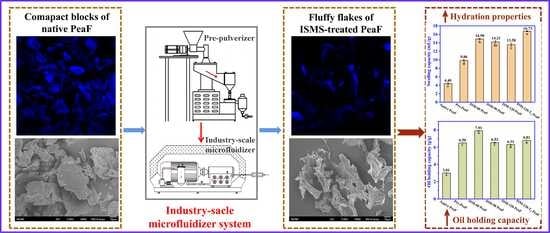
Disintegrating the Structure and Improving the Functionalities of Pea Fiber by Industry-Scale Microfluidizer System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. ISMS Treatment of Pea Fiber
2.3. Particle Size
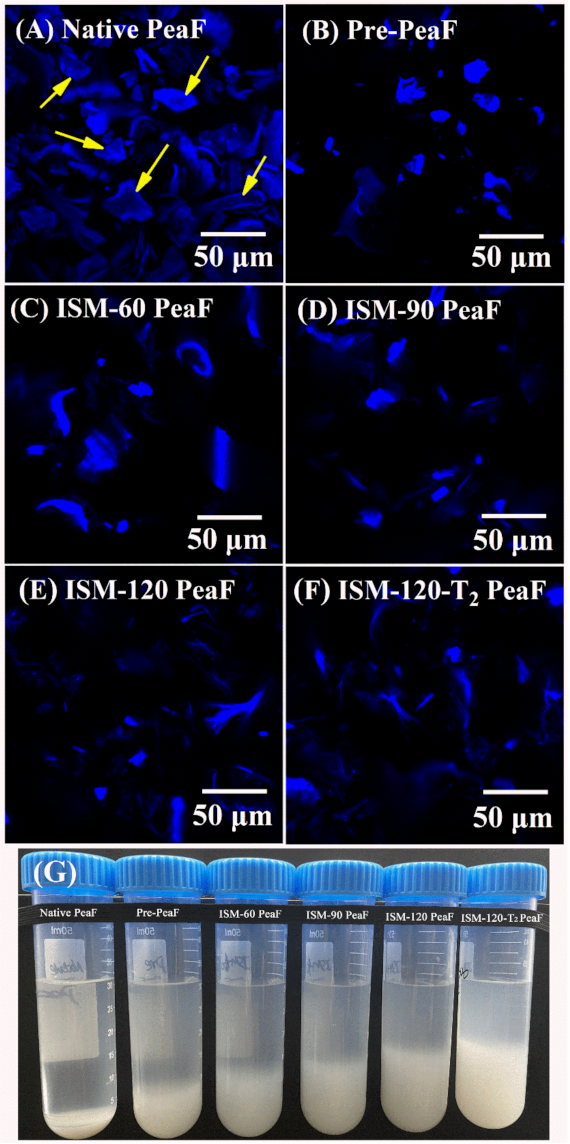
2.4. Confocal Laser Scanning Microscopy (CLSM) Analysis
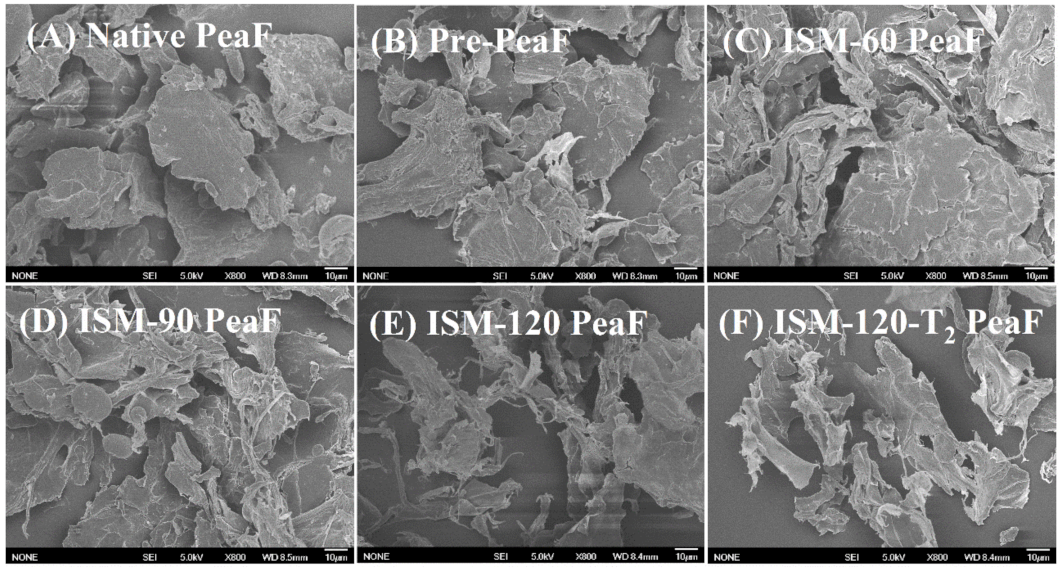
2.5. Scanning Electron Microscopy (SEM) Analysis
2.6. Bulk Density Analysis
2.7. X-ray Diffraction (XRD) Analysis
2.8. Measurement of Hydration Properties
2.9. Measurement of Oil Holding Capacity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Particle Size Characteristics
3.2. CLSM
3.3. Morphology
3.4. Bulk Density
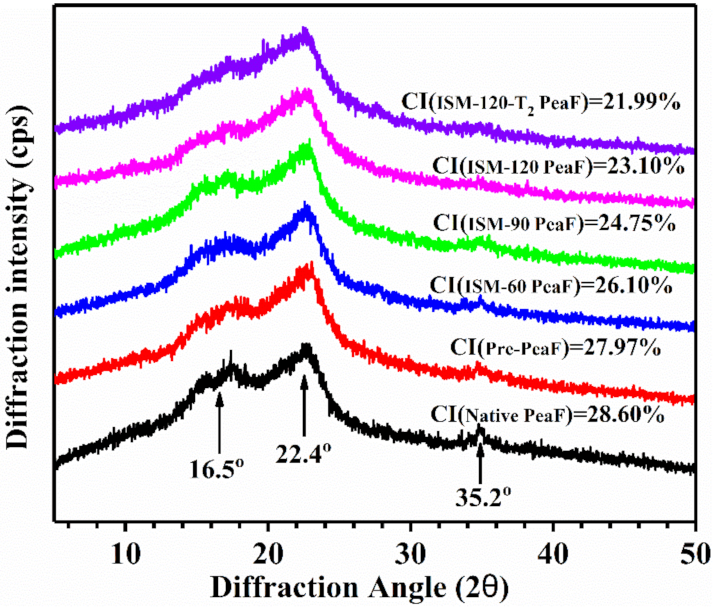
3.5. XRD Analysis
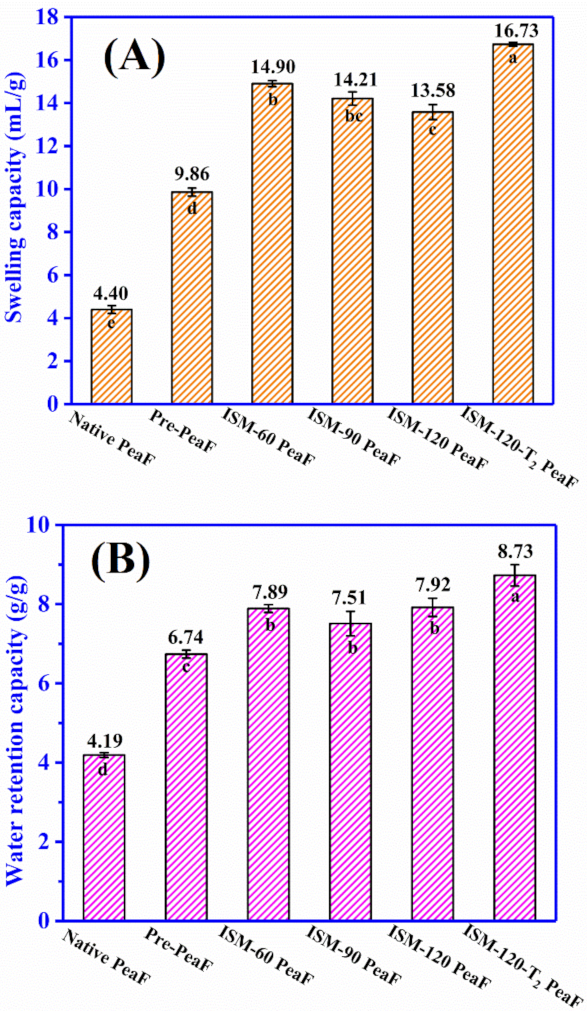
3.6. Hydration Properties
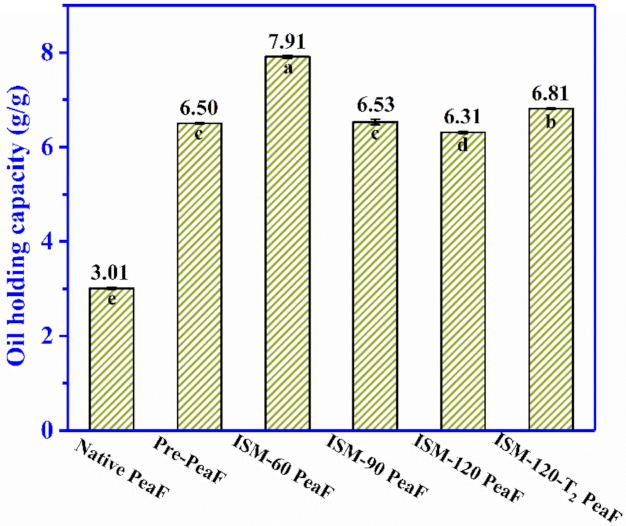
3.7. Oil Holding Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutohrlein, F.; Morales-Medina, R.; Boje, A.L.; Drusch, S.; Schalow, S. Modulating the hydration properties of pea hull fibre by its composition as affected by mechanical processing and various extraction procedures. Food Hydrocoll. 2020, 107, 105958. [Google Scholar] [CrossRef]
- Muneer, F.; Johansson, E.; Hedenqvist, M.S.; Plivelic, T.S.; Markedal, K.E.; Petersen, I.L.; Sorensen, J.C.; Kuktaite, R. The impact of newly produced protein and dietary fiber rich fractions of yellow pea (Pisum sativum L.) on the structure and mechanical properties of pasta-like sheets. Food Res. Int. 2018, 106, 607–618. [Google Scholar] [CrossRef]
- Gan, J.P.; Xie, L.; Peng, G.Y.; Xie, J.H.; Chen, Y.; Yu, Q. Systematic review on modification methods of dietary fiber. Food Hydrocoll. 2021, 119, 106872. [Google Scholar] [CrossRef]
- Su, D.B.; Zhu, X.D.; Wang, Y.; Li, D.; Wang, L.J. Effect of high-pressure homogenization on rheological properties of citrus fiber. LWT-Food Sci. Technol. 2020, 127, 109366. [Google Scholar] [CrossRef]
- Bruno, E.; Lupi, F.R.; Martin-Pinero, M.J.; Girimonte, R.; Baldino, N.; Munoz, J.; Gabriele, D. Influence of different dispersing systems on rheological and microstructural properties of citrus fiber suspensions. LWT-Food Sci. Technol. 2021, 152, 112270. [Google Scholar] [CrossRef]
- Xie, F.; Li, M.; Lan, X.H.; Zhang, W.; Gong, S.X.; Wu, J.H.; Wang, Z.W. Modification of dietary fibers from purple-fleshed potatoes (Heimeiren) with high hydrostatic pressure and high pressure homogenization processing: A comparative study. Innov. Food Sci. Emerg. Technol. 2017, 42, 157–164. [Google Scholar] [CrossRef]
- Luo, X.L.; Wang, Q.; Fang, D.Y.; Zhuang, W.J.; Chen, C.H.; Jiang, W.T.; Zheng, Y.F. Modification of insoluble dietary fibers from bamboo shoot shell: Structural characterization and functional properties. Int. J. Biol. Macromol. 2018, 120, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.Y.; Bei, J.; Zhao, J.; Li, Q.H.; Cheng, C. Modification of carrot (Daucus carota Linn. var. Sativa Hoffm.) pomace insoluble dietary fiber with complex enzyme method, ultrafine comminution, and high hydrostatic pressure. Food Chem. 2018, 257, 333–340. [Google Scholar] [CrossRef]
- Guo, X.J.; Chen, M.S.; Li, Y.T.; Dai, T.T.; Shuai, X.X.; Chen, J.; Liu, C.M. Modification of food macromolecules using dynamic high pressure microfluidization: A review. Trends Food Sci. Technol. 2020, 100, 223–234. [Google Scholar] [CrossRef]
- Ozturk, O.K.; Turasan, H. Latest developments in the applications of microfluidization to modify the structure of macromolecules leading to improved physicochemical and functional properties. Crit. Rev. Food Sci. Nutr. 2021, 1–23. [Google Scholar] [CrossRef]
- Morales-Medina, R.; Dong, D.; Schalow, S.; Drusch, S. Impact of microfluidization on the microstructure and functional properties of pea hull fibre. Food Hydrocoll. 2020, 103, 105660. [Google Scholar] [CrossRef]
- Guo, X.J.; McClements, D.J.; Chen, J.; He, X.M.; Liu, W.; Dai, T.T.; Liu, C.M. The nutritional and physicochemical properties of whole corn slurry prepared by a novel industry-scale microfluidizer system. LWT-Food Sci. Technol. 2021, 144, 111096. [Google Scholar] [CrossRef]
- He, X.H.; Luo, S.J.; Chen, M.S.; Xia, W.; Chen, J.; Liu, C.M. Effect of industry-scale microfluidization on structural and physicochemical properties of potato starch. Innov. Food Sci. Emerg. Technol. 2020, 60, 102278. [Google Scholar] [CrossRef]
- Li, Y.T.; Chen, M.S.; Deng, L.Z.; Liang, Y.Z.; Liu, Y.K.; Liu, W.; Chen, J.; Liu, C.M. Whole soybean milk produced by a novel industry-scale micofluidizer system without soaking and filtering. J. Food Eng. 2021, 291, 110228. [Google Scholar] [CrossRef]
- Chen, J.L.; Gao, D.X.; Yang, L.T.; Gao, Y.X. Effect of microfluidization process on the functional properties of insoluble dietary fiber. Food Res. Int. 2013, 54, 1821–1827. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, S.R.; Guan, X.; Li, C.; Li, S.; Liu, Y.Y.; Shi, J.L. Effect of the oat beta-glucan on the development of functional quinoa (Chenopodium quinoa wild) milk. Food Chem. 2021, 349, 129201. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sun, X.H.; Zhou, Z.X.; Chen, G.B. Effects of microfluidization process on physicochemical properties of wheat bran. Food Res. Int. 2012, 48, 742–747. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhang, H.B.; Yi, C.P.; Quan, K.; Lin, B.P. Chemical composition, structure, physicochemical and functional properties of rice bran dietary fiber modified by cellulase treatment. Food Chem. 2021, 342, 128352. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Mateos-Peinado, C.; Ruperez, P. High hydrostatic pressure improves the functionality of dietary fibre in okara by-product from soybean. Innov. Food Sci. Emerg. Technol. 2010, 11, 445–450. [Google Scholar] [CrossRef]
- Jiang, G.H.; Bai, X.S.; Wu, Z.G.; Li, S.J.; Zhao, C.; Ramachandraiah, K. Modification of ginseng insoluble dietary fiber through alkaline hydrogen peroxide treatment and its impact on structure, physicochemical and functional properties. LWT-Food Sci. Technol. 2021, 150, 111956. [Google Scholar] [CrossRef]
- Meng, X.M.; Liu, F.; Xiao, Y.; Cao, J.W.; Wang, M.; Duan, X.C. Alterations in physicochemical and functional properties of buckwheat straw insoluble dietary fiber by alkaline hydrogen peroxide treatment. Food Chem. X 2019, 3, 100029. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Yin, T.; Xiong, S.B.; Zhang, J.; Din, Z.U.; Zhang, M.L. Structural characteristics and physicochemical properties of okara (soybean residue) insoluble dietary fiber modified by high-energy wet media milling. LWT-Food Sci. Technol. 2017, 82, 15–22. [Google Scholar] [CrossRef]
- Wang, T.; Sun, X.H.; Raddatz, J.; Chen, G.B. Effects of microfluidization on microstructure and physicochemical properties of corn bran. J. Cereal Sci. 2013, 58, 355–361. [Google Scholar] [CrossRef]
- Ma, M.M.; Mu, T.H. Effects of extraction methods and particle size distribution on the structural, physicochemical, and functional properties of dietary fiber from deoiled cumin. Food Chem. 2016, 194, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.F.; Cai, Y.J.; Liu, T.X.; Huang, L.H.; Deng, X.L.; Zhao, Q.Z.; Zhao, M.M. Improvements in physicochemical and emulsifying properties of insoluble soybean fiber by physical-chemical treatments. Food Hydrocoll. 2019, 93, 167–175. [Google Scholar] [CrossRef]
- Sun, C.C.; Wu, X.F.; Chen, X.J.; Li, X.J.; Zheng, Z.; Jiang, S.W. Production and characterization of okara dietary fiber produced by fermentation with Monascus Anka. Food Chem. 2020, 316, 126243. [Google Scholar] [CrossRef]
- Chau, C.F.; Wen, Y.L.; Wang, Y.T. Effects of micronisation on the characteristics and physicochemical properties of insoluble fibres. J. Sci. Food Agric. 2006, 86, 2380–2386. [Google Scholar] [CrossRef]
- Deleris, I.; Wallecan, J. Relationship between processing history and functionality recovery after rehydration of dried cellulose-based suspensions: A critical review. Adv. Colloid Interface Sci. 2017, 246, 1–12. [Google Scholar] [CrossRef]
- Gupta, P.; Premavalli, K.S. Effect of particle size reduction on physicochemical properties of ashgourd (Benincasa hispida) and radish (Raphanus sativus) fibres. Int. J. Food Sci. Nutr. 2010, 61, 18–28. [Google Scholar] [CrossRef]
- Raghavendra, S.N.; Swamy, S.R.R.; Rastogi, N.K.; Raghavarao, K.; Kumar, S.; Tharanathan, R.N. Grinding characteristics and hydration properties of coconut residue: A source of dietary fiber. J. Food Eng. 2006, 72, 281–286. [Google Scholar] [CrossRef]
- Chen, J.J.; Zhao, Q.S.; Wang, L.W.; Zha, S.H.; Zhang, L.J.; Zhao, B. Physicochemical and functional properties of dietary fiber from maca (Lepidium meyenii Walp.) liquor residue. Carbohydr. Polym. 2015, 132, 509–512. [Google Scholar] [CrossRef] [PubMed]
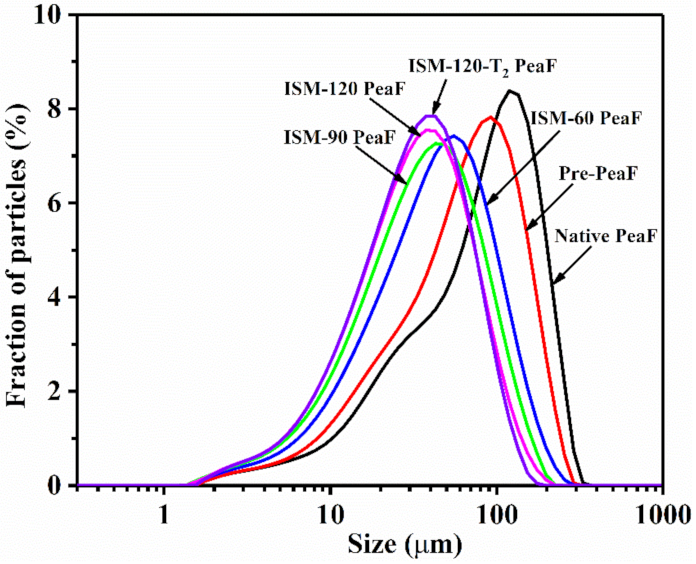

| Samples | Specific Surface Area (m2/kg) | D[3,2] (μm) | D[4,3] (μm) | D(50) (μm) | D(90) (μm) | Span |
|---|---|---|---|---|---|---|
| native PeaF | 173.6 ± 1.7e | 34.5 ± 0.4a | 92.6 ± 0.5a | 82.8 ± 1.2a | 185.0 ± 1.4a | 2.03 ± 0.05b |
| Pre-PeaF | 197.3 ± 3.1d | 30.4 ± 0.4b | 72.7 ± 3.3b | 64.2 ± 1.0b | 145.0 ± 9.9b | 2.03 ± 0.13b |
| ISM-60 PeaF | 256.6 ± 0.1c | 23.5 ± 0.1c | 52.9 ± 0.4c | 43.1 ± 0.1c | 109.0 ± 1.4c | 2.26 ± 0.03a |
| ISM-90 PeaF | 293.4 ± 0.4b | 20.4 ± 0.1d | 45.0 ± 0.1d | 36.1 ± 0.1d | 93.2 ± 0.14d | 2.31 ± 0.01a |
| ISM-120 PeaF | 309.4 ± 1.3a | 19.4 ± 0.1e | 40.5 ± 0.4e | 32.7 ± 0.1e | 82.5 ± 1.0e | 2.23 ± 0.02a |
| ISM-T2-120 PeaF | 310.0 ± 2.8a | 19.3 ± 0.2e | 38.3 ± 0.6e | 32.1 ± 0.3e | 76.2 ± 1.3e | 2.08 ± 0.01b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Dai, T.; Sun, J.; Liang, R.; Liu, W.; Chen, M.; Chen, J.; Liu, C. Disintegrating the Structure and Improving the Functionalities of Pea Fiber by Industry-Scale Microfluidizer System. Foods 2022, 11, 418. https://doi.org/10.3390/foods11030418
He X, Dai T, Sun J, Liang R, Liu W, Chen M, Chen J, Liu C. Disintegrating the Structure and Improving the Functionalities of Pea Fiber by Industry-Scale Microfluidizer System. Foods. 2022; 11(3):418. https://doi.org/10.3390/foods11030418
Chicago/Turabian StyleHe, Xiaohong, Taotao Dai, Jian Sun, Ruihong Liang, Wei Liu, Mingshun Chen, Jun Chen, and Chengmei Liu. 2022. "Disintegrating the Structure and Improving the Functionalities of Pea Fiber by Industry-Scale Microfluidizer System" Foods 11, no. 3: 418. https://doi.org/10.3390/foods11030418
APA StyleHe, X., Dai, T., Sun, J., Liang, R., Liu, W., Chen, M., Chen, J., & Liu, C. (2022). Disintegrating the Structure and Improving the Functionalities of Pea Fiber by Industry-Scale Microfluidizer System. Foods, 11(3), 418. https://doi.org/10.3390/foods11030418