Impact of Cold Atmospheric Plasma (CAP) Treatments on the Oxidation of Pistachio Kernel Lipids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Plasma Treatments
2.2. Total Lipid Extraction and Lipid Analyses
2.3. Analysis of Volatile Components
2.4. Data Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UN Food and Agriculture Organization. Corporate Statistical Database (FAOSTAT). Available online: https://www.fao.org/faostat/en/#home (accessed on 18 December 2021).
- Wilson, J.W.; Petino, G.; Knudsen, D.C. Geographic context of the Green Pistachio of Bronte, a protected designation of origin product. J. Maps 2018, 14, 144–150. [Google Scholar] [CrossRef]
- Soares Mateus, A.R.; Barros, S.; Pena, A.; Sanches Silva, A. Mycotoxins in Pistachios (Pistacia vera L.): Methods for Determination, Occurrence, Decontamination. Toxins 2021, 13, 682. [Google Scholar] [CrossRef] [PubMed]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; EisenBrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin 2016, 32, 179–205. [Google Scholar] [CrossRef] [PubMed]
- Niemira, B.A. Cold plasma decontamination of foods. Annu. Rev. Food Technol. 2012, 3, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Singh, A.; Pratap Singh, A. Recent developments in cold plasma decontamination technology in the food industry. Trends Food Sci. Technol. 2018, 80, 93–103. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Ezhilarasi, P.N.; Rajauria, G. Application of cold plasma on food matrices: A review on current and future prospects. J. Food Process Preserv. 2020, e15070. [Google Scholar] [CrossRef]
- Capelli, F.; Tappi, S.; Gritti, T.; de Aguiar Saldanha Pinheiro, A.C.; Laurita, R.; Tylewicz, U.; Spataro, F.; Braschi, G.; Lanciotti, R.; Gómez Galindo, F.; et al. Decontamination of Food Packages from SARS-CoV-2 RNA with a Cold Plasma-Assisted System. Appl. Sci. 2021, 11, 4177. [Google Scholar] [CrossRef]
- Basaran, P.; Basaran-Akgul, N.; Oksuz, L. Elimination of Aspergillus parasiticus from nut surface with low pressure cold plasma (LPCP) treatment. Food Microbiol. 2008, 25, 626–632. [Google Scholar] [CrossRef]
- Makari, M.; Hojjati, M.; Shahbazi, S.; Askari, H. Elimination of Aspergillus flavus from pistachio nuts with Dielectric Barrier Discharge (DBD) cold plasma and its impacts on biochemical indices. J. Food Qual. 2021, 2021, 9968711. [Google Scholar] [CrossRef]
- Ghorashi, A.H.; Tasouji, M.A.R.; Kargarian, A. Optimum cold plasma generating device for treatment of Aspergillus flavus from nuts surface. J. Food Sci. Technol. 2020, 3988–3994. [Google Scholar] [CrossRef]
- Tasouji, M.A.; Ghorashi, A.H.; Hamedmoosavian, M.T.; Mahmoudi, M.B. Inactivation of pistachio contaminant Aspergillus flavus by Atmospheric Pressure Capacitive Coupled Plasma (AP-CCP). J. Microbiol. Biotechnol. Food Sci. 2018, 8, 668–671. [Google Scholar] [CrossRef] [Green Version]
- Sohbatzadeh, F.; Mirzanejhad, S.; Shokri, H.; Nikpour, M. Inactivation of Aspergillus flavus spores in a sealed package by cold plasma streamers. J. Theor. Appl. Phys. 2016, 10, 99–106. [Google Scholar] [CrossRef]
- Pignata, C.; D’Angelo, D.; Basso, D.; Cavallero, M.C.; Beneventi, S.; Tartaro, D.; Meineri, V.; Gilli, G. Low-temperature, low-pressure gas plasma application on Aspergillus brasiliensis, Escherichia coli and pistachios. J. Appl. Microbiol. 2014, 116, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, Z.; Hosseinzadeh Samani, B.; Nemati, A.; Nazari, F.; Rostami, S. Development of novel green pesticide system by using cold plasma to control Plodia interpunctella in pistachio. J. Food Process. Preserv. 2021, 45, e15621. [Google Scholar] [CrossRef]
- Thirumdas, R.; Sarangapani, C.; Annapure, U.S. Cold Plasma: A novel non-thermal technology for food processing. Food Biophys 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Moisan, M.; Barbeau, J.; Crevier, M.C.; Pelletier, J.; Philip, N.; Saoudi, B. Plasma sterilization: Methods and mechanisms. Pure Appl. Chem. 2002, 74, 349–358. [Google Scholar] [CrossRef]
- Arena, E.; Campisi, S.; Fallico, B.; Maccarone, E. Distribution of fatty acids and phytosterols as a criterion to discriminate geographic origin of pistachio seeds. Food Chem. 2007, 104, 403–408. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- AOCS (American Oil Chemists’ Society) Official Method Cd 8b-90. Available online: https://www.aocs.org/attain-lab-services/methods (accessed on 4 September 2021).
- Pegg, R.B. Spectrophotometric Measurement of Secondary Lipid Oxidation Products. Curr. Protoc. Food Anal. Chem. 2001, 1, D2.4.1–D2.4.18. [Google Scholar] [CrossRef]
- Tavoletti, S.; Foligni, R.; Mozzon, M.; Pasquini, M. Comparison between fatty acid profiles of old and modern varieties of T. turgidum and T. aestivum: A case study in central Italy. J. Cereal. Sci. 2018, 82, 198–205. [Google Scholar] [CrossRef]
- Tavoletti, S.; Pasquini, M.; Mozzon, M.; Servadio, D.; Merletti, A.; Mannozzi, C.; Foligni, R. Discrimination among varieties of Triticum turgidum subspecies (dicoccon, turanicum and durum) based on the fatty acid profile. J. Cereal Sci. 2021, 99, 103213. [Google Scholar] [CrossRef]
- Larkeson, B.; Dutta, P.C.; Hansson, I. Effects of frying and storage on cholesterol oxidation in minced meat products. J. Am. Oil Chem. Soc. 2000, 77, 675–680. [Google Scholar] [CrossRef]
- Belleggia, L.; Milanović, V.; Ferrocino, I.; Cocolin, L.; Haouet, M.N.; Scuota, S.; Maoloni, A.; Garofalo, C.; Cardinali, F.; Aquilanti, L.; et al. Is there any still undisclosed biodiversity in Ciauscolo salami? A new glance into the microbiota of an artisan production as revealed by high-throughput sequencing. Meat Sci. 2020, 165, 108128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozzon, M.; Foligni, R.; Mannozzi, C. Brewing Quality of Hop Varieties Cultivated in Central Italy Based on Multivolatile Fingerprinting and Bitter Acid Content. Foods 2020, 9, 541. [Google Scholar] [CrossRef]
- Yahyavi, F.; Alizadeh-Khaledabad, M.; Azadmard-Damirchi, S. Oil quality of pistachios (Pistacia vera L.) grown in East Azarbaijan, Iran. NFS J. 2020, 18, 12–18. [Google Scholar] [CrossRef]
- Salhi, M.; Gharsallaoui, M.; Gabsi, S.; Tunisian Pistacia atlantica Desf. Extraction Process: Impact on Chemical and Nutritional Characteristics. Eur. J. Lipid Sci. Technol. 2021, 123, 2100013. [Google Scholar] [CrossRef]
- Catalán, L.; Alvarez-Ortí, M.; Pardo-Giménez, A.; Gómez, R.; Rabadán, A.; Pardo, J.E. Pistachio oil: A review on its chemical composition, extraction systems, and uses. Eur. J. Lipid Sci. Technol. 2017, 119, 1600126. [Google Scholar] [CrossRef]
- Mozzon, M.; Pacetti, D.; Frega, N.G.; Lucci, P. Crude palm oil from interspecific hybrid Elaeis oleifera × E. guineensis: Alcoholic constituents of unsaponifiable matter. J. Am. Oil Chem. Soc. 2015, 92, 717–724. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Wu, X.; Zhao, Y.; Wu, L.; Lu, B. Quantitative determination of free and esterified phytosterol profile in nuts and seeds commonly consumed in China by SPE/GC–MS. LWT 2019, 100, 355–361. [Google Scholar] [CrossRef]
- Mozzon, M.; Foligni, R.; Mannozzi, C. Current knowledge on interspecific hybrid palm oil as food and food ingredient. Foods 2020, 9, 631. [Google Scholar] [CrossRef]
- Lucarini, M.; D’Evoli, L.; Lombardi-Boccia, G. Phytosterols and phytosterol oxides in Bronte’s Pistachio (Pistacia vera L.) and in processed pistachio products. Eur. Food Res. Technol. 2020, 246, 307–314. [Google Scholar] [CrossRef]
- Derewiaka, D.; Obiedzijski, M. Phytosterol oxides content in selected thermally processed products. Eur. Food Res. Technol. 2012, 234, 703–712. [Google Scholar] [CrossRef]
- Menéndez-Carreño, M.; Knol, D.; Janssen, H.G. Development and validation of methodologies for the quantification of phytosterols and phytosterol oxidation products in cooked and baked food products. J. Chromatogr. A 2016, 1428, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Grandgirard, A.; Martine, L.; Joffre, C.; Juaneda, P.; Berdeaux, O. Gas chromatographic separation and mass spectrometric identification of mixtures of oxyphytosterol and oxycholesterol derivatives: Application to a phytosterol-enriched food. J. Chromatogr. A 2004, 1040, 239–250. [Google Scholar] [CrossRef]
- Brewer, M.S. Irradiation effects on meat flavor: A review. Meat Sci. 2009, 81, 1–14. [Google Scholar] [CrossRef]
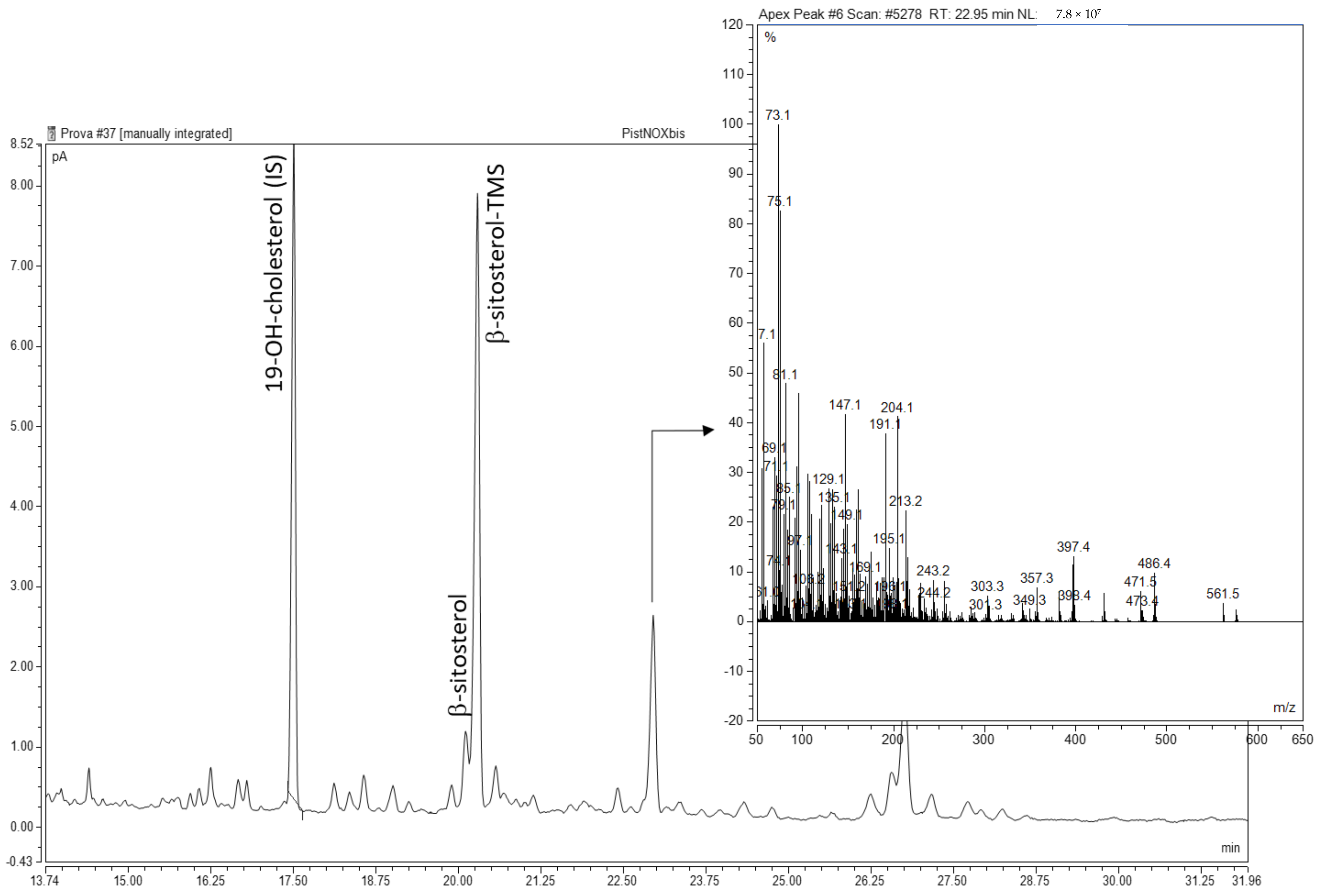
| Parameter | C 1 | O3 | O3(+) |
|---|---|---|---|
| Peroxide value [mEq O2/kg oil] | 3.36 ± 0.69 | 3.00 ± 0.42 | 4.22 ± 0.78 |
| TBARS [mg TEP/g oil] | 0.072 ± 0.004 b | 0.600 ± 0.037 a | 0.109 ± 0.007 b |
| Total FAMEs composition [%] 2 | |||
| C14:0 | 0.13 ± 0.06 | 0.19 ± 0.05 | 0.10 ± 0.05 |
| C16:0 | 9.68 ± 0.51 | 11.17 ± 0.74 | 9.86 ± 0.59 |
| C16:1 | 0.73 ± 0.07 b | 1.07 ± 0.14 a | 0.90 ± 0.14 ab |
| C17:0 | 0.03 ± 0.01 | 0.37 ± 0.54 | 0.05 ± 0.02 |
| C18:1Δ9 | 52.21 ± 1.50 | 50.70 ± 1.52 | 50.03 ± 1.99 |
| C18:1Δ11 | 2.03 ± 0.18 | 2.08 ± 0.19 | 1.68 ± 0.17 |
| C18:2Δ9,12 | 34.07 ± 1.88 | 33.19 ± 2.64 | 36.66 ± 0.73 |
| C18:3Δ9,12,15 | 0.52 ± 0.05 | 0.47 ± 0.07 | 0.61 ± 0.07 |
| C20:0 | 0.10 ± 0.03 | 0.14 ± 0.06 | 0.06 ± 0.03 |
| C20:1Δ11 | 0.38 ± 0.01 | 0.46 ± 0.13 | 0.37 ± 0.01 |
| C21:0 | 0.07 ± 0.02 | 0.12 ± 0.03 | 0.10 ± 0.03 |
| C20:2Δ11,14 | 0.03 ± 0.00 | 0.04 ± 0.01 | 0.03 ± 0.01 |
| Unsaponifiable matter components | |||
| cholesterol [mg/100 g oil] | 6.1 ± 0.0 a (2.0 ± 0.3) | 3.2 ± 0.4 b (1.1 ± 0.2) | 5.6 ± 0.2 a (1.8 ± 0.3) |
| campesterol [mg/100 g oil] | 14.1 ± 1.5 (4.5 ± 0.1) 3 | 15.7 ± 2.2 (5.2 ± 0.6) | 14.3 ± 1.9 (4.6 ± 0.2) |
| stigmasterol [mg/100 g oil] | 3.0 ± 0.6 (1.0 ± 0.1) | 3.6 ± 0.2 (1.2 ± 0.1) | 3.2 ± 0.6 (1.0 ± 0.0) |
| β-sitosterol [mg/100 g oil] | 266.0 ± 33.6 (85.8 ± 0.1) | 259.5 ± 18.8 (85.6 ± 0.8) | 269.1 ± 50.3 (86.5 ± 0.9) |
| Δ5-avenasterol [mg/100 g oil] | 20.9 ± 4.0 (6.7 ± 0.4) | 20.8 ± 1.6 (6.9 ± 0.8) | 18.8 ± 2.0 (6.1 ± 0.4) |
| Total 4-desmethylsterols [mg/100 g oil] | 310.2 ± 39.7 | 302.9 ± 19.3 | 310.9 ± 55.1 |
| cycloartenol [mg/100 g oil] | 8.9 ± 0.2 | 8.4 ± 0.8 | 8.6 ± 1.9 |
| 24-methylenecycloartanol [mg/100 g oil] | 9.5 ± 1.3 | 11.1 ± 0.7 | 9.6 ± 2.2 |
| Total triterpenols [mg/100 g oil] | 18.4 ± 1.1 | 19.5 ± 1.4 | 18.1 ± 4.1 |
| citrostadienol [mg/100 g oil] | 5.9 ± 1.4 | 6.1 ± 1.1 | 7.9 ± 2.4 |
| γ-tocopherol [mg/100 g oil] | 32.3 ± 3.0 b | 35.6 ± 7.9 ab | 50.0 ± 6.8 a |
| POPs [μg/g oil] | 14.47 ± 3.72 | 14.43 ± 5.32 | 17.20 ± 5.54 |
| RI 3 | Kernel Oils | Ground Kernels | |||||
|---|---|---|---|---|---|---|---|
| C 2 | O3 | O3(+) | C | O3 | O3(+) | ||
| 794 | 2-butenoic acid, (E)- | 1374 ± 18 | 1250 ± 354 | 2566 ± 564 | |||
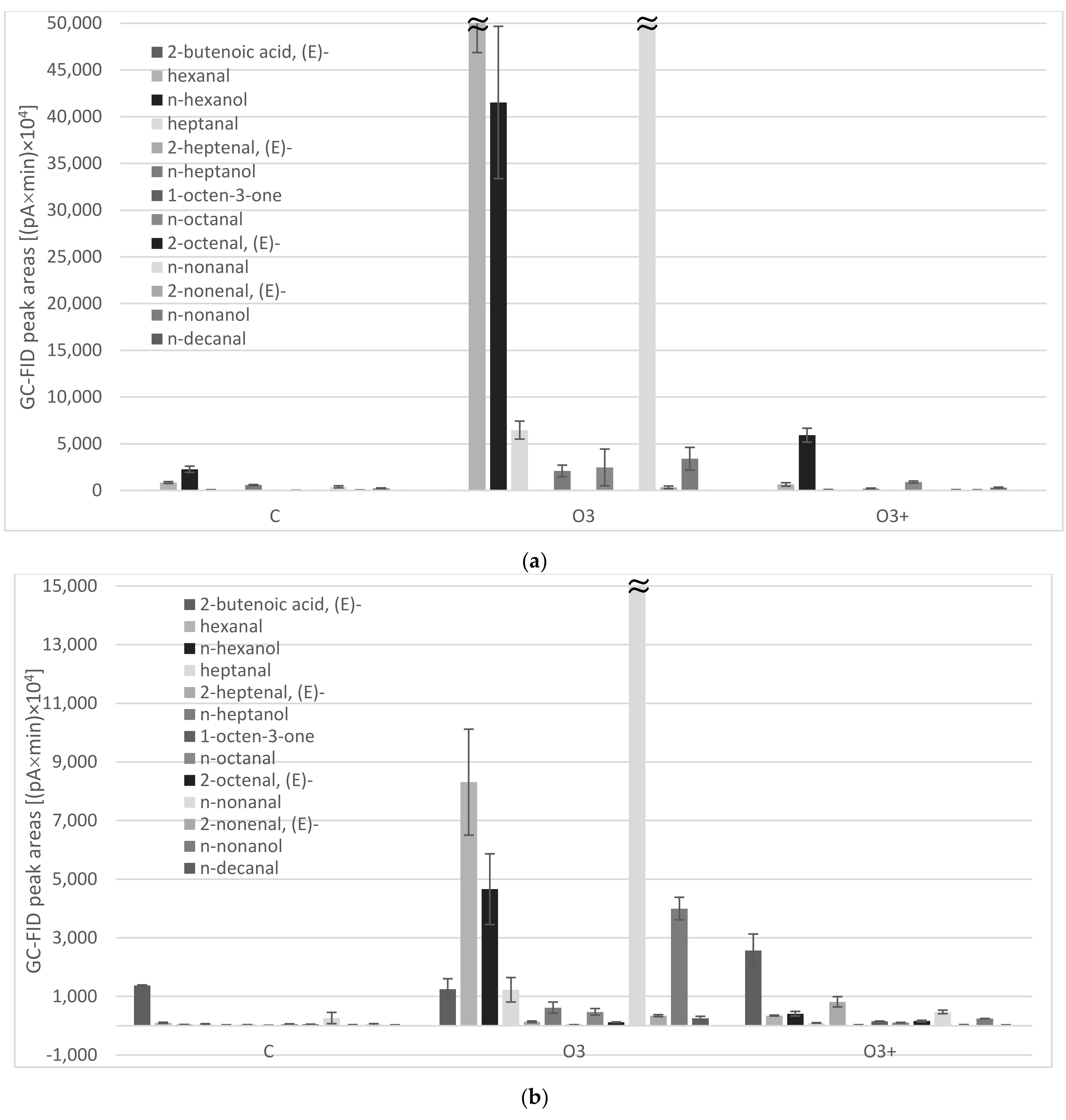
| 799 | hexanal | 101 ± 18 b | 8310 ± 1808 a | 347 ± 17 b | 849 ± 101 b | 54,606 ± 7741 a | 651 ± 187 b |
| 841 | 2-pentanone, 4-hydroxy-4-methyl- | 128 ± 17 b | 113 ± 14 b | 234 ± 18 a | |||
| 870 | n-hexanol | 33 ± 12 b | 4659 ± 1206 a | 410 ± 78 b | 2274 ± 332 b | 41,530 ± 8147 a | 5922 ± 740 b |
| 902 | heptanal | 48 ± 23 b | 1226 ± 417 a | 95 ± 9 b | 75 ± 13 b | 6463 ± 964 a | 105 ± 3 b |
| 909 | butyrolactone | 206 ± 17 | 296 ± 66 | 387 ± 59 | |||
| 930 | β-thujene | 265 ± 7 b | 620 ± 30 a | 657 ± 7 a | |||
| 939 | α-pinene | 996 ± 18 b | 1036 ± 50 b | 1615 ± 221 a | 4390 ± 304 b | 4943 ± 277 b | 17742 ± 322 a |
| 960 | 2-heptenal, (E)- | 28 ± 5 b | 133 ± 30 b | 817 ± 174 a | |||
| 967 | benzaldehyde | 147 ± 15 | 77 ± 26 | 78 ± 16 | |||
| 972 | n-heptanol | 36 ± 2 b | 618 ± 190 a | 26 ± 8 b | 585 ± 57 ab | 2094 ± 623 a | 198 ± 65 b |
| 979 | 1-octen-3-one | 19 ± 1 c | 33 ± 3 b | 158 ± 3 a | |||
| 995 | 3,5-dimethyl-2(5H)-furanone | 214 ± 123 | 143 ± 5 | 276 ± 18 | |||
| 1000 | n-decane | 97 ± 69 | 22 ± 5 | 111 ± 8 | |||
| 1003 | n-octanal | 56 ± 9 c | 475 ± 112 a | 106 ± 8 b | 31 ± 7 c | 2473 ± 1962 a | 902 ± 112 b |
| 1012 | 3-carene | 242 ± 22 | 413 ± 147 | 403 ± 30 | |||
| 1020 | methyl 5-oxohexanoate | 223 ± 31 b | 256 ± 49 ab | 438 ± 67 a | |||
| 1025 | p-cymene | 898 ± 122 a | 843 ± 96 a | 301 ± 7 b | |||
| 1035 | limonene | 629 ± 62 | 943 ± 170 | 806 ± 158 | 7058 ± 698 ab | 10,867 ± 1545 a | 5491 ± 266 b |
| 1038 | β-ocimene | 254 ± 37b | 598 ± 98a | 302 ± 19b | |||
| 1062 | 2-octenal, (E)- | 49 ± 5 b | 119 ± 11 ab | 160 ± 27 a | |||
| 1080 | terpinolene | 468 ± 75 b | 2076 ± 440 a | 872 ± 8 b | |||
| 1100 | n-undecane | 271 ± 32 | 304 ± 64 | 524 ± 93 | 1507 ± 308 | 992 ± 204 | 1511 ± 23 |
| 1105 | n-nonanal | 263 ± 193 b | 36,123 ± 9035 a | 472 ± 60 b | 395 ± 112 b | 72,676 ± 20,053 a | 90 ± 4 b |
| 1163 | 2-nonenal, (E)- | 28 ± 8 b | 344 ± 35 a | 31 ± 8 b | 51 ± 13 b | 337 ± 140 a | 64 ± 17 b |
| 1174 | n-nonanol | 40 ± 33 b | 3998 ± 385 a | 246 ± 1 b | 243 ± 38 b | 3402 ± 1213 a | 315 ± 49 b |
| 1200 | n-dodecane | 292 ± 70 | 484 ± 84 | 545 ± 93 | 1065 ± 257 | 1135 ± 301 | 868 ± 37 |
| 1207 | n-decanal | 22 ± 8 b | 253 ± 69 a | 26 ± 5 b | |||
| 1300 | n-tridecane | 223 ± 2 b | 305 ± 35 ab | 335 ± 16 a | 362 ± 109 | 418 ± 139 | 197 ± 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foligni, R.; Mannozzi, C.; Ismaiel, L.; Capelli, F.; Laurita, R.; Tappi, S.; Dalla Rosa, M.; Mozzon, M. Impact of Cold Atmospheric Plasma (CAP) Treatments on the Oxidation of Pistachio Kernel Lipids. Foods 2022, 11, 419. https://doi.org/10.3390/foods11030419
Foligni R, Mannozzi C, Ismaiel L, Capelli F, Laurita R, Tappi S, Dalla Rosa M, Mozzon M. Impact of Cold Atmospheric Plasma (CAP) Treatments on the Oxidation of Pistachio Kernel Lipids. Foods. 2022; 11(3):419. https://doi.org/10.3390/foods11030419
Chicago/Turabian StyleFoligni, Roberta, Cinzia Mannozzi, Lama Ismaiel, Filippo Capelli, Romolo Laurita, Silvia Tappi, Marco Dalla Rosa, and Massimo Mozzon. 2022. "Impact of Cold Atmospheric Plasma (CAP) Treatments on the Oxidation of Pistachio Kernel Lipids" Foods 11, no. 3: 419. https://doi.org/10.3390/foods11030419
APA StyleFoligni, R., Mannozzi, C., Ismaiel, L., Capelli, F., Laurita, R., Tappi, S., Dalla Rosa, M., & Mozzon, M. (2022). Impact of Cold Atmospheric Plasma (CAP) Treatments on the Oxidation of Pistachio Kernel Lipids. Foods, 11(3), 419. https://doi.org/10.3390/foods11030419












