Development of a Stepwise Algorithm for Supercooling Storage of Pork Belly and Chicken Breast and Its Effect on Freshness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
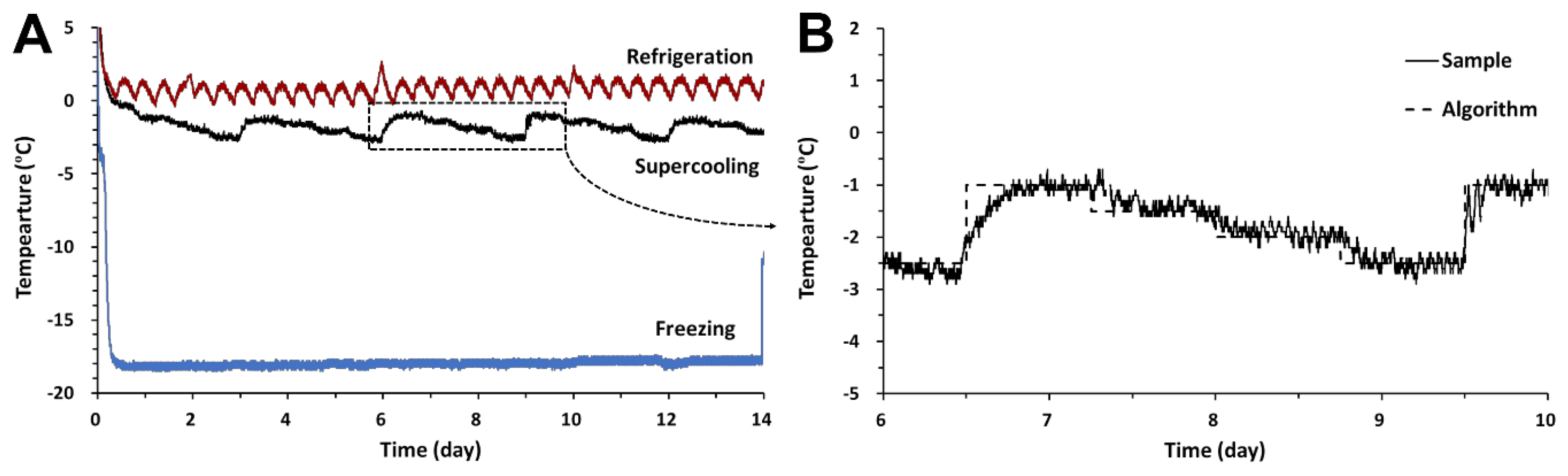
2.2. Storage Temperature Conditions
2.3. Total Volatile Basic Nitrogen (TVBN)
2.4. Thioabarbituric Acid Reactive Substances (TBARS)
2.5. Microbial Analysis
2.6. Drip Loss
2.7. Water Holding Capacity (WHC)
2.8. Cooking Loss
2.9. Color
2.10. Statistical Analysis
3. Results and Discussion
3.1. Time–Temperature Profile
3.2. Chemical Quality Properties
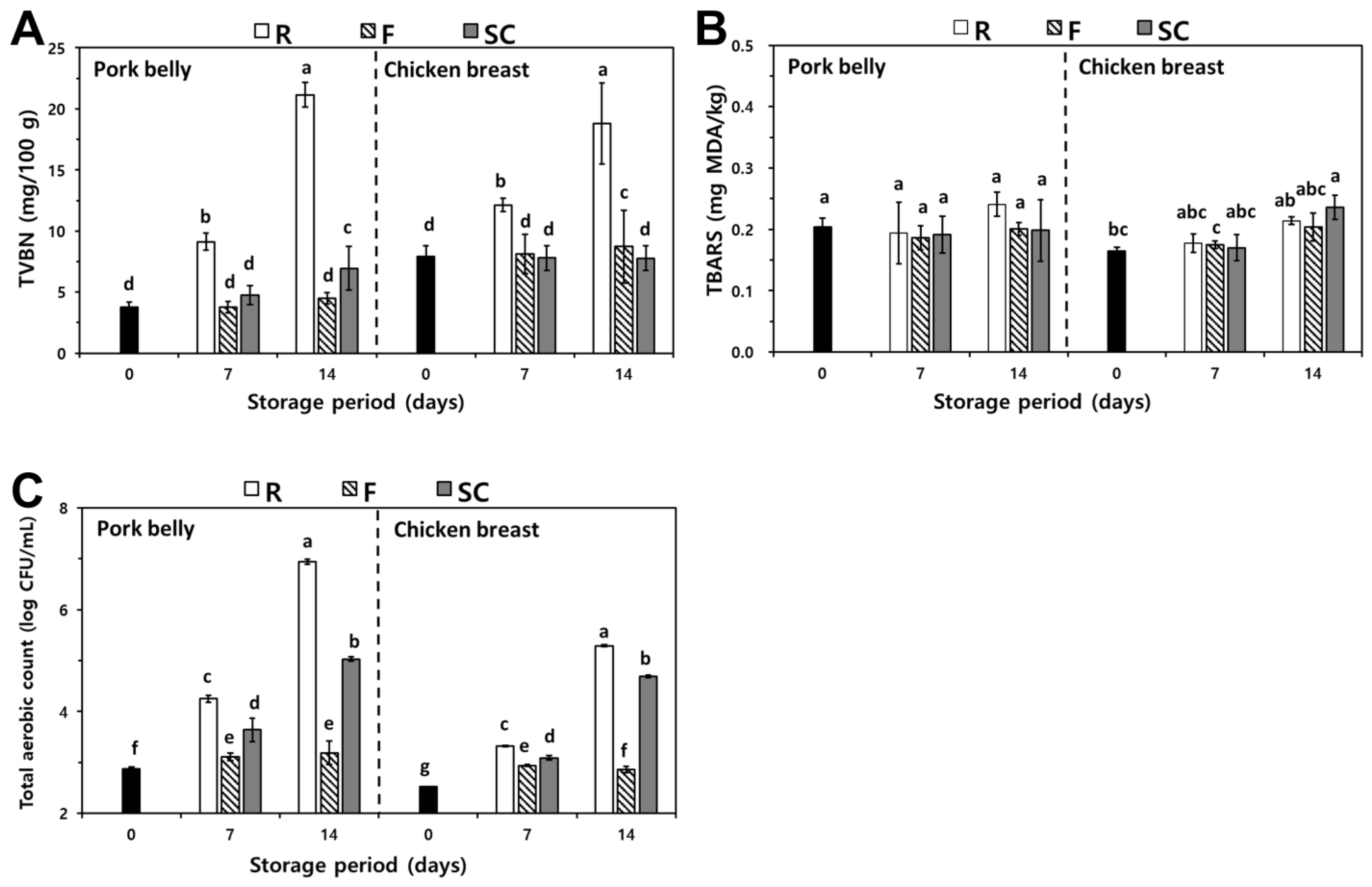
3.2.1. TVBN
3.2.2. TBARS
3.2.3. TAC
3.3. Physical Properties Quality
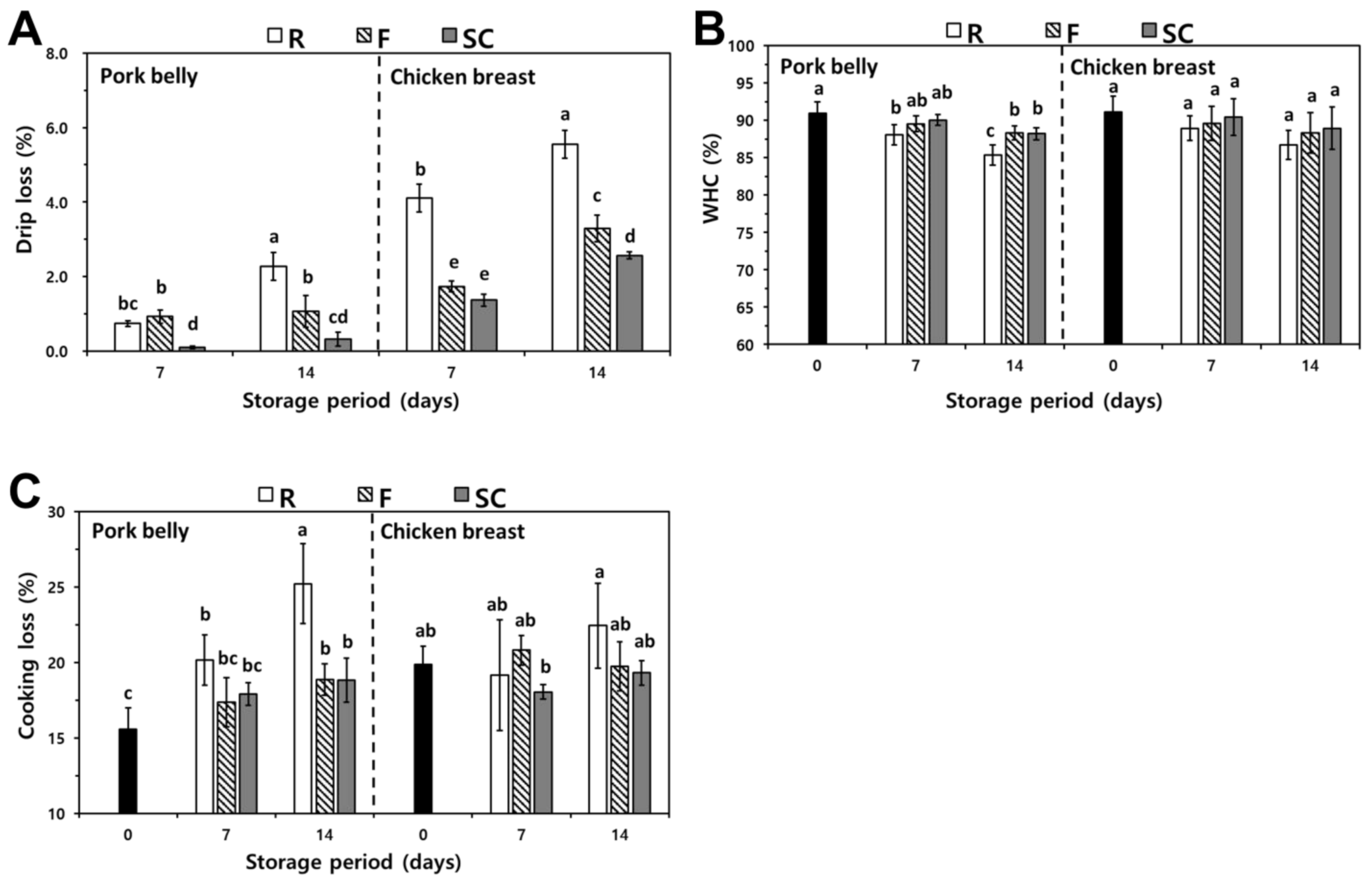
3.3.1. Drip Loss
3.3.2. WHC
3.3.3. Cooking Loss
3.3.4. Color and Appearance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hwang, Y.-H.; Sabikun, N.; Ismail, I.; Joo, S.-T. Comparison of Meat Quality Characteristics of Wet and Dry–aging Pork Belly and Shoulder Blade. Korean J. Food Sci. Anim. Resour. 2018, 38, 950–958. [Google Scholar] [CrossRef] [Green Version]
- Ha, J.-H.; Lee, J.-H.; Lee, J.-J.; Choi, Y.-I.; Lee, H.-J. Effects of Whey Protein Injection as a Curing Solution on Chicken Breast Meat. Food Sci. Anim. Resour. 2019, 39, 494–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, Y.; Her, J.-Y.; Shafel, T.; Kang, T.; Jun, S. Supercooling preservation on quality of beef steak. J. Food Eng. 2019, 274, 109840. [Google Scholar] [CrossRef]
- Stonehouse, G.; Evans, J. The use of supercooling for fresh foods: A review. J. Food Eng. 2015, 148, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Kang, T.; You, Y.; Jun, S. Supercooling preservation technology in food and biological samples: A review focused on electric and magnetic field applications. Food Sci. Biotechnol. 2020, 29, 303–321. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-J.; Abduzukhurov, T.; Park, D.H.; Kim, E.J.; Hong, G.-P. Effects of Deep Freezing Temperature for Long-term Storage on Quality Characteristics and Freshness of Lamb Meat. Food Sci. Anim. Resour. 2018, 38, 959–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuma, Y.; Yamane, A.; Itoh, T.; Tsukamasa, Y.; Ando, M. Application of supercooling to long-term storage of fish meat. Fish. Sci. 2012, 78, 451–461. [Google Scholar] [CrossRef]
- Lee, S.Y. Feasible Strategies to Improve Freshness and Wholesomeness of Pork Loin During Extended Storage. Ph.D. Thesis, Konkuk University, Seoul, Korea, 2020. [Google Scholar]
- Dalvi-Isfahan, M.; Hamdami, N.; Xanthakis, E.; Le-Bail, A. Review on the control of ice nucleation by ultrasound waves, electric and magnetic fields. J. Food Eng. 2017, 195, 222–234. [Google Scholar] [CrossRef]
- Berendsen, T.A.; Bruinsma, B.G.; Puts, C.F.; Saeidi, N.; Usta, O.B.; Uygun, B.E.; Izamis, M.-L.; Toner, M.; Yarmush, M.L.; Uygun, K. Supercooling enables long-term transplantation survival following 4 days of liver preservation. Nat. Med. 2014, 20, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Lee, S.; Park, D.H.; Kim, H.; Choi, M.-J. Physicochemical Properties of Pork Neck and Chicken Leg Meat under Various Freezing Temperatures in a Deep Freezer. Food Sci. Anim. Resour. 2020, 40, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hong, G.-P. Effects of Artificial Supercooling Followed by Slow Freezing on the Microstructure and Qualities of Pork Loin. Food Sci. Anim. Resour. 2016, 36, 650–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, D.; Zhou, C.; Ge, X.; Ye, K.; Wang, P.; Bai, Y.; Zhou, G. The effect of different degrees of superchilling on shelf life and quality of pork during storage. J. Food Process. Preserv. 2020, 44. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Liang, L.; Xia, W.; Regenstein, J.M.; Zhou, P. Biochemical and physical changes of grass carp (Ctenopharyngodon idella) fillets stored at −3 and 0 °C. Food Chem. 2013, 140, 105–114. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, L.; Yang, H.; Xiao, Y.; Xiong, Y.L. Super-chilling (−0.7 °C) with high-CO2 packaging inhibits biochemical changes of microbial origin in catfish (Clarias gariepinus) muscle during storage. Food Chem. 2016, 206, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.B.; da Silva, M.V.; Lannes, S.C.D.S. Lipid oxidation in meat: Mechanisms and protective factors—A review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.I.; Cho, H.G.; Kim, I.S. Animal products and processing: A study on the physicochemical and storage characteristics of domestic chilled pork. Korean J. Anim. Sci. 1998, 40, 59–68. [Google Scholar]
- Kim, S.K.; Lee, M.S.; Lee, K.T.; Park, S.K.; Song, K.B. Changes in quality of pork and beef during storage and electronic nose analysis. Korean J. Food Preserv. 2004, 11, 441–447. [Google Scholar]
- Lu, X.; Zhang, Y.; Zhu, L.; Luo, X.; Hopkins, D. Effect of superchilled storage on shelf life and quality characteristics of M. longissimus lumborum from Chinese Yellow cattle. Meat Sci. 2018, 149, 79–84. [Google Scholar] [CrossRef]
- Dawson, P.L.; Chaves, B.D.; Northcutt, J.K.; Han, I.Y. Quality and Shelf Life of Fresh Chicken Breasts Subjected to Crust Freezing with and without Skin. J. Food Qual. 2013, 36, 361–368. [Google Scholar] [CrossRef]
- Hong, H.; Luo, Y.; Zhu, S.; Shen, H. Application of the general stability index method to predict quality deterioration in bighead carp (Aristichthys nobilis) heads during storage at different temperatures. J. Food Eng. 2012, 113, 554–558. [Google Scholar] [CrossRef]
- Reid, R.; Fanning, S.; Whyte, P.; Kerry, J.; Lindqvist, R.; Yu, Z.; Bolton, D. The microbiology of beef carcasses and primals during chilling and commercial storage. Food Microbiol. 2017, 61, 50–57. [Google Scholar] [CrossRef]
- Choi, Y.S.; Park, B.Y.; Lee, S.K.; Kim, I.S.; Kim, B.C. Composition and physico-chemical properties of vacuum packaged Korean pork loins for export during cold storage. Food Sci. Anim. Resour. 2002, 22, 151–157. [Google Scholar]
- Bellés, M.; Alonso, V.; Roncalés, P.; Beltrán, J. The combined effects of superchilling and packaging on the shelf life of lamb. Meat Sci. 2017, 133, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Ngapo, T.; Babare, I.; Reynolds, J.; Mawson, R. Freezing and thawing rate effects on drip loss from samples of pork. Meat Sci. 1999, 53, 149–158. [Google Scholar] [CrossRef]
- Kim, Y.B.; Woo, S.M.; Jeong, J.Y.; Ku, S.K.; Jeong, J.W.; Kum, J.S.; Kim, E.M. Temperature Changes during Freezing and Effect of Physicochemical Properties after Thawing on Meat by Air Blast and Magnetic Resonance Quick Freezing. Korean J. Food Sci. Anim. Resour. 2013, 33, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Duun, A.; Rustad, T. Quality of superchilled vacuum packed Atlantic salmon (Salmo salar) fillets stored at −1.4 and −3.6 °C. Food Chem. 2008, 106, 122–131. [Google Scholar] [CrossRef]
- Hoff-Lonergan, E.; Sosnicki, A. Water holding capacity of fresh meat. American Meat Science Association Fact Sheet No. 04669, Des Moines, IA 20002, National Pork Producers Council.
- Liu, Z.; Xiong, Y.L.; Chen, J. Protein Oxidation Enhances Hydration but Suppresses Water-Holding Capacity in Porcine Longissimus Muscle. J. Agric. Food Chem. 2010, 58, 10697–10704. [Google Scholar] [CrossRef]
- Warner, R.D. Chapter 14–The eating of meat–Ⅳ Water-holding capacity and juiciness. In Lawrie’s Meat Science, 8th ed.; National Pork Producers Council: London, UK, 2017; pp. 419–458. [Google Scholar]
- Kaale, L.D.; Eikevik, T.M.; Rustad, T.; Nordtvedt, T.S. Changes in water holding capacity and drip loss of Atlantic salmon (Salmo salar) muscle during superchilled storage. LWT 2014, 55, 528–535. [Google Scholar] [CrossRef] [Green Version]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Mok, J.H.; Her, J.-Y.; Kang, T.; Hoptowit, R.; Jun, S. Effects of Pulsed Electric Field (PEF) and Oscillating Magnetic Field (OMF) combination technology on the extension of supercooling for chicken breasts. J. Food Eng. 2017, 196, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Aaslyng, M.D.; Bejerholm, C.; Ertbjerg, P.; Bertram, H.C.; Andersen, H.J. Cooking loss and juiciness of pork in relation to raw meat quality and cooking procedure. Food Qual. Prefer. 2003, 14, 277–288. [Google Scholar] [CrossRef]
- Heymann, H.; Hedrick, H.; Karrasch, M.; Eggeman, M.; Ellersieck, M. Sensory and Chemical Characteristics of Fresh Pork Roasts Cooked to Different Endpoint Temperatures. J. Food Sci. 1990, 55, 613–617. [Google Scholar] [CrossRef]
- Bentley, D.; Reagan, J.; Miller, M. Effects of Gas Atmosphere, Storage Temperature and Storage Time on the Shelflife and Sensory Attributes of Vacuum Packaged Ground Beef Patties. J. Food Sci. 1989, 54, 284–286. [Google Scholar] [CrossRef]
- Straadt, I.; Rasmussen, M.; Andersen, H.J.; Bertram, H.C. Aging-induced changes in microstructure and water distribution in fresh and cooked pork in relation to water-holding capacity and cooking loss—A combined confocal laser scanning microscopy (CLSM) and low-field nuclear magnetic resonance relaxation study. Meat Sci. 2007, 75, 687–695. [Google Scholar] [CrossRef]
- Ye, K.; Ding, D.; Zhu, X.; Wu, Z.; Hu, Q.; Li, R. Modified atmosphere packaging with a small change in gas ratio could maintain pork quality during −3 °C storage. Food Control 2019, 109, 106943. [Google Scholar] [CrossRef]
- Buys, E.; Nortje, G.; Steyn, P. The effect of wholesale vacuum and 100% CO2 storage on the subsequent microbiological, colour and acceptability attributes of PVC-overwrapped pork loin chops. Food Res. Int. 1993, 26, 421–429. [Google Scholar] [CrossRef]

| Color | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Storage Temperature (°C) | Storage Period (Days) | CIE L* | CIE a* | CIE b* | ΔE | ||||||||
| Pork belly | Control | - | 44.33 | ± | 1.52 a | 11.45 | ± | 0.92 ab | 4.19 | ± | 0.80 bc | - | ||
| Refrigeration (3) | 7 | 44.58 | ± | 4.96 a | 9.86 | ± | 2.83 b | 3.18 | ± | 0.66 c | 18.34 | ± | 7.21 b | |
| 14 | 42.95 | ± | 0.90 a | 4.84 | ± | 1.39 c | 4.63 | ± | 1.36 bc | 30.25 | ± | 6.40 a | ||
| Supercooling (−2.5) | 7 | 41.92 | ± | 3.61 a | 10.64 | ± | 2.00 ab | 5.11 | ± | 1.16 bc | 9.36 | ± | 4.74 bc | |
| 14 | 41.41 | ± | 3.78 a | 10.39 | ± | 1.72 b | 5.60 | ± | 1.41 ab | 19.65 | ± | 5.95 b | ||
| Freezing (−18) | 7 | 42.65 | ± | 2.68 a | 13.90 | ± | 2.22 a | 6.38 | ± | 0.79 a | 4.37 | ± | 2.68 c | |
| 14 | 39.79 | ± | 2.63 a | 8.77 | ± | 1.10 b | 3.28 | ± | 0.55 c | 13.26 | ± | 6.54 bc | ||
| Chicken breast | Control | - | 51.23 | ± | 1.58 ab | 2.13 | ± | 0.90 ab | 4.03 | ± | 0.60 ab | - | ||
| Refrigeration (3) | 7 | 55.42 | ± | 7.90 ab | −0.34 | ± | 0.93 c | 3.51 | ± | 1.91 ab | 7.74 | ± | 5.33 a | |
| 14 | 54.58 | ± | 3.32 ab | 1.34 | ± | 0.87 b | 3.01 | ± | 1.07 b | 4.25 | ± | 2.69 a | ||
| Supercooling (−2.5) | 7 | 58.72 | ± | 6.21 a | 0.48 | ± | 0.78 bc | 4.03 | ± | 1.32 ab | 9.77 | ± | 4.30 a | |
| 14 | 57.57 | ± | 3.76 a | 1.44 | ± | 1.30 b | 4.07 | ± | 1.33 ab | 6.76 | ± | 3.14 a | ||
| Freezing (−18) | 7 | 48.23 | ± | 2.57 b | 3.15 | ± | 0.69 a | 5.64 | ± | 1.36 a | 4.13 | ± | 1.44 a | |
| 14 | 52.85 | ± | 4.12 ab | 1.51 | ± | 0.58 b | 4.72 | ± | 1.33 ab | 3.80 | ± | 2.17 a | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.H.; Lee, S.; Kim, E.J.; Jo, Y.-J.; Choi, M.-J. Development of a Stepwise Algorithm for Supercooling Storage of Pork Belly and Chicken Breast and Its Effect on Freshness. Foods 2022, 11, 380. https://doi.org/10.3390/foods11030380
Park DH, Lee S, Kim EJ, Jo Y-J, Choi M-J. Development of a Stepwise Algorithm for Supercooling Storage of Pork Belly and Chicken Breast and Its Effect on Freshness. Foods. 2022; 11(3):380. https://doi.org/10.3390/foods11030380
Chicago/Turabian StylePark, Dong Hyeon, SangYoon Lee, Eun Jeong Kim, Yeon-Ji Jo, and Mi-Jung Choi. 2022. "Development of a Stepwise Algorithm for Supercooling Storage of Pork Belly and Chicken Breast and Its Effect on Freshness" Foods 11, no. 3: 380. https://doi.org/10.3390/foods11030380
APA StylePark, D. H., Lee, S., Kim, E. J., Jo, Y.-J., & Choi, M.-J. (2022). Development of a Stepwise Algorithm for Supercooling Storage of Pork Belly and Chicken Breast and Its Effect on Freshness. Foods, 11(3), 380. https://doi.org/10.3390/foods11030380





