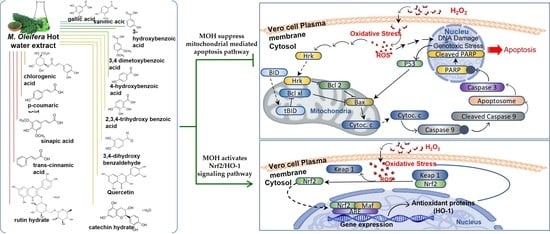
Moringa oleifera Hot Water Extract Protects Vero Cells from Hydrogen Peroxide-Induced Oxidative Stress by Regulating Mitochondria-Mediated Apoptotic Pathway and Nrf2/HO-1 Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Collection and Extraction
2.3. Compositional Analysis of MOH
2.4. High-Performance Liquid Chromatography (HPLC) Analysis of MOH
2.5. Radical Absorbance Capacity of MOH
2.6. Cell Culture
2.7. Cell Viability and ROS Production Analysis
2.8. Evaluation of Apoptotic Body Formation
2.9. Cell Cycle Analysis
2.10. Mitochondrial Depolarization Analysis by JC-1 Assay
2.11. Western Blot Analysis
2.12. Statistical Analysis
3. Results
3.1. Extraction Yield and Proximate Composition of MOH
3.2. Composition of Antioxidant Phytochemicals in MOH
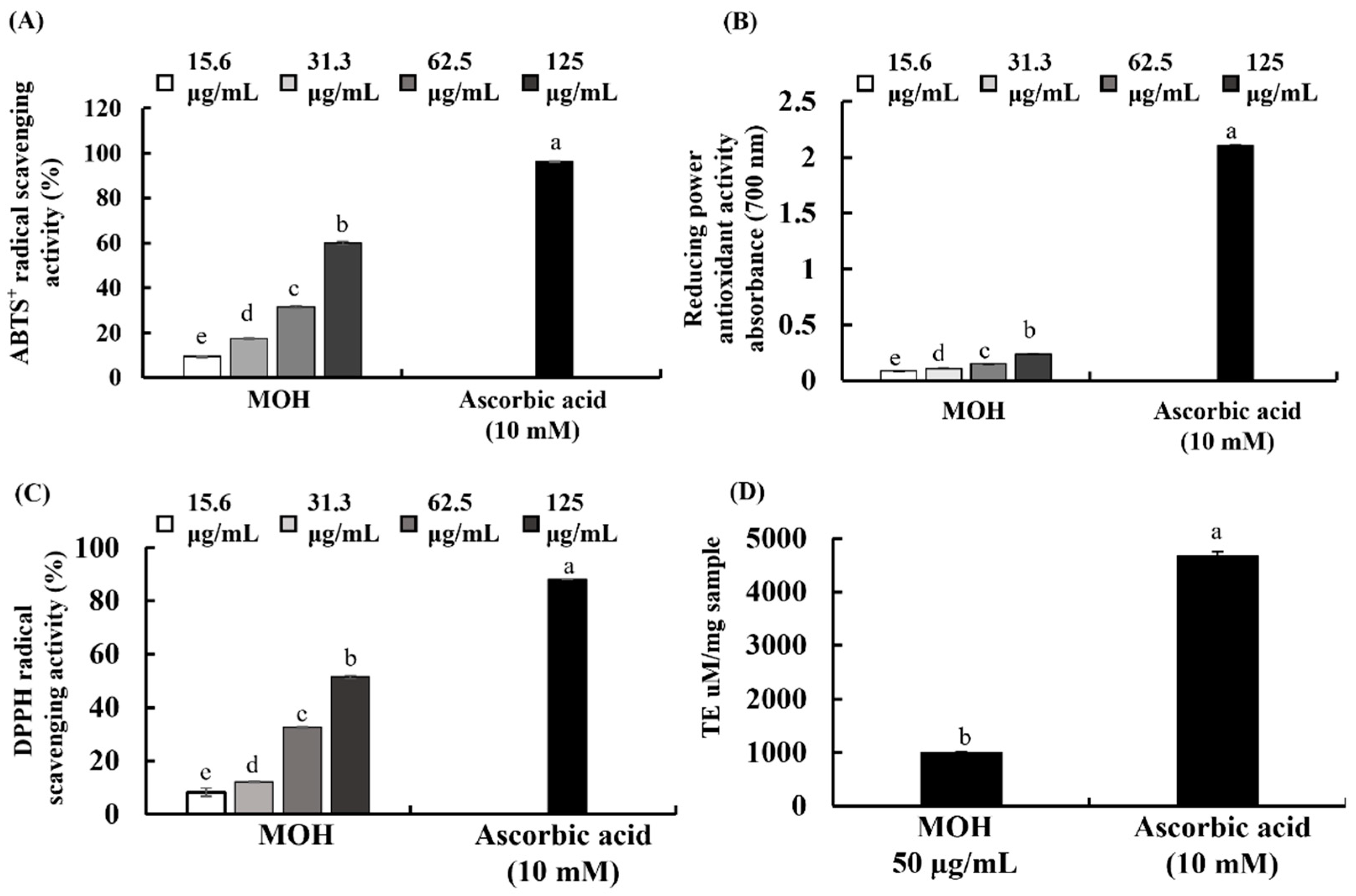
3.3. Antioxidant Activities of MOH
3.4. Effects of MOH on Cell Viability and Intracellular ROS Production
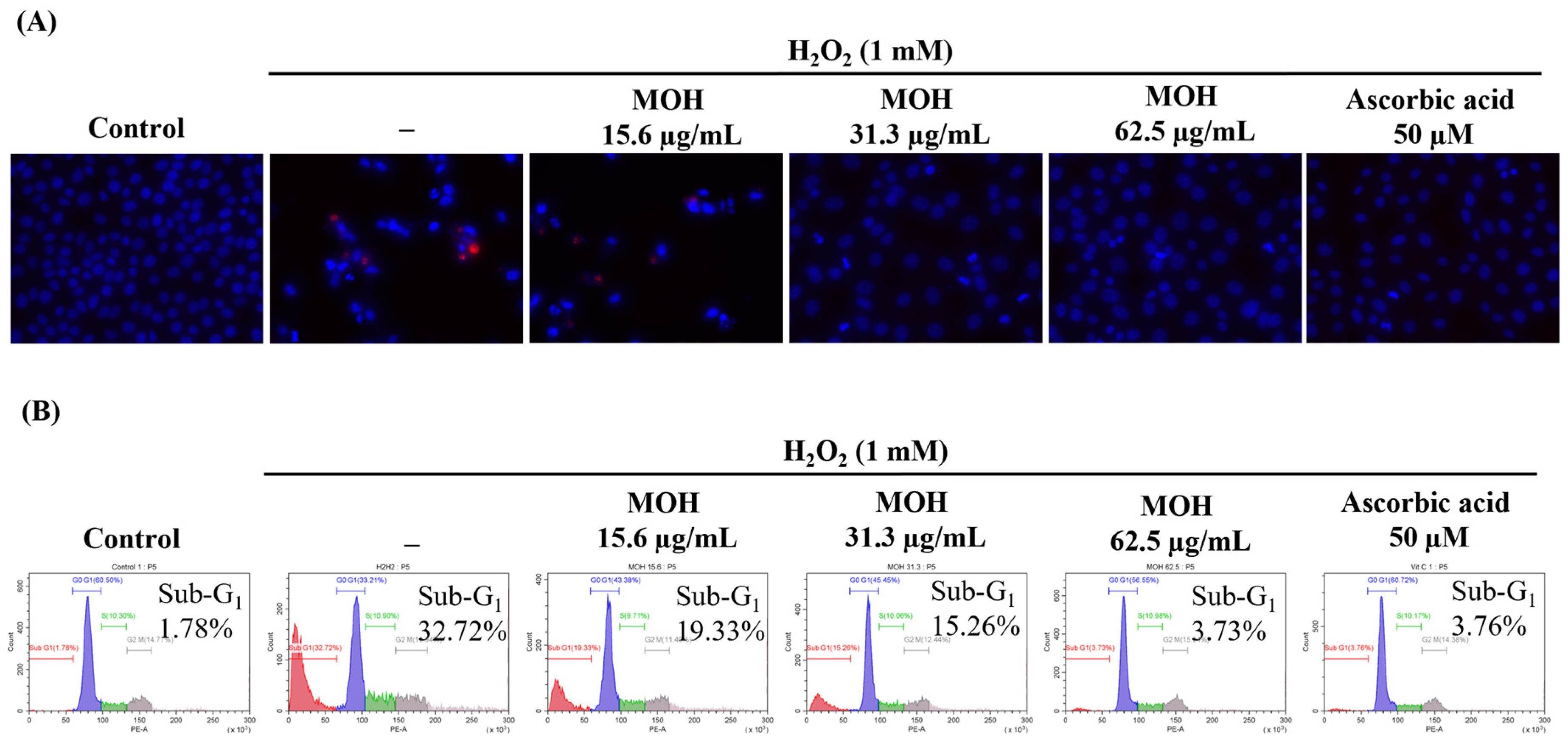
3.5. Effect of MOH on H2O2-Induced Apoptosis
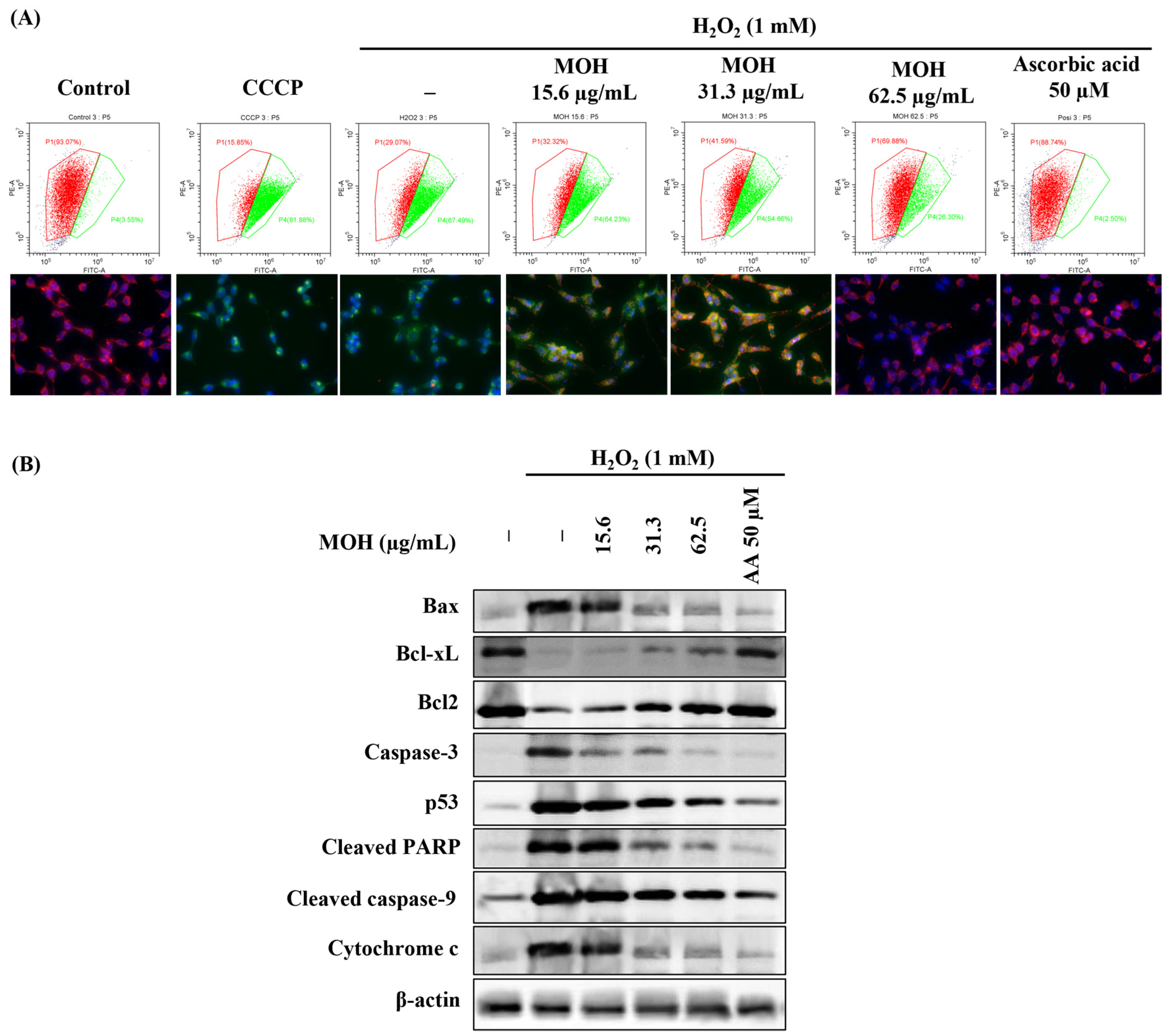
3.6. Effect of MOH on Mitochondrial Depolarization and Expression Levels of the Proteins Related to Mitochondrial Apoptosis Pathway
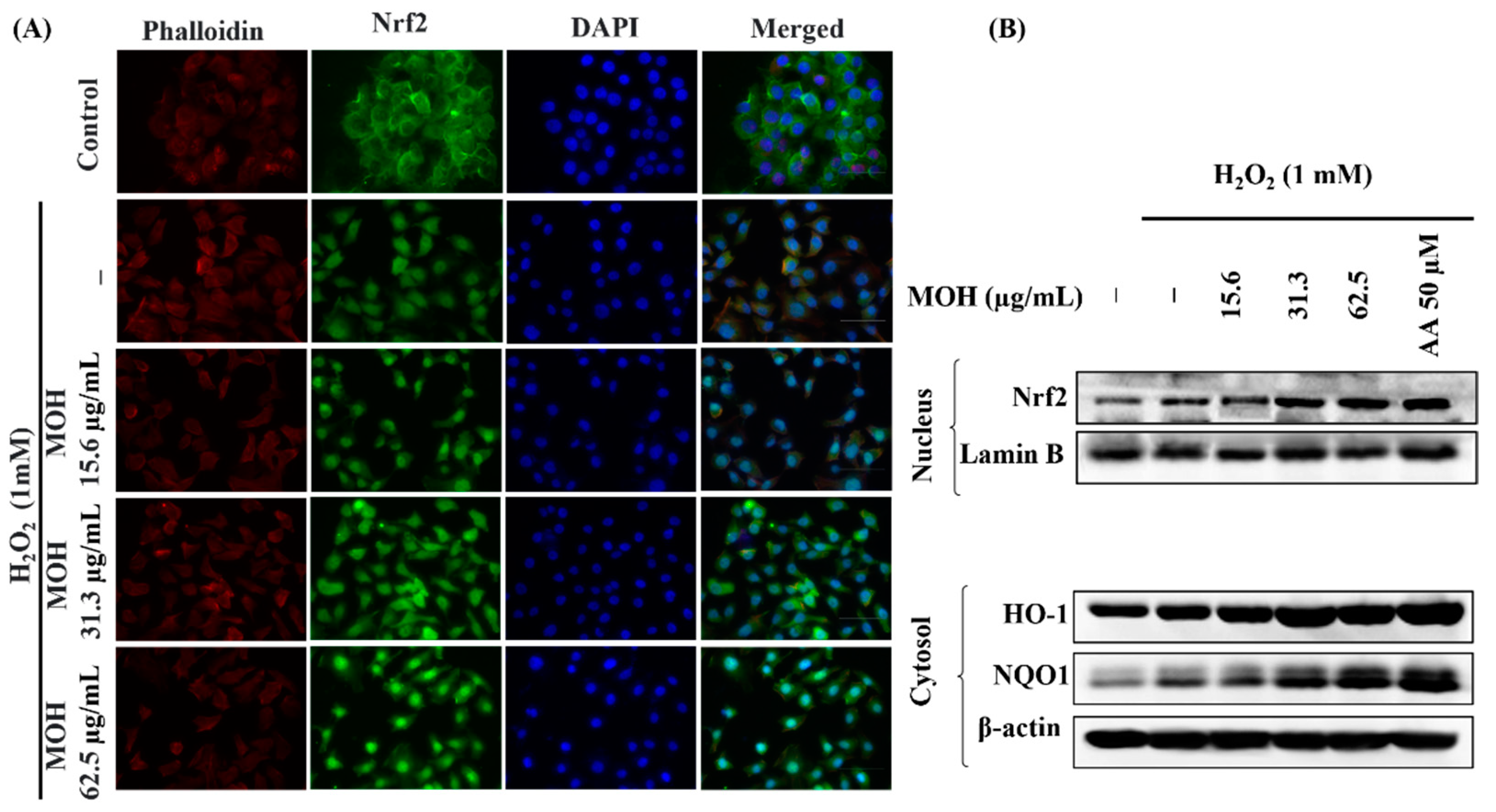
3.7. Effect of MOH on Activation of Nrf2/HO-1/NQO1 Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and oxidative stress in human diseases: From molecular mechanisms to novel treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyedsadjadi, N.; Grant, R. The Potential Benefit of Monitoring Oxidative Stress and Inflammation in the Prevention of Non-Communicable Diseases (NCDs). Antioxidants 2021, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Tabriziani, H.; Lipkowitz, M.S.; Vuong, N. Chronic kidney disease, kidney transplantation and oxidative stress: A new look to successful kidney transplantation. Clin. Kidney J. 2018, 11, 130–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suraweera, T.L.; Rupasinghe, H.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE pathway by dietary flavonoids: A friend or foe for cancer management? Antioxidants 2020, 9, 973. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Oh, J.Y.; Kim, H.S.; Lee, W.; Cui, Y.; Lee, H.G.; Kim, Y.-T.; Ko, J.Y.; Jeon, Y.-J. Protective effect of polysaccharides from Celluclast-assisted extract of Hizikia fusiforme against hydrogen peroxide-induced oxidative stress in vitro in Vero cells and in vivo in zebrafish. Int. J. Biol. Macromol. 2018, 112, 483–489. [Google Scholar] [CrossRef]
- Ozbek, E. Induction of oxidative stress in kidney. Int. J. Nephrol. 2012, 2012, 465897. [Google Scholar] [CrossRef] [Green Version]
- Honda, T.; Hirakawa, Y.; Nangaku, M. The role of oxidative stress and hypoxia in renal disease. Kidney Res. Clin. Pract. 2019, 38, 414. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.-B.; Liu, H.-T.; Chen, S.-Y.; Lin, P.-T.; Lai, C.-Y.; Huang, Y.-C. Changes of oxidative stress, glutathione, and its dependent antioxidant enzyme activities in patients with hepatocellular carcinoma before and after tumor resection. PLoS ONE 2017, 12, e0170016. [Google Scholar] [CrossRef]
- Basile, D.P.; Bonventre, J.V.; Mehta, R.; Nangaku, M.; Unwin, R.; Rosner, M.H.; Kellum, J.A.; Ronco, C. Progression after AKI: Understanding maladaptive repair processes to predict and identify therapeutic treatments. J. Am. Soc. Nephrol. 2016, 27, 687–697. [Google Scholar] [CrossRef]
- Nangaku, M.; Hirakawa, Y.; Mimura, I.; Inagi, R.; Tanaka, T. Epigenetic changes in the acute kidney injury-to-chronic kidney disease transition. Nephron 2017, 137, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Tucker, P.S.; Scanlan, A.T.; Dalbo, V.J. Chronic kidney disease influences multiple systems: Describing the relationship between oxidative stress, inflammation, kidney damage, and concomitant disease. Oxid. Med. Cell. Longev. 2015, 2015, 806358. [Google Scholar] [CrossRef] [PubMed]
- Engwa, G.A. Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases. In Phytochemicals: Source of Antioxidants and Role in Disease Prevention; Asaduzzaman, M., Asao, T., Eds.; IntechOpen: London, UK, 2018; Volume 7, pp. 49–74. [Google Scholar]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; da Silva Lannes, S.C.; da Silva, M.V. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Zhang, J.; Chen, X. Bioactive flavonoids in Moringa oleifera and their health-promoting properties. J. Funct. Foods 2018, 47, 469–479. [Google Scholar] [CrossRef]
- Chumark, P.; Khunawat, P.; Sanvarinda, Y.; Phornchirasilp, S.; Morales, N.P.; Phivthong-Ngam, L.; Ratanachamnong, P.; Srisawat, S.; Klai-upsorn, S.P. The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam. leaves. J. Ethnopharmacol. 2008, 116, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Vongsak, B.; Gritsanapan, W.; Wongkrajang, Y.; Jantan, I. In vitro inhibitory effects of Moringa oleifera leaf extract and its major components on chemiluminescence and chemotactic activity of phagocytes. Nat. Prod. Commun. 2013, 8, 1934578X1300801115. [Google Scholar] [CrossRef] [Green Version]
- Nouman, W.; Anwar, F.; Gull, T.; Newton, A.; Rosa, E.; Domínguez-Perles, R. Profiling of polyphenolics, nutrients and antioxidant potential of germplasm’s leaves from seven cultivars of Moringa oleifera Lam. Ind. Crops Prod. 2016, 83, 166–176. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, M.; Bhowmick, S.; Hussain, J.; Hasan, M.; Hossain, S. Hot Water Extract of Moringa oleifera Leaves Protects Erythrocytes from Hemolysis and Major Organs from Oxidative Stress in vitro. J. Basic Appl. Res. Biomed. 2017, 3, 120–126. [Google Scholar]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Jeon, Y.J. Squalene isolated from marine macroalgae Caulerpa racemosa and its potent antioxidant and anti-inflammatory activities. J. Food Biochem. 2018, 42, e12628. [Google Scholar] [CrossRef]
- Matshediso, P.G.; Cukrowska, E.; Chimuka, L. Development of pressurised hot water extraction (PHWE) for essential compounds from Moringa oleifera leaf extracts. Food Chem. 2015, 172, 423–427. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Packer, L., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Mæhre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I.-J. Protein determination—method matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.S. Phenol-sulfuric acid method for total carbohydrates. In Food Analysis Laboratory Manual; Springer: Boston, MA, USA, 2010; pp. 47–53. [Google Scholar]
- Um, J.H.; Kim, E.-A.; Lee, W.; Kang, N.; Han, E.J.; Oh, J.Y.; Park, S.Y.; Jeon, Y.-J.; Lee, S.-H.; Ahn, G. Protective effects of an enzymatic hydrolysate from Octopus ocellatus meat against hydrogen peroxide-induced oxidative stress in Chang liver cells and zebrafish embryo. In Taurine 10; Lee, D.-H., Schaffer, S.W., Park, E., Kim, H.W., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 603–620. [Google Scholar]
- Fernando, I.S.; Sanjeewa, K.A.; Ann, Y.-S.; Ko, C.-I.; Lee, S.-H.; Lee, W.W.; Jeon, Y.-J. Apoptotic and antiproliferative effects of Stigmast-5-en-3-ol from Dendronephthya gigantea on human leukemia HL-60 and human breast cancer MCF-7 cells. Toxicol. Vitr. 2018, 52, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.-F.; Tsimidou, M.; Zhang, H.-Y. Estimation of scavenging activity of phenolic compounds using the ABTS•+ assay. J. Agric. Food Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-N.; Ham, Y.M.; Moon, J.-Y.; Kim, M.-J.; Jung, Y.-H.; Jeon, Y.-J.; Lee, N.H.; Kang, N.; Yang, H.-M.; Kim, D. Acanthoic acid induces cell apoptosis through activation of the p38 MAPK pathway in HL-60 human promyelocytic leukaemia. Food Chem. 2012, 135, 2112–2117. [Google Scholar] [CrossRef] [PubMed]
- Han, E.-J.; Fernando, I.P.S.; Kim, H.-S.; Lee, D.-S.; Kim, A.; Je, J.-G.; Seo, M.-J.; Jee, Y.-H.; Jeon, Y.-J.; Kim, S.-Y.; et al. (–)-Loliolide Isolated from Sargassum horneri Suppressed Oxidative Stress and Inflammation by Activating Nrf2/HO-1 Signaling in IFN-γ/TNF-α-Stimulated HaCaT Keratinocytes. Antioxidants 2021, 10, 856. [Google Scholar] [CrossRef]
- Kim, D.S.; Choi, M.H.; Shin, H.J. Extracts of Moringa oleifera leaves from different cultivation regions show both antioxidant and antiobesity activities. J. Food Biochem. 2020, 44, e13282. [Google Scholar] [CrossRef]
- Sreelatha, S.; Padma, P. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum. Nutr. 2009, 64, 303–311. [Google Scholar] [CrossRef]
- Uddin, R.; Saha, M.R.; Subhan, N.; Hossain, H.; Jahan, I.A.; Akter, R.; Alam, A. HPLC-analysis of polyphenolic compounds in Gardenia jasminoides and determination of antioxidant activity by using free radical scavenging assays. Adv. Pharm. Bull. 2014, 4, 273. [Google Scholar] [PubMed]
- Stohs, S.J.; Hartman, M.J. Review of the safety and efficacy of Moringa oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Pawlowska, E.; Szczepanska, J.; Koskela, A.; Kaarniranta, K.; Blasiak, J. Dietary Polyphenols in Age-Related Macular Degeneration: Protection against Oxidative Stress and Beyond. Oxid. Med. Cell. Longev. 2019, 2019, 9682318. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Can Plant Phenolic Compounds Protect the Skin from Airborne Particulate Matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanner, J. Polyphenols by Generating H2O2, Affect Cell Redox Signaling, Inhibit PTPs and Activate Nrf2 Axis for Adaptation and Cell Surviving: In Vitro, In Vivo and Human Health. Antioxidants 2020, 9, 797. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, H.-S.; Jeon, Y.-J.; Jee, Y.; Kim, K.-N.; Lee, K.; Fernando, I.P.S.; Ahn, G. Sargassum horneri (Turner) C. Agardh ethanol extract attenuates fine dust-induced inflammatory responses and impaired skin barrier functions in HaCaT keratinocytes. J. Ethnopharmacol. 2021, 273, 114003. [Google Scholar] [CrossRef]
- Sherma, J.; Fried, B. Handbook of Thin-Layer Chromatography; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Miliauskas, G.; Venskutonis, P.; Van Beek, T. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Singh, S.P.; Häder, D.-P.; Sinha, R.P. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′, 7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem. Biophys. Res. Commun. 2010, 397, 603–607. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compost. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Barzegar, A. Antioxidant activity of polyphenolic myricetin in vitro cell-free and cell-based systems. Mol. Biol. Res. Commun. 2016, 5, 87. [Google Scholar] [PubMed]
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Oxidative stress in apoptosis and cancer: An update. Arch. Toxicol. 2012, 86, 1649–1665. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Mu, T.; Wang, G.; Jiang, X. Mitochondria-mediated apoptosis in mammals. Protein Cell 2014, 5, 737–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.-J.; Lee, K.; Cheong, S.H.; Han, Y.S.; Park, S.R. Human keratinocyte UVB-protective effects of a low molecular weight fucoidan from Sargassum horneri purified by step gradient ethanol precipitation. Antioxidants 2020, 9, 340. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Jia, Z.; Zhu, H. Regulation of Nrf2 signaling. React. Oxyg. Species 2019, 8, 312. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.-J.; Ahn, G. Fucoidan refined by Sargassum confusum indicate protective effects suppressing photo-oxidative stress and skin barrier perturbation in UVB-induced human keratinocytes. Int. J. Biol. Macromol. 2020, 164, 149–161. [Google Scholar] [CrossRef]
- Erlank, H.; Elmann, A.; Kohen, R.; Kanner, J. Polyphenols activate Nrf2 in astrocytes via H2O2, semiquinones, and quinones. Free Radic. Biol. Med. 2011, 51, 2319–2327. [Google Scholar] [CrossRef]

| MOH | Composition (%) |
|---|---|
| Yield | 35.67 ± 0.44 |
| Protein | 19.01 ± 0.27 |
| Polysaccharide | 44.95 ± 0.66 |
| Polyphenol | 5.24 ± 0.07 |
| Phenolic Compound | μmol/100 g |
|---|---|
| Gallic acid | 78.24 ± 0.18 |
| D-mandelic acid | Not detected |
| 2,3,4-trihydroxybenzoic acid | 79.00 ± 0.06 |
| 3,4-dehydroxybenzaldehyde | 96.87 ± 0.43 |
| 4-hydroxybenzoic acid | 97.31 ± 0.14 |
| Gentisic acid sodium salt hydrate | Not detected |
| Catechin hydrate | 43.34 ± 0.26 |
| Vanillic acid | 79.52 ± 0.36 |
| 3-hydroxy benzoic acid | 95.64 ± 0.36 |
| Chlorogenic acid | 35.73 ± 0.03 |
| Syringic acid | Not detected |
| p-coumaric acid | 81.07 ± 0.12 |
| 3,4 dimethoxy benzoic acid | 18.33 ± 1.26 |
| Sinapic acid | 60.08 ± 0.13 |
| Rutin hydrate | 1.11 ± 0.43 |
| Trans-cinnamic acid | 91.66 ± 0.00 |
| Quercetin | 45.89 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirindage, K.G.I.S.; Fernando, I.P.S.; Jayasinghe, A.M.K.; Han, E.-J.; Dias, M.K.H.M.; Kang, K.-P.; Moon, S.-I.; Shin, T.-S.; Ma, A.; Ahn, G. Moringa oleifera Hot Water Extract Protects Vero Cells from Hydrogen Peroxide-Induced Oxidative Stress by Regulating Mitochondria-Mediated Apoptotic Pathway and Nrf2/HO-1 Signaling. Foods 2022, 11, 420. https://doi.org/10.3390/foods11030420
Kirindage KGIS, Fernando IPS, Jayasinghe AMK, Han E-J, Dias MKHM, Kang K-P, Moon S-I, Shin T-S, Ma A, Ahn G. Moringa oleifera Hot Water Extract Protects Vero Cells from Hydrogen Peroxide-Induced Oxidative Stress by Regulating Mitochondria-Mediated Apoptotic Pathway and Nrf2/HO-1 Signaling. Foods. 2022; 11(3):420. https://doi.org/10.3390/foods11030420
Chicago/Turabian StyleKirindage, Kirinde Gedara Isuru Sandanuwan, Ilekuttige Priyan Shanura Fernando, Arachchige Maheshika Kumari Jayasinghe, Eui-Jeong Han, Mawalle Kankanamge Hasitha Madhawa Dias, Kyung-Pil Kang, Sung-Ig Moon, Tai-Sun Shin, Ayeong Ma, and Ginnae Ahn. 2022. "Moringa oleifera Hot Water Extract Protects Vero Cells from Hydrogen Peroxide-Induced Oxidative Stress by Regulating Mitochondria-Mediated Apoptotic Pathway and Nrf2/HO-1 Signaling" Foods 11, no. 3: 420. https://doi.org/10.3390/foods11030420
APA StyleKirindage, K. G. I. S., Fernando, I. P. S., Jayasinghe, A. M. K., Han, E.-J., Dias, M. K. H. M., Kang, K.-P., Moon, S.-I., Shin, T.-S., Ma, A., & Ahn, G. (2022). Moringa oleifera Hot Water Extract Protects Vero Cells from Hydrogen Peroxide-Induced Oxidative Stress by Regulating Mitochondria-Mediated Apoptotic Pathway and Nrf2/HO-1 Signaling. Foods, 11(3), 420. https://doi.org/10.3390/foods11030420