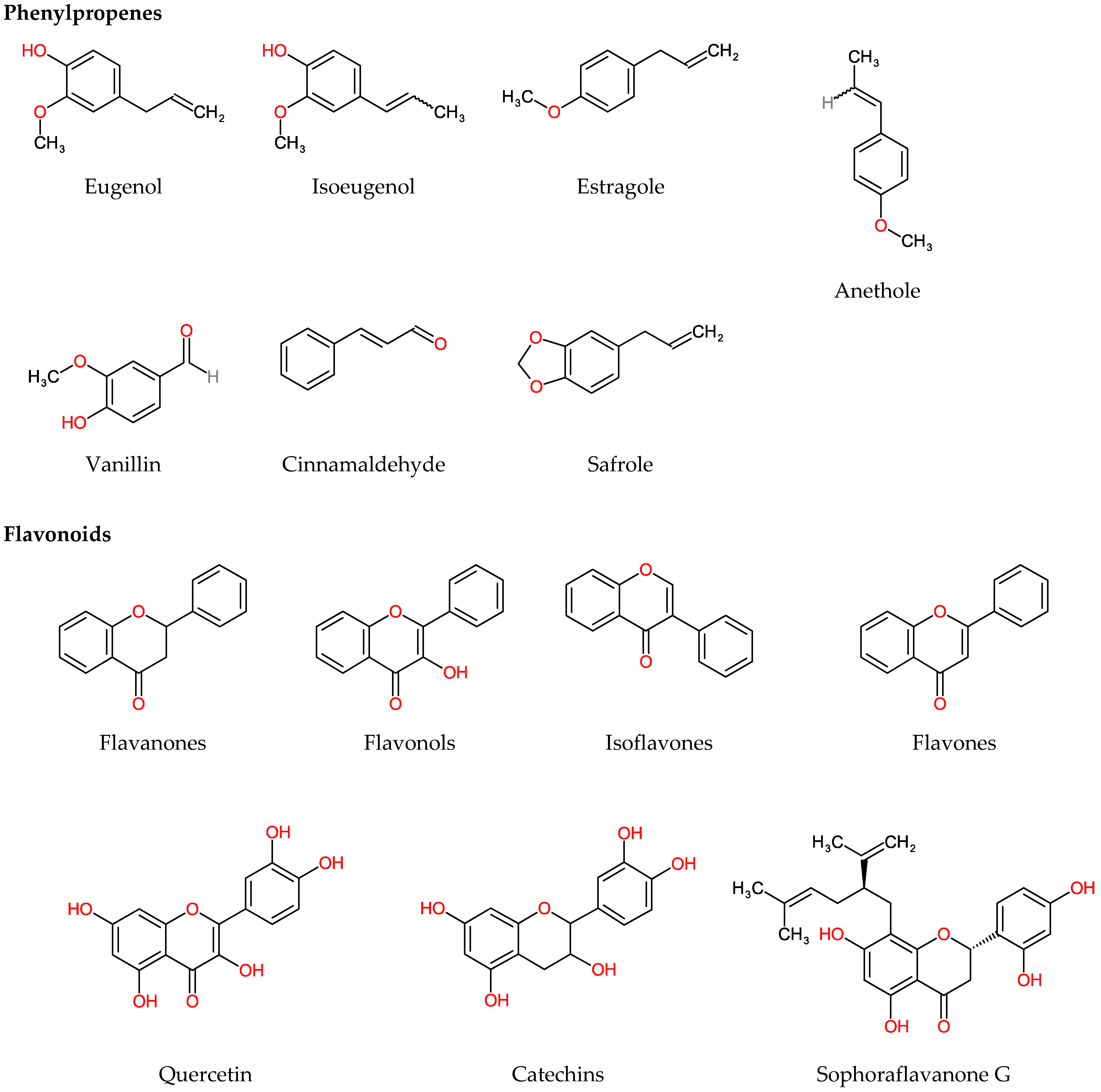
4.2. Polyphenols
4.2.1. Phenylpropenes
4.2.2. Flavonoids
5. Application of Essential Oils in Food Products
Preservation
| Essential Oil | Pathogen | Food | Method Used | Concentration Applied | References |
|---|---|---|---|---|---|
| Thyme (Thymus vulgaris) | E. coli O157:H7 | Cherry tomatoes | Dipping | 0.0625, 0.125 mg/mL | [148] |
| Clove (Syzygium aromaticum) | E. coli O157:H7, S. aureus | Beef jerkies | Treated with EO and dried for 2 hrs | 0.50%, 1.00%, 1.50% | [154] |
| Ajwain (Trachyspermum ammi) | L. monocytogenes | Turkey fillets | Coating | 8, 4, 2 mg/mL | [161] |
| May chang (Litsea cubeba) | E. coli O157:H7 | Bitter gourd, cucumber, carrot, and spinach juice | Inoculation | 0.5, 0.25 mg/mL | [152] |
| Felon herb (Artemisia persica Boiss) | L. monocytogenes, E. coli O157:H7 | Probiotic doogh | Addition of EO and mixing | 75 ppm, 150 ppm | [164] |
| Rosemary (Rosmarinus officinalis), Lavender (Lavandula), Mint (Mentha piperita) | Penicillium crustosum | Bread | Exposing bread to a disk loaded with EO | 125, 250, 500 µL/L | [165] |
| Thyme (Thy), Cinnamon (CN) (Cinnamomum verum), Clove (CV) | P. fluorescens | Chicken breast | Coated by dipping in EO emulsion for 5 min | Thy- 0.560 g/L, CN- 0.042, 0.170 g/L, CV- 0.078, 0.312 g/L | [159] |
| Ginger (Zingiber officinale), Clove, Thyme | S. aureus, P. aeruginosa, E. coli, E. faecalis, P. fluorescens, C. albicans and Aspergillus parasiticus | Fortified cheese | EO added and stirred | 0.01% | [166] |
| Tea tree (Melaleuca alternifolia) | TVC, Psychrophilic, Coliform, Salmonella, Yeast, and mould count | Beef steaks | Addition of EO and mixing | 0.1%, 0.5% | [155] |
| Cranberry extract (Vaccinium macrocarpon) | Listeria sp. | Chicken breast | Dipped in extract solution | 4, 8 mg/mL | [170] |
| Thyme | Thermotolerant coliforms and Escherichia coli | Hamburger | Addition of EO and mixing | 0.1 g/100 g of thyme EO 1 g/100 g of encapsulated thyme EO | [169] |
| Cinnamon leaf EO nanoemulsion | L. monocytogenes, E. coli O157:H7 | Kale leaves | Washing | 50 ppm | [150] |
| Thymol, Eugenol, Carvacrol | E. coli O157:H7 | Lettuce leaves | Rinsing | 0.63 mg/mL | [151] |
| Chrysanthemum (Chrysanthemum indicum) | L. monocytogenes | Beef | Packed into membrane (Chitosan nanofiber loaded with EO) | 1.5% | [156] |
| Pistachio (Pistacia vera) | Total viable count (TVC) | Ground beef | EO added to meat and stomached for 1 min | 1.5% (v/w) | [157] |
| Black cumin (Bunium persicum) | E. coli O157:H7 | Rainbow trout fillet | Coated by dipping in nanoemulsion for 15 min | 0.5% | [162] |
| Cranberry extract (Vaccinium macrocarpon) | Aerobic mesophilic count, Brochothrix thermosphacta, P. putida, L. mesenteroides, L. monocytogenes, C. jejuni | Pork meat slurry, hamburger, cooked ham | Mixed in meat | 3.3%, 1.65%, 0.83%, 0.42% | [171] |
| Anise (Pimpinella anisum) | TVC, Psychotropic count, Enterobacteriaceae, Lactic acid bacteria, Pseudomonas sp. | Minced beef | EO added using micropipette and massaged manually for 2min | 0.1%, 0.3%, 0.5% (v/w) | [172] |
| Coriander (Coriandrum sativum) | TVC, sulphite-reducing clostridia, Salmonella sp., E. coli, L. monocytogenes | Pork sausage | Mixed in sausage | 0.000, 0.075, 0.100, 0.125, 0.150 μL/g | [173] |
| Cinnamon | TVC, Enterobacteriaceae | Italian pork sausage | Mixed in sausage | 0.1%, 0.5% (v/m) | [174] |
| Ginger | Psychrophilic, Yeast and mould count | Chicken breast fillet | Coated by dipping in emulsion | 3%, 6% | [163] |
| Cranberry extract | E. coli, Salmonella enterica serovar Enteritidis, L. monocytogenes, S. aureus | Minced pork | 2.5 g/100 g | [175] | |
| Thyme | E. coli, S. enterica serovar Typhimurium, S. aureus, and P. aeruginosa | Minced beef meat | EO added to meat and stomached for 5 min | 0.001%, 0.05%, 3% of EO in 10% DMSO (v/w) | [160] |
| Cinnamon EO (CEO) and grape seed extract (GSE) | TVC, Lactic acid bacteria, Psychotropic count, Yeast, and mould count | Sausage | Mixed in sausage and packed in polyamide bags | CEO (0.02% and 0.04%) and GSE (0.08% and 0.16%) | [176] |
| Apple mint (Mentha suaveolens) | E. coli, S. aureus | Turkey sausage | 2, 5, 10 mg/g | [177] | |
| Isoeugenol | L. monocytogenes, S. aureus, Leuconostoc mesenteroides, P. fluorescens | Carrot juice | Inoculation | 702, 1580 mg/mL | [153] |
| Thyme | Salmonella enterica serovar Enteritidis, S. enterica serovar Typhimurium, S. Montevideo and S. Infantis | Minced pork | Mixed in minced meat and vacuum packed | 0.3%, 0.6%, 0.9% | [178] |
| Thyme | L. monocytogenes | Beef and pork sausage | Mixed and vacuum packed | 100 ppm | [179] |
| Clove, Cinnamon | L. monocytogenes | Ground beef | Adding and mixing | Clove—5%, 10% Cinnamon—2.5%, 5% | [158] |
| Spanish origanum oil, Spanish marjoram oil and coriander oil | L. monocytogenes | Fresh cut vegetables | Immersing in EO solution | 0.1%, 0.4%, 0.9% | [135] |
| Peppermint (Mentha piperita) | Vibrio spp. | Cheese | Applying on surface | 5–15 µL/mL | [180] |
6. Food Regulations on Applications of Essential Oils
7. Conclusions and Future Prospects
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Antimicrobial potential of pomegranate peel: A review. Int. J. Food Sci. Technol. 2019, 54, 959–965. [Google Scholar] [CrossRef]
- Asbahani, A.E.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; Mousadik, A.E.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Rios, J.L. Essential Oils: What They Are and How the Terms Are Used and Defined. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; Chapter 1; pp. 3–10. [Google Scholar]
- Martucci, J.F.; Gende, L.B.; Neira, L.M.; Ruseckaite, R.A. Oregano and lavender essential oils as antioxidant and antimicrobial additives of biogenic gelatin films. Ind. Crop. Prod. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Garzoli, S.; Petralito, S.; Ovidi, E.; Turchetti, G.; Laghezza Masci, V.; Tiezzi, A.; Trilli, J.; Cesa, S.; Casadei, M.A.; Giacomello, P.; et al. Lavandula × intermedia essential oil and hydrolate: Evaluation of chemical composition and antibacterial activity before and after formulation in nanoemulsion. Ind. Crop. Prod. 2020, 145, 112068. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Aleksic Sabo, V.; Knezevic, P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind. Crop. Prod. 2019, 132, 413–429. [Google Scholar] [CrossRef]
- Papadochristopoulos, A.; Kerry, J.P.; Fegan, N.; Burgess, C.M.; Duffy, G. Natural anti-microbials for enhanced microbial safety and shelf-life of processed packaged meat. Foods 2021, 10, 1598. [Google Scholar] [CrossRef]
- Joshi, V.; Kumar, A.; Kumar, V. Antimicrobial, antioxidant and phyto-chemicals from fruit and vegetable wastes: A review. Int. J. Food Ferment. Technol. 2012, 2, 123–136. [Google Scholar]
- Chanda, S.; Barvaliya, Y.; Kaneria, M.; Rakholiya, K. Fruit and vegetable peels—Strong natural source of antimicrobics. In Current Research, Technology and Education Topics in Apllied Microbiology and Microbial Biotechnology; Mendez, V.A., Ed.; Formatex Research Center: Badajoz, Spain, 2010; Volume 1, pp. 444–450. [Google Scholar]
- Saleem, M.; Saeed, M.T. Potential application of waste fruit peels (orange, yellow lemon and banana) as wide range natural antimicrobial agent. J. King Saud Univ. Sci. 2020, 32, 805–810. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Rosas-Domínguez, C.; Vega-Vega, V.; González-Aguilar, G.A. Antioxidant enrichment and antimicrobial protection of fresh-cut fruits using their own byproducts: Looking for integral exploitation. J. Food Sci. 2010, 75, R175–R181. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Mahmood, Z.; Shoaib, A.; Javaid, D.A. Biocidal activity of citrus peel essential oils against some food spoilage bacteria. J. Med. Plants Res. 2011, 5, 2868–2872. [Google Scholar]
- Alsaraf, S.; Hadi, Z.; Al-Lawati, W.M.; Al Lawati, A.A.; Khan, S.A. Chemical composition, in vitro antibacterial and antioxidant potential of Omani Thyme essential oil along with in silico studies of its major constituent. J. King Saud Univ. Sci. 2020, 32, 1021–1028. [Google Scholar] [CrossRef]
- Smigielski, K.; Prusinowska, R.; Stobiecka, A.; Kunicka-Styczyñska, A.; Gruska, R. Biological Properties and Chemical Composition of Essential Oils from Flowers and Aerial Parts of Lavender (Lavandula angustifolia). J. Essent. Oil Bear. Plants 2018, 21, 1303–1314. [Google Scholar] [CrossRef]
- Purkait, S.; Bhattacharya, A.; Bag, A.; Chattopadhyay, R.R. Synergistic antibacterial, antifungal and antioxidant efficacy of cinnamon and clove essential oils in combination. Arch. Microbiol. 2020, 202, 1439–1448. [Google Scholar] [CrossRef]
- Meng, F.C.; Zhou, Y.-Q.; Ren, D.; Wang, R.; Wang, C.; Lin, L.G.; Zhang, X.Q.; Ye, W.-C.; Zhang, Q.W. Turmeric: A Review of Its Chemical Composition, Quality Control, Bioactivity, and Pharmaceutical Application. In Natural and Artificial Flavoring Agents and Food Dyes; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Chapter 10; pp. 299–350. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. The inhibitory effect of plant essential oils on foodborne pathogenic bacteria in food. Crit. Rev. Food Sci. Nutr. 2019, 59, 3281–3292. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, C.M.S.; Ikeda, N.Y.; Miano, A.C.; Saldaña, E.; Moreno, A.M.; Stashenko, E.; Contreras-Castillo, C.J.; Da Gloria, E.M. Unraveling the selective antibacterial activity and chemical composition of citrus essential oils. Sci. Rep. 2019, 9, 17719. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Liu, K.; Cao, S.; Sun, J.; Zhong, B.; Chun, J. Chemical Composition, Antimicrobial, Antioxidant, and Antiproliferative Properties of Grapefruit Essential Oil Prepared by Molecular Distillation. Molecules 2020, 25, 217. [Google Scholar] [CrossRef] [Green Version]
- Geraci, A.; Di Stefano, V.; Di Martino, E.; Schillaci, D.; Schicchi, R. Essential oil components of orange peels and antimicrobial activity. Nat. Prod. Res. 2017, 31, 653–659. [Google Scholar] [CrossRef]
- Lockwood, G.B. Techniques for gas chromatography of volatile terpenoids from a range of matrices. J. Chromatogr. A 2001, 936, 23–31. [Google Scholar] [CrossRef]
- Tranchida, P.Q.; Bonaccorsi, I.; Dugo, P.; Mondello, L.; Dugo, G. Analysis of Citrus essential oils: State of the art and future perspectives. A review. Flavour Fragr. J. 2012, 27, 98–123. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Impact of different storage conditions on the quality of selected essential oils. Food Res. Int. 2012, 46, 341–353. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Guimaraes, A.; Meireles, L.; Lemos, M.; Guimaraes, M.; Endringer, D.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [Green Version]
- Lyu, X.; Lee, J.; Chen, W.N. Potential Natural Food Preservatives and Their Sustainable Production in Yeast: Terpenoids and Polyphenols. J. Agric. Food Chem. 2019, 67, 4397–4417. [Google Scholar] [CrossRef]
- Cutrim, C.S.; Cortez, M.A.S. A review on polyphenols: Classification, beneficial effects and their application in dairy products. Int. J. Dairy Technol. 2018, 71, 564–578. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Gorniak, I.; Bartoszewski, R.; Kroliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Q.; Liu, K.; Deng, W.; Zhong, B.; Yang, W.; Chun, J. Chemical composition and antimicrobial activity of Gannan navel orange (Citrus sinensis Osbeck cv. Newhall) peel essential oils. Food Sci. Nutr. 2018, 6, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Uysal, B.; Sozmen, F.; Aktas, O.; Oksal, B.S.; Kose, E.O. Essential oil composition and antibacterial activity of the grapefruit (Citrus Paradisi. L) peel essential oils obtained by solvent-free microwave extraction: Comparison with hydrodistillation. Int. J. Food Sci. Technol. 2011, 46, 1455–1461. [Google Scholar] [CrossRef]
- Ou, M.C.; Liu, Y.H.; Sun, Y.W.; Chan, C.F. The Composition, Antioxidant and Antibacterial Activities of Cold-Pressed and Distilled Essential Oils of Citrus paradisi and Citrus grandis (L.) Osbeck. Evid.-Based Complement. Altern. Med. 2015, 2015, 804091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, N.G.; Liu, Y.J. Chemical Composition and Antimicrobial Activity of the Essential Oil from the Peel of Shatian Pummelo (Citrus Grandis Osbeck). Int. J. Food Prop. 2012, 15, 709–716. [Google Scholar] [CrossRef]
- Hosni, K.; Zahed, N.; Chrif, R.; Abid, I.; Medfei, W.; Kallel, M.; Brahim, N.B.; Sebei, H. Composition of peel essential oils from four selected Tunisian Citrus species: Evidence for the genotypic influence. Food Chem. 2010, 123, 1098–1104. [Google Scholar] [CrossRef]
- Hou, H.S.; Bonku, E.M.; Zhai, R.; Zeng, R.; Hou, Y.L.; Yang, Z.H.; Quan, C. Extraction of essential oil from Citrus reticulate Blanco peel and its antibacterial activity against Cutibacterium acnes (formerly Propionibacterium acnes). Heliyon 2019, 5, e02947. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Bishop, K.S.; Quek, S.Y. Compositional analysis and aroma evaluation of Feijoa essential oils from New Zealand grown cultivars. Molecules 2019, 24, 2053. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial susceptibility and antibacterial mechanism of limonene against Listeria monocytogenes. Molecules 2020, 25, 33. [Google Scholar] [CrossRef] [Green Version]
- Fancello, F.; Petretto, G.L.; Zara, S.; Sanna, M.L.; Addis, R.; Maldini, M.; Foddai, M.; Rourke, J.P.; Chessa, M.; Pintore, G. Chemical characterization, antioxidant capacity and antimicrobial activity against food related microorganisms of Citrus limon var. pompia leaf essential oil. LWT Food Sci. Technol. 2016, 69, 579–585. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid.-Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Phillips, C. Potential antimicrobial uses of essential oils in food: Is citrus the answer? Trends Food Sci. Technol. 2008, 19, 156–164. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substance. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Al-Fekaiki, D.; Niamah, A.; Al-Sahlany, S. Extraction and identification of essential oil from Cinnamomum Zeylanicum barks and study the antibacterial activity. J. Microbiol. Biotechnol. Food Sci. 2017, 7, 312–316. [Google Scholar] [CrossRef]
- Abd-Elwahab, S.M.; El-Tanbouly, N.D.; Moussa, M.Y.; Abdel-Monem, A.R.; Fayek, N.M. Antimicrobial and Antiradical Potential of Four Agro-waste Citrus Peels Cultivars. J. Essent. Oil-Bear. Plants 2016, 19, 1932–1942. [Google Scholar] [CrossRef]
- Bendaha, H.; Bouchal, B.; El Mounsi, I.; Salhi, A.; Berrabeh, M.; El Bellaoui, M.; Mimouni, M. Chemical composition, antioxidant, antibacterial and antifungal activities of peel essential oils of citrus aurantium grown in Eastern Morocco. Der Pharm. Lett. 2016, 8, 239–245. [Google Scholar]
- Gupta, M.; Gularia, P.; Singh, D.; Gupta, S. Analysis of aroma active constituents, antioxidant and antimicrobial activity of C. Sinensis, Citrus limetta and C. Limon fruit peel oil by GC-MS. Biosci. Biotechnol. Res. Asia 2014, 11, 895–899. [Google Scholar] [CrossRef] [Green Version]
- Tao, N.G.; Liu, Y.J.; Zhang, M.L. Chemical composition and antimicrobial activities of essential oil from the peel of bingtang sweet orange (Citrus sinensis Osbeck). Int. J. Food Sci. Technol. 2009, 44, 1281–1285. [Google Scholar] [CrossRef]
- Ali, J.; Abbas, S.; Khan, F.A.; Rehman, S.U.; Shah, J.; Rahman, Z.U.; Rahman, I.U.; Paracha, G.M.U.; Khan, M.A.; Shahid, M. Biochemical and antimicrobial properties of Citrus peel waste. Pharmacologyonline 2016, 3, 98–103. [Google Scholar]
- Nwachukwu, B.C.; Taiwo, M.O.; Olisemeke, J.K.; Obero, O.J.; Abibu, W.A. Qualitative Properties and Antibacterial Activity of Essential Oil obtained from Citrus sinensis Peel on Three Selected Bacteria. Biomed. J. Sci. Tech. Res. 2019, 19. [Google Scholar] [CrossRef]
- Kirbaslar, G.F.; Tavman, A.; Dulger, B.; Turker, G. Antimicrobial activity of Turkish Citrus peel oils. Pak. J. Bot. 2009, 41, 3207–3212. [Google Scholar]
- Van de Vel, E.; Sampers, I.; Raes, K. A review on influencing factors on the minimum inhibitory concentration of essential oils. Crit. Rev. Food Sci. Nutr. 2019, 59, 357–378. [Google Scholar] [CrossRef]
- Faleiro, M.L.; Miguel, M.G.; Ladeiro, F.; Venâncio, F.; Tavares, R.; Brito, J.C.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Antimicrobial activity of essential oils isolated from Portuguese endemic species of Thymus. Lett. Appl. Microbiol. 2003, 36, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diep, T.T.; Yoo, M.J.Y.; Pook, C.; Sadooghy-Saraby, S.; Gite, A.; Rush, E. Volatile Components and Preliminary Antibacterial Activity of Tamarillo (Solanum betaceum Cav.). Foods 2021, 10, 2212. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bennett, R.N.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C.B.; Gasson, M.J.; Narbad, A. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J. Appl. Microbiol. 2007, 103, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Hindi, N.; Chabuck, Z. Antimicrobial activity of different aqueous lemon extracts. J. Appl. Pharm. Sci. 2013, 3, 74–78. [Google Scholar]
- El-Hawary, S.; Taha, K.; Abdel-Monem, A.; Kirollos, F.; Mohamed, A. Chemical composition and biological activities of peels and leaves essential oils of four cultivars of Citrus deliciosa var. tangarina. Am. J. Essent. Oils Nat. Prod. 2013, 1, 1–6. [Google Scholar]
- Al-Saman, M.A.; Abdella, A.; Mazrou, K.E.; Tayel, A.A.; Irmak, S. Antimicrobial and antioxidant activities of different extracts of the peel of kumquat (Citrus japonica Thunb). J. Food Meas. Charact. 2019, 13, 3221–3229. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Chaliha, M.; Sultanbawa, Y.; Netzel, M.E. Nutritional characteristics and antimicrobial activity of Australian grown feijoa (Acca sellowiana). Foods 2019, 8, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, R.; Alves, E.S.S.; Santos, M.P.; Aquije, G.M.F.V.; Fernandes, A.A.R.; Dos Santos, R.B.; Ventura, J.A.; Fernandes, P.M.B. Antimicrobial activity and potential use of monoterpenes as tropical fruits preservatives. Braz. J. Microbiol. 2008, 39, 163–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabuck, Z.A.G.; Al-Charrakh, A.H.; Hindi, N.K.K.; Hindi, S.K.K. Antimicrobial effect of aqueous banana peel extract. Res. Gate Pharmceutical Sci. 2013, 1, 73–75. [Google Scholar]
- Mahmud, S.; Saleem, M.; Siddique, S.; Ahmed, R.; Khanum, R.; Perveen, Z. Volatile components, antioxidant and antimicrobial activity of Citrus acida var. sour lime peel oil. J. Saudi Chem. Soc. 2009, 13, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Malviya, S.; Arvind, J.A.; Hettiarachchy, N. Antioxidant and antibacterial potential of pomegranate peel extracts. J. Food Sci. Technol. 2014, 51, 4132–4137. [Google Scholar] [CrossRef] [Green Version]
- Matook, S.M.; Fumio, H. Antibacterial and Antioxidant Activities of Banana (Musa, AAA cv. Cavendish) Fruits Peel. Am. J. Biochem. Biotechnol. 2005, 1, 125–131. [Google Scholar]
- Mehmood, T.; Afzal, A.; Anwar, F.; Iqbal, M.; Afzal, M.; Qadir, R. Variations in the Composition, Antibacterial and Haemolytic Activities of Peel Essential Oils from Unripe and Ripened Citrus limon (L.) Osbeck Fruit. J. Essent. Oil-Bear. Plants 2019, 22, 159–168. [Google Scholar] [CrossRef]
- Okunowo, W.O.; Oyedeji, O.; Afolabi, L.O.; Matanmi, E. Essential oil of grape fruit (Citrus paradisi) peels and its antimicrobial activities. Am. J. Plant Sci. 2013, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Fawole, O.A.; Makunga, N.P.; Opara, U.L. Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement. Altern. Med. 2012, 12, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.G.; Kang, O.H.; Lee, Y.S.; Chae, H.S.; Oh, Y.C.; Brice, O.O.; Kim, M.S.; Sohn, D.H.; Kim, H.S.; Park, H.; et al. In Vitro and In Vivo Antibacterial Activity of Punica granatum peel Ethanol Extract against Salmonella. Evid.-Based Complement. Altern. Med. 2011, 2011, 690518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhanavade, D.M.; Jalkute, D.C.; Ghosh, J.; Sonawane, K. Study Antimicrobial Activity of Lemon (Citrus lemon L.) Peel Extract. Br. J. Pharmacol. Toxicol. 2011, 2, 119–122. [Google Scholar]
- Kanatt, S.; Chander, R.; Sharma, A. Antioxidant and antimicrobial activity of pomegranate peel extract improves the shelf life of chicken products. Int. J. Food Sci. Technol. 2010, 45, 216–222. [Google Scholar] [CrossRef]
- Al-Zoreky, N.S. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. Int. J. Food Microbiol. 2009, 134, 244–248. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Baek, K.-H.; Kang, S.C. Control of Salmonella in foods by using essential oils: A review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils-Present Status and Future Perspectives. Med. Basel Switz. 2017, 4, 58. [Google Scholar] [CrossRef] [Green Version]
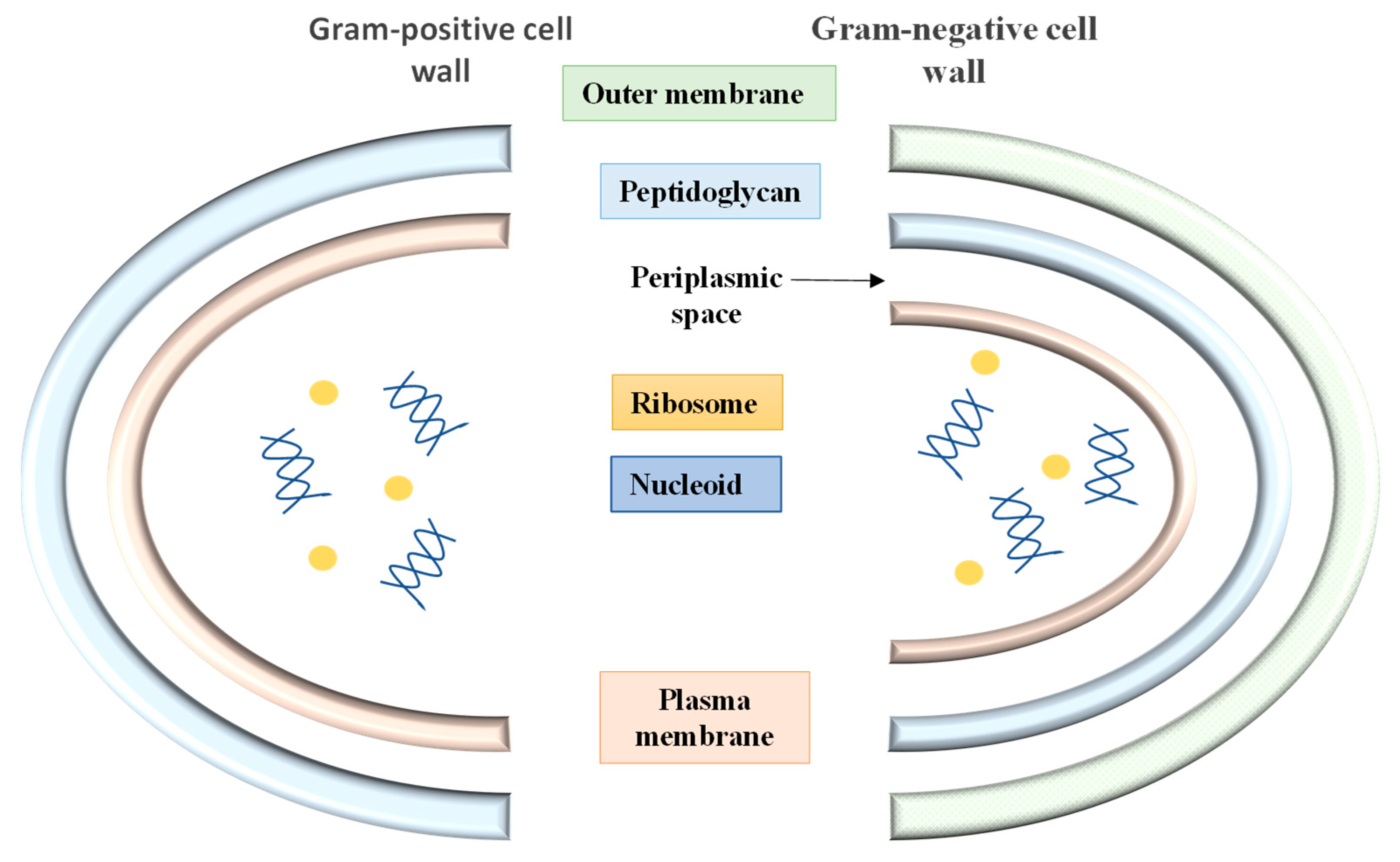
- Plesiat, P.; Nikaido, H. Outer membranes of Gram-negative bacteria are permeable to steroid probes. Mol. Microbiol. 1992, 6, 1323–1333. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crop. Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Nikaido, H. Prevention of drug access to bacterial targets: Permeability barriers and active efflux. Science 1994, 264, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Antibacterial Activity and Mechanism of Ginger Essential Oil against Escherichia coli and Staphylococcus aureus. Molecules 2020, 25, 3955. [Google Scholar] [CrossRef]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus Essential Oils (CEOs) and Their Applications in Food: An Overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Cho, T.; Park, S.M.; Yu, H.; Seo, G.; Kim, H.; Kim, S.A.; Rhee, M. Recent Advances in the Application of Antibacterial Complexes Using Essential Oils. Molecules 2020, 25, 1752. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Marshall, M.R.; Wei, C.I. Antibacterial Activity of Some Essential Oil Components against Five Foodborne Pathogens. J. Agric. Food Chem. 1995, 43, 2839–2845. [Google Scholar] [CrossRef]
- Griffin, S.G.; Wyllie, S.G.; Markham, J.L.; Leach, D.N. The role of structure and molecular properties of terpenoids in determining their antimicrobial activity. Flavour Fragr. J. 1999, 14, 322–332. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and Antioxidant Activity of Essential Oil Terpenes against Pathogenic and Spoilage-Forming Bacteria and Cell Structure-Activity Relationships Evaluated by SEM Microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Togashi, N.; Hamashima, H.; Shiraishi, A.; Inoue, Y.; Takano, A. Antibacterial activities against Staphylococcus aureus of terpene alcohols with aliphatic carbon chains. J. Essent. Oil Res. 2010, 22, 263–269. [Google Scholar] [CrossRef]
- Togashi, N.; Inoue, Y.; Hamashima, H.; Takano, A. Effects of Two Terpene Alcohols on the Antibacterial Activity and the Mode of Action of Farnesol against Staphylococcus aureus. Molecules 2008, 13, 3069–3076. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, H.; Oono, T.; Huh, W.K.; Yamasaki, O.; Ogawa, S.; Katsuyama, M.; Ichikawa, H.; Iwatsuki, K. Actions of farnesol and xylitol against Staphylococcus aureus. Chemotherapy 2002, 48, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.I.A.; Teixeira, P.; Azeredo, J.; Oliveira, R. Effect of farnesol on planktonic and biofilm cells of Staphylococcus epidermidis. Curr. Microbiol. 2009, 59, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Jabra-Rizk, M.A.; Meiller, T.F.; James, C.E.; Shirtliff, M.E. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob. Agents Chemother. 2006, 50, 1463–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
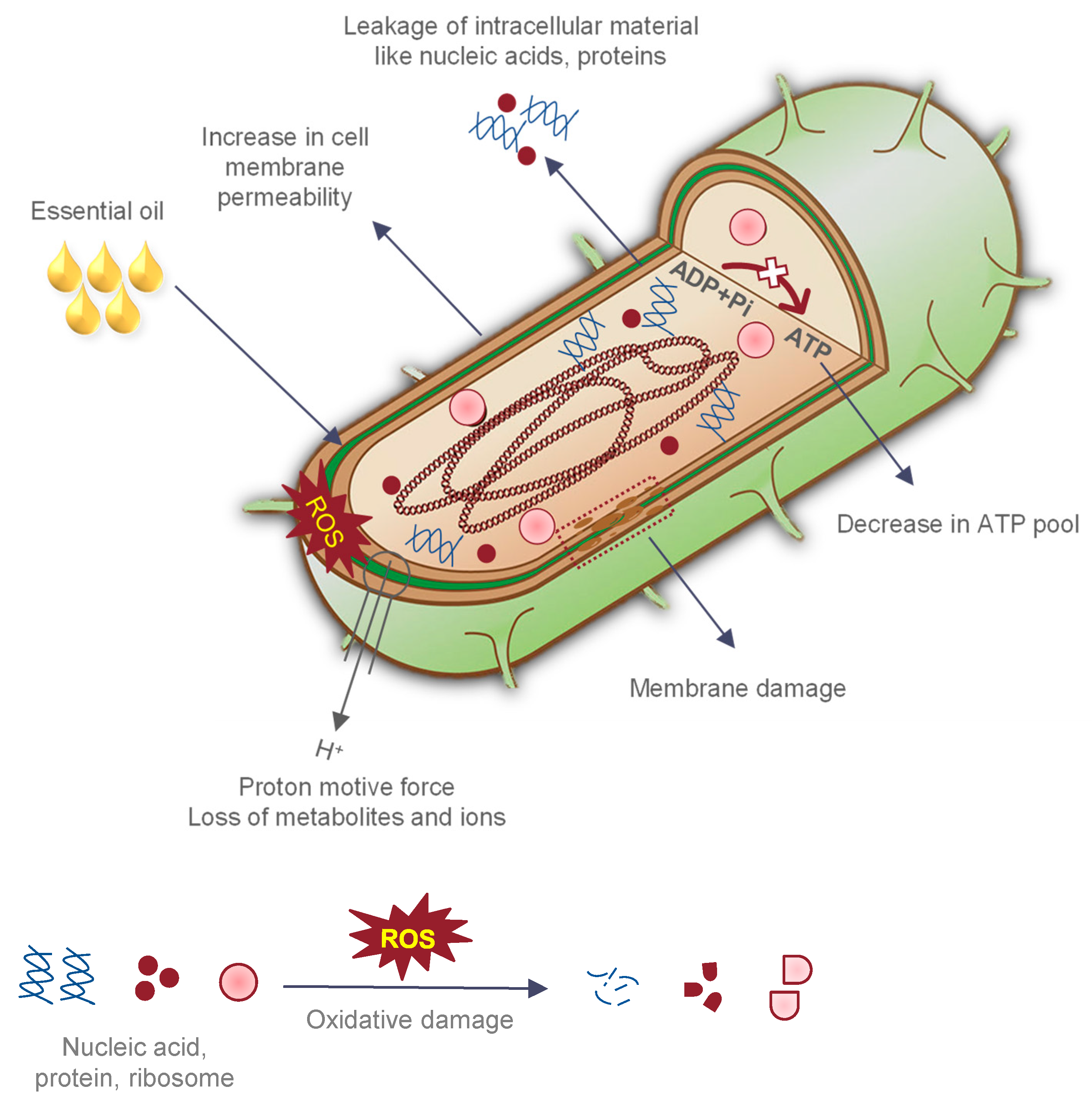
- Saad, N.Y.; Muller, C.D.; Lobstein, A. Major bioactivities and mechanism of action of essential oils and their components. Flavour Fragr. J. 2013, 28, 269–279. [Google Scholar] [CrossRef]
- Lorenzi, V.; Muselli, A.; Bernardini, A.F.; Berti, L.; Pagès, J.M.; Amaral, L.; Bolla, J.M. Geraniol restores antibiotic activities against multidrug-resistant isolates from gram-negative species. Antimicrob. Agents Chemother. 2009, 53, 2209–2211. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.A.; Devaki, T. Geraniol, a component of plant essential oils-a review of its pharmacological activities. Int. J. Pharm. Pharm. Sci. 2015, 7, 67–70. [Google Scholar]
- Lieutaud, A.; Guinoiseau, E.; Lorenzi, V.; Giuliani, M.C.; Lome, V.; Brunel, J.M.; Luciani, A.; Casanova, J.; Pagès, J.M.; Berti, L.; et al. Inhibitors of antibiotic efflux by AcrAB-TolC in enterobacter aerogenes. Anti-Infect. Agents 2013, 11, 168–178. [Google Scholar] [CrossRef]
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef]
- Gao, Z.; Van Nostrand, J.D.; Zhou, J.; Zhong, W.; Chen, K.; Guo, J. Anti-listeria Activities of Linalool and Its Mechanism Revealed by Comparative Transcriptome Analysis. Front. Microbiol. 2019, 10, 2947. [Google Scholar] [CrossRef]
- Wang, J.-N.; Chen, W.-X.; Chen, R.-H.; Zhang, G.-F. Antibacterial activity and mechanism of limonene against Pseudomonas aeruginosa. J. Food Sci. Technol 2018, 39, 1–5. [Google Scholar]
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef]
- Silva, F.; Domingues, F.C. Antimicrobial activity of coriander oil and its effectiveness as food preservative. Crit. Rev. Food Sci. Nutr. 2017, 57, 35–47. [Google Scholar] [CrossRef]
- Silva, F.; Ferreira, S.; Queiroz, J.A.; Domingues, F.C. Coriander (Coriandrum sativum L.) essential oil: Its antibacterial activity and mode of action evaluated by flow cytometry. J. Med Microbiol. 2011, 60, 1479–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Romero, J.C.; González-Ríos, H.; Borges, A.; Simões, M. Antibacterial Effects and Mode of Action of Selected Essential Oils Components against Escherichia coli and Staphylococcus aureus. Evid.-Based Complement. Altern. Med. 2015, 2015, 795435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broniatowski, M.; Mastalerz, P.; Flasiński, M. Studies of the interactions of ursane-type bioactive terpenes with the model of Escherichia coli inner membrane—Langmuir monolayer approach. Biochim. Biophys. Acta 2015, 1848, 469–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Park, S.J.; Lee, E.J.; Cerbo, R.M.; Lee, S.M.; Ryu, C.H.; Kim, G.S.; Kim, J.O.; Ha, Y.L. Antibacterial compounds from Rose Bengal-sensitized photooxidation of β-caryophyllene. J. Food Sci. 2008, 73, C540–C545. [Google Scholar] [CrossRef]
- Coman, M.M.; Oancea, A.M.; Verdenelli, M.C.; Cecchini, C.; Bahrim, G.E.; Orpianesi, C.; Cresci, A.; Silvi, S. Polyphenol content and in vitro evaluation of antioxidant, antimicrobial and prebiotic properties of red fruit extracts. Eur. Food Res. Technol. 2018, 244, 735–745. [Google Scholar] [CrossRef]
- Gharib, R.; Najjar, A.; Auezova, L.; Charcosset, C.; Greige-Gerges, H. Interaction of Selected Phenylpropenes with Dipalmitoylphosphatidylcholine Membrane and Their Relevance to Antibacterial Activity. J. Membr. Biol. 2017, 250, 259–271. [Google Scholar] [CrossRef]
- Ferreira, L.; Perestrelo, R.; Caldeira, M.; Câmara, J.S. Characterization of volatile substances in apples from Rosaceae family by headspace solid-phase microextraction followed by GC-qMS. J. Sep. Sci. 2009, 32, 1875–1888. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, Y.; Yusa, T.; Sawabe, A.; Iizuka, Y.; Okamoto, K. Structure and Physiological Activity of Phenyl Propanoid Glycosides in Lemon (Citrus limon Burm. f.) Peel. Agric. Biol. Chem. 1991, 55, 647–650. [Google Scholar]
- Voo, S.S.; Grimes, H.D.; Lange, B.M. Assessing the Biosynthetic Capabilities of Secretory Glands in Citrus Peel. Plant Physiol. 2012, 159, 81–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, R.G. Phenylpropenes: Occurrence, Distribution, and Biosynthesis in Fruit. J. Agric. Food Chem. 2018, 66, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Sun, Z.; Wang, T.; Yang, M.; Liu, M.; Zhang, J.; Li, Y. Antimicrobial activity of eugenol against carbapenem-resistant Klebsiella pneumoniae and its effect on biofilms. Microb. Pathog. 2020, 139, 103924. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antimicrobial mechanism of clove oil on Listeria monocytogenes. Food Control 2018, 94, 140–146. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Mizan, M.F.R.; Ha, A.J.-w.; Park, S.H.; Ha, S.-D. Antibacterial and antibiofilm mechanism of eugenol against antibiotic resistance Vibrio parahaemolyticus. Food Microbiol. 2020, 91, 103500. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Doble, M. Synergistic interaction of eugenol with antibiotics against Gram negative bacteria. Phytomedicine 2009, 16, 997–1005. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Piotrowska, R.; Foss, M.; Meyer, R. Isoeugenol has a non-disruptive detergent-like mechanism of action. Front. Microbiol. 2015, 6, 754. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Auezova, L.; Najjar, A.; Kfoury, M.; Fourmentin, S.; Greige-Gerges, H. Antibacterial activity of free or encapsulated selected phenylpropanoids against Escherichia coli and Staphylococcus epidermidis. J. Appl. Microbiol. 2020, 128, 710–720. [Google Scholar] [CrossRef]
- Albano, M.; Crulhas, B.P.; Alves, F.C.B.; Pereira, A.F.M.; Andrade, B.F.M.T.; Barbosa, L.N.; Furlanetto, A.; Lyra, L.P.d.S.; Rall, V.L.M.; Júnior, A.F. Antibacterial and anti-biofilm activities of cinnamaldehyde against S. epidermidis. Microb. Pathog. 2019, 126, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhang, T.; Yuan, Y.; Lin, S.; Xu, J.; Ye, H. Effects of cinnamaldehyde on Escherichia coli and Staphylococcus aureus membrane. Food Control 2015, 47, 196–202. [Google Scholar] [CrossRef]
- Yin, L.; Chen, J.; Wang, K.; Geng, Y.; Lai, W.; Huang, X.; Chen, D.; Guo, H.; Fang, J.; Chen, Z.; et al. Study the antibacterial mechanism of cinnamaldehyde against drug-resistant Aeromonas hydrophila in vitro. Microb. Pathog. 2020, 145, 104208. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Pal, R.; Hameed, S.; Fatima, Z. Antimycobacterial mechanism of vanillin involves disruption of cell-surface integrity, virulence attributes, and iron homeostasis. Int. J. Mycobacteriol. 2016, 5, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Fathima, A.; Rao, J.R. Selective toxicity of Catechin—A natural flavonoid towards bacteria. Appl. Microbiol. Biotechnol. 2016, 100, 6395–6402. [Google Scholar] [CrossRef]
- Cushnie, T.; Taylor, P.; Nagaoka, Y.; Uesato, S.; Hara, Y.; Lamb, A. Investigation of the antibacterial activity of 3-O-octanoyl-(-)-epicatechin. J. Appl. Microbiol. 2008, 105, 1461–1469. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Iinuma, M. Reduction of membrane fluidity by antibacterial sophoraflavanone G isolated from Sophora exigua. Phytomedicine 2000, 7, 161–165. [Google Scholar] [CrossRef]
- Dave, D.; Ghaly, A.E. Meat spoilage mechanisms and preservation techniques: A critical review. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar]
- Jayasena, D.D.; Jo, C. Essential oils as potential antimicrobial agents in meat and meat products: A review. Trends Food Sci. Technol. 2013, 34, 96–108. [Google Scholar] [CrossRef]
- Lucera, A.; Costa, C.; Conte, A.; Del Nobile, M.A. Food applications of natural antimicrobial compounds. Front. Microbiol. 2012, 3, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasniewska, K.; Kosakowska, O.; Pobiega, K.; Gniewosz, M. The influence of two-component mixtures from Spanish Origanum oil with Spanish Marjoram oil or coriander oil on antilisterial activity and sensory quality of a fresh cut vegetable mixture. Foods 2020, 9, 1740. [Google Scholar] [CrossRef] [PubMed]
- Karagozlu, N.; Ergonul, B.; Ozcan, D. Determination of antimicrobial effect of mint and basil essential oils on survival of E. coli O157:H7 and S. typhimurium in fresh-cut lettuce and purslane. Food Control 2011, 22, 1851–1855. [Google Scholar] [CrossRef]
- Siddiqua, S.; Anusha, B.A.; Ashwini, L.S.; Negi, P.S. Antibacterial activity of cinnamaldehyde and clove oil: Effect on selected foodborne pathogens in model food systems and watermelon juice. J. Food Sci. Technol. 2015, 52, 5834–5841. [Google Scholar] [CrossRef] [Green Version]
- Huq, T.; Vu, K.D.; Riedl, B.; Bouchard, J.; Lacroix, M. Synergistic effect of gamma (γ)-irradiation and microencapsulated antimicrobials against Listeria monocytogenes on ready-to-eat (RTE) meat. Food Microbiol. 2015, 46, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Petrou, S.; Tsiraki, M.; Giatrakou, V.; Savvaidis, I.N. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. Int. J. Food Microbiol. 2012, 156, 264–271. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Trigui, M.; Mansour, R.B.; Jarraya, R.M.; Damak, M.; Jaoua, S. Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat. Int. J. Food Microbiol. 2011, 148, 66–72. [Google Scholar] [CrossRef]
- Hulankova, R.; Borilova, G.; Steinhauserova, I. Combined antimicrobial effect of oregano essential oil and caprylic acid in minced beef. Meat Sci. 2013, 95, 190–194. [Google Scholar] [CrossRef]
- Vasilatos, G.C.; Savvaidis, I.N. Chitosan or rosemary oil treatments, singly or combined to increase turkey meat shelf-life. Int. J. Food Microbiol. 2013, 166, 54–58. [Google Scholar] [CrossRef]
- Fernandez-Lopez, J.; Viuda-Martos, M. Introduction to the Special Issue: Application of Essential Oils in Food Systems. Foods 2018, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Gupta, T.B.; Bronlund, J.; Kaur, L. The potential of rosemary as a functional ingredient for meat products—A review. Food Rev. Int. 2021, 1–21. [Google Scholar] [CrossRef]
- Huang, X.; Lao, Y.; Pan, Y.; Chen, Y.; Zhao, H.; Gong, L.; Xie, N.; Mo, C.-H. Synergistic antimicrobial effectiveness of plant essential oil and its application in seafood preservation: A review. Molecules 2021, 26, 307. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential oils and their application on active packaging systems: A review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Guo, M.; Jin, T.Z.; Arabi, S.A.; Liu, D. Ultrasound improves the decontamination effect of thyme essential oil nanoemulsions against Escherichia coli O157: H7 on cherry tomatoes. Int. J. Food Microbiol. 2021, 337, 108936. [Google Scholar] [CrossRef]
- Kang, J.-H.; Song, K.B. Inhibitory effect of plant essential oil nanoemulsions against Listeria monocytogenes, Escherichia coli O157:H7, and Salmonella typhimurium on red mustard leaves. Innov. Food Sci. Emerg. Technol. 2018, 45, 447–454. [Google Scholar] [CrossRef]
- Kang, J.-H.; Park, S.-J.; Park, J.-B.; Song, K.B. Surfactant type affects the washing effect of cinnamon leaf essential oil emulsion on kale leaves. Food Chem. 2019, 271, 122–128. [Google Scholar] [CrossRef]
- Yuan, W.; Teo, C.H.M.; Yuk, H.-G. Combined antibacterial activities of essential oil compounds against Escherichia coli O157:H7 and their application potential on fresh-cut lettuce. Food Control 2019, 96, 112–118. [Google Scholar] [CrossRef]
- Dai, J.; Li, C.; Cui, H.; Lin, L. Unraveling the anti-bacterial mechanism of Litsea cubeba essential oil against E. coli O157:H7 and its application in vegetable juices. Int. J. Food Microbiol. 2021, 338, 108989. [Google Scholar] [CrossRef]
- Krogsgård Nielsen, C.; Kjems, J.; Mygind, T.; Snabe, T.; Schwarz, K.; Serfert, Y.; Meyer, R.L. Antimicrobial effect of emulsion-encapsulated isoeugenol against biofilms of food pathogens and spoilage bacteria. Int. J. Food Microbiol. 2017, 242, 7–12. [Google Scholar] [CrossRef]
- Yoo, J.H.; Baek, K.H.; Heo, Y.S.; Yong, H.I.; Jo, C. Synergistic bactericidal effect of clove oil and encapsulated atmospheric pressure plasma against Escherichia coli O157:H7 and Staphylococcus aureus and its mechanism of action. Food Microbiol. 2021, 93, 103611. [Google Scholar] [CrossRef] [PubMed]
- Krichen, F.; Hamed, M.; Karoud, W.; Bougatef, H.; Sila, A.; Bougatef, A. Essential oil from pistachio by-product: Potential biological properties and natural preservative effect in ground beef meat storage. J. Food Meas. Charact. 2020, 14, 3020–3030. [Google Scholar] [CrossRef]
- Lin, L.; Mao, X.; Sun, Y.; Rajivgandhi, G.; Cui, H. Antibacterial properties of nanofibers containing chrysanthemum essential oil and their application as beef packaging. Int. J. Food Microbiol. 2019, 292, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.d.S.; Figueiredo, H.M.d.; Stamford, T.L.M.; Silva, L.H.M.d. Inhibition of Listeria monocytogenes by Melaleuca alternifolia (tea tree) essential oil in ground beef. Int. J. Food Microbiol. 2019, 293, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Khaleque, M.A.; Keya, C.A.; Hasan, K.N.; Hoque, M.M.; Inatsu, Y.; Bari, M.L. Use of cloves and cinnamon essential oil to inactivate Listeria monocytogenes in ground beef at freezing and refrigeration temperatures. LWT 2016, 74, 219–223. [Google Scholar] [CrossRef]
- Chaichi, M.; Mohammadi, A.; Badii, F.; Hashemi, M. Triple synergistic essential oils prevent pathogenic and spoilage bacteria growth in the refrigerated chicken breast meat. Biocatal. Agric. Biotechnol. 2021, 32, 101926. [Google Scholar] [CrossRef]
- Jayari, A.; El Abed, N.; Jouini, A.; Mohammed Saed Abdul-Wahab, O.; Maaroufi, A.; Ben Hadj Ahmed, S. Antibacterial activity of Thymus capitatus and Thymus algeriensis essential oils against four food-borne pathogens inoculated in minced beef meat. J. Food Saf. 2018, 38, e12409. [Google Scholar] [CrossRef]
- Kazemeini, H.; Azizian, A.; Adib, H. Inhibition of Listeria monocytogenes growth in turkey fillets by alginate edible coating with Trachyspermum ammi essential oil nano-emulsion. Int. J. Food Microbiol. 2021, 344, 109104. [Google Scholar] [CrossRef]
- Kazemeini, H.; Azizian, A.; Shahavi, M.H. Effect of chitosan nano-gel/emulsion containing Bunium Persicum essential oil and nisin as an edible biodegradable coating on Escherichia coli O 157:H 7 in rainbow trout fillet. J. Water Environ. Nanotechnol. 2019, 4, 343–349. [Google Scholar]
- Noori, S.; Zeynali, F.; Almasi, H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control 2018, 84, 312–320. [Google Scholar] [CrossRef]
- Khezri, S.; Khezerlou, A.; Dehghan, P. Antibacterial activity of Artemisia persica Boiss essential oil against Escherichia coli O157: H7 and Listeria monocytogenes in probiotic Doogh. J. Food Process. Preserv. 2021, 45, e15446. [Google Scholar] [CrossRef]
- Valkova, V.; Duranova, H.; Galovicova, L.; Vukovic, N.L.; Vukic, M.; Kacaniova, M. In vitro antimicrobial activity of lavender, mint, and rosemary essential oils and the effect of their vapours on growth of Penicillium spp. In a bread model system. Molecules 2021, 26, 3859. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.I.; Ibrahim, N.; Abdel-Salam, A.B.; Fahim, K.M. Potential application of ginger, clove and thyme essential oils to improve soft cheese microbial safety and sensory characteristics. Food Biosci. 2021, 42, 101177. [Google Scholar] [CrossRef]
- Santos, M.I.S.; Martins, S.R.; Veríssimo, C.S.C.; Nunes, M.J.C.; Lima, A.I.G.; Ferreira, R.M.S.B.; Pedroso, L.; Sousa, I.; Ferreira, M.A.S.S. Essential oils as antibacterial agents against food-borne pathogens: Are they really as useful as they are claimed to be? J. Food Sci. Technol. 2017, 54, 4344–4352. [Google Scholar] [CrossRef]
- Lages, L.Z.; Radünz, M.; Gonçalves, B.T.; Silva da Rosa, R.; Fouchy, M.V.; de Cássia dos Santos da Conceição, R.; Gularte, M.A.; Barboza Mendonça, C.R.; Gandra, E.A. Microbiological and sensory evaluation of meat sausage using thyme (Thymus vulgaris, L.) essential oil and powdered beet juice (Beta vulgaris L., Early Wonder cultivar). LWT 2021, 148, 111794. [Google Scholar] [CrossRef]
- Radunz, M.; dos Santos Hackbart, H.C.; Camargo, T.M.; Nunes, C.F.P.; de Barros, F.A.P.; Dal Magro, J.; Filho, P.J.S.; Gandra, E.A.; Radünz, A.L.; da Rosa Zavareze, E. Antimicrobial potential of spray drying encapsulated thyme (Thymus vulgaris) essential oil on the conservation of hamburger-like meat products. Int. J. Food Microbiol. 2020, 330, 108696. [Google Scholar] [CrossRef]
- Diarra, M.; Hassan, Y.; Block, G.; Drover, J.; Delaquis, P.; Oomah, B.D. Antibacterial activities of a polyphenolic-rich extract prepared from American cranberry (Vaccinium macrocarpon) fruit pomace against Listeria spp. LWT 2020, 123, 109056. [Google Scholar] [CrossRef]
- Tamkutė, L.; Gil, B.M.; Carballido, J.R.; Pukalskienė, M.; Venskutonis, P.R. Effect of cranberry pomace extracts isolated by pressurized ethanol and water on the inhibition of food pathogenic/spoilage bacteria and the quality of pork products. Food Res. Int. 2019, 120, 38–51. [Google Scholar] [CrossRef]
- Khanjari, A.; Bahonar, A.; Noori, N.; Siahkalmahaleh, M.R.; Rezaeigolestani, M.; Asgarian, Z.; Khanjari, J. In vitro antibacterial activity of Pimpinella anisum essential oil and its influence on microbial, chemical, and sensorial properties of minced beef during refrigerated storage. J. Food Saf. 2019, 39, e12626. [Google Scholar] [CrossRef]
- Sojic, B.; Pavlic, B.; Ikonić, P.; Tomovic, V.; Ikonic, B.; Zekovic, Z.; Kocic-Tanackov, S.; Jokanovic, M.; Skaljac, S.; Ivic, M. Coriander essential oil as natural food additive improves quality and safety of cooked pork sausages with different nitrite levels. Meat Sci. 2019, 157, 107879. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Li, X.; Sun, Y.; Pan, D.; Wang, Y.; Cao, J. Effect of cinnamon essential oil on the microbiological and physiochemical characters of fresh Italian style sausage during storage. Anim. Sci. J. 2019, 90, 435–444. [Google Scholar] [CrossRef]
- Gniewosz, M.; Stobnicka, A. Bioactive components content, antimicrobial activity, and foodborne pathogen control in minced pork by cranberry pomace extracts. J. Food Saf. 2018, 38, e12398. [Google Scholar] [CrossRef] [Green Version]
- Aminzare, M.; Tajik, H.; Aliakbarlu, J.; Hashemi, M.; Raeisi, M. Effect of cinnamon essential oil and grape seed extract as functional-natural additives in the production of cooked sausage-impact on microbiological, physicochemical, lipid oxidation and sensory aspects, and fate of inoculated Clostridium perfringens. J. Food Saf. 2018, 38, e12459. [Google Scholar] [CrossRef]
- Ed-Dra, A.; Rhazi Filali, F.; Bou-Idra, M.; Zekkori, B.; Bouymajane, A.; Moukrad, N.; Benhallam, F.; Bebtayeb, A. Application of Mentha suaveolens essential oil as an antimicrobial agent in fresh turkey sausages. J. Appl. Biol. Biotechnol. 2018, 6, 7–12. [Google Scholar]
- Boskovic, M.; Djordjevic, J.; Ivanovic, J.; Janjic, J.; Zdravkovic, N.; Glisic, M.; Glamoclija, N.; Baltic, B.; Djordjevic, V.; Baltic, M. Inhibition of Salmonella by thyme essential oil and its effect on microbiological and sensory properties of minced pork meat packaged under vacuum and modified atmosphere. Int. J. Food Microbiol. 2017, 258, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Lizarazo, C.M.; Betancourt-Cortés, R.; Lombana, A.; Carrillo-Castro, K.; Sotelo-Díaz, I. Listeria monocytogenes behaviour and quality attributes during sausage storage affected by sodium nitrite, sodium lactate and thyme essential oil. Food Sci. Technol. Int. 2017, 23, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Al-Sahlany, S.T.G. Effect of Mentha piperita essential oil against Vibrio spp. isolated from local cheeses. Pak. J. Food Sci. 2016, 26, 65–71. [Google Scholar]
- European Commission. Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on Flavourings and Certain Food Ingredients with Flavouring Properties for Use in and on Foods and Amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. 2008. Available online: https://eur-lex.europa.eu/eli/reg/2008/1334/oj (accessed on 1 January 2022).
- Ribeiro-Santos, R.; Andrade, M.; Melo, N.R.d.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Benkhaira, N.; Koraichi, S.I.; Fikri-Benbrahim, K. In vitro methods to study antioxidant and some biological activities of essential oils: A review. Biointerface Res. Appl. Chem. 2022, 12, 3332–3347. [Google Scholar]
- Yang, T.; Qin, W.; Zhang, Q.; Luo, J.; Lin, D.; Chen, H. Essential-oil capsule preparation and its application in food preservation: A review. Food Rev. Int. 2022, 1–35. [Google Scholar] [CrossRef]
- Roda, R.; Taboada-Rodríguez, A.; Valverde-Franco, M.; Marín-Iniesta, F. Antimicrobial Activity of Vanillin and Mixtures with Cinnamon and Clove Essential Oils in Controlling Listeria monocytogenes and Escherichia coli O157:H7 in Milk. Food Bioprocess Technol. 2010, 5, 2120–2131. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyung, K.H. Antimicrobial properties of Allium species. Curr. Opin. Biotechnol. 2012, 23, 142–147. [Google Scholar] [CrossRef]
- Ruzauskas, M.; Bartkiene, E.; Stankevicius, A.; Bernatoniene, J.; Zadeike, D.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Grigas, J.; Zokaityte, E.; et al. The influence of essential oils on gut microbial profiles in pigs. Animals 2020, 10, 1734. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. https://doi.org/10.3390/foods11030464
Angane M, Swift S, Huang K, Butts CA, Quek SY. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods. 2022; 11(3):464. https://doi.org/10.3390/foods11030464
Chicago/Turabian StyleAngane, Manasweeta, Simon Swift, Kang Huang, Christine A. Butts, and Siew Young Quek. 2022. "Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry" Foods 11, no. 3: 464. https://doi.org/10.3390/foods11030464
APA StyleAngane, M., Swift, S., Huang, K., Butts, C. A., & Quek, S. Y. (2022). Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods, 11(3), 464. https://doi.org/10.3390/foods11030464








