Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases
Abstract
1. Introduction
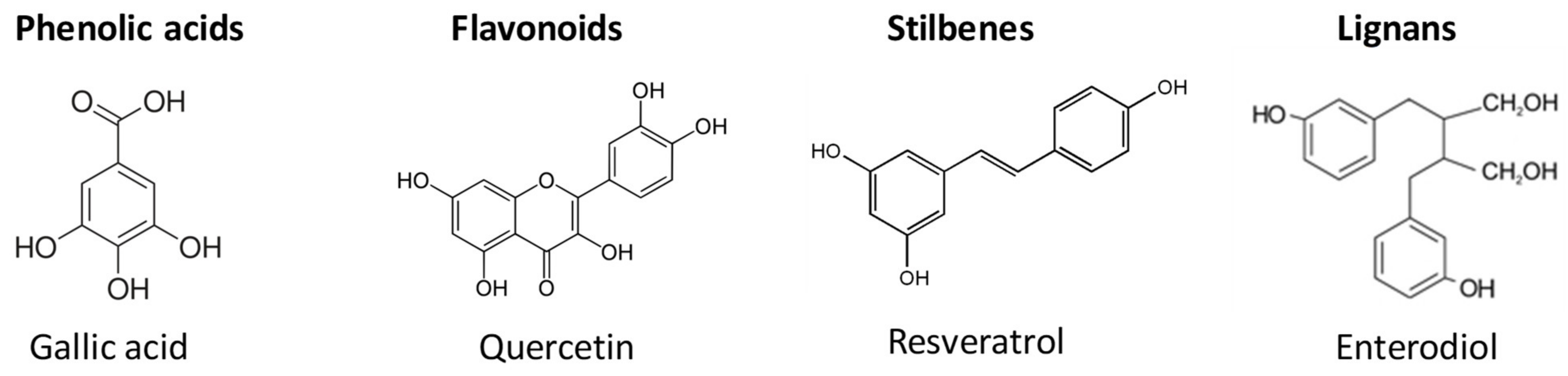
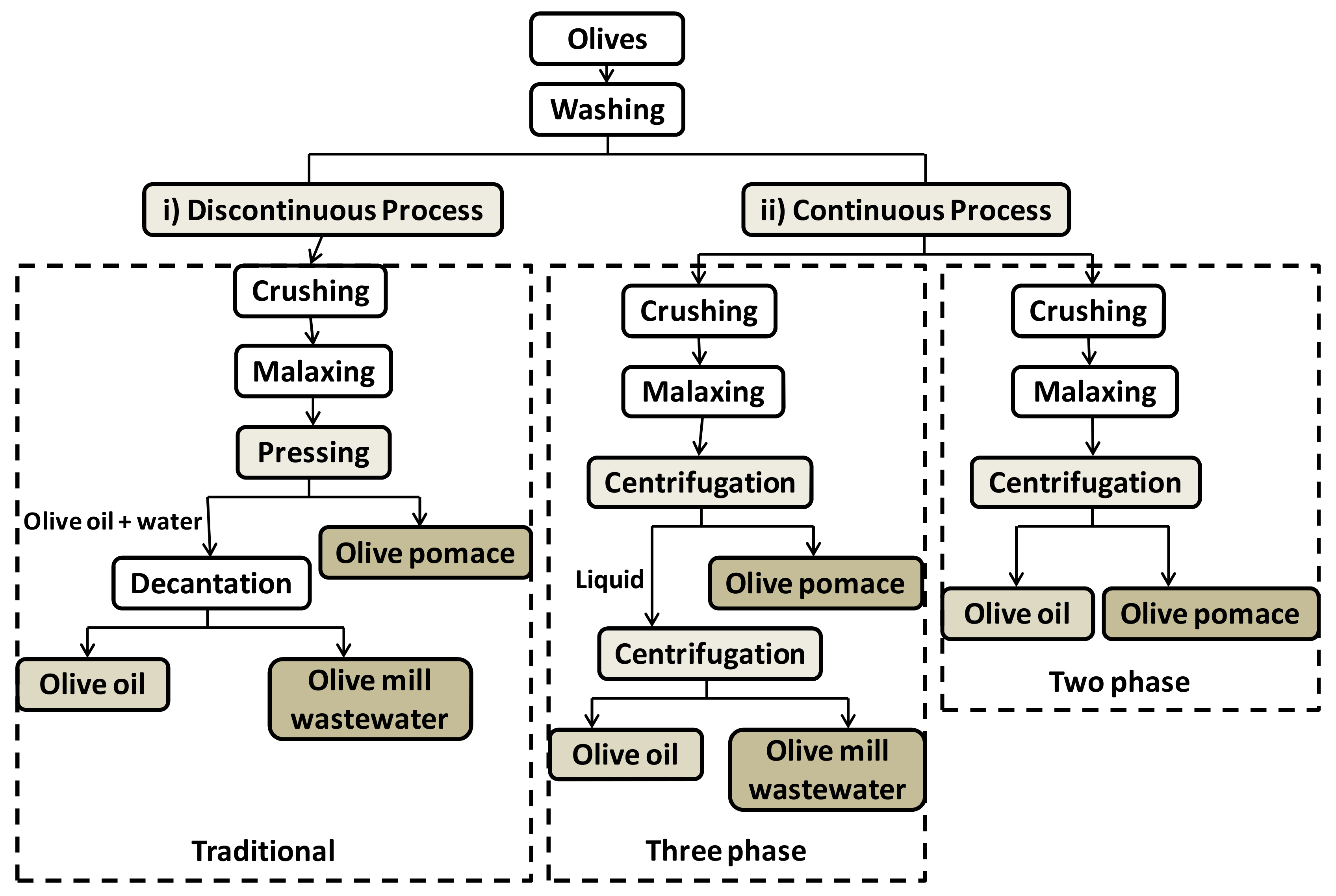
2. Polyphenols from Olive Oil Production Wastes: Source and Applications
Applications of Polyphenols from Olive Oil Industrial Wastes
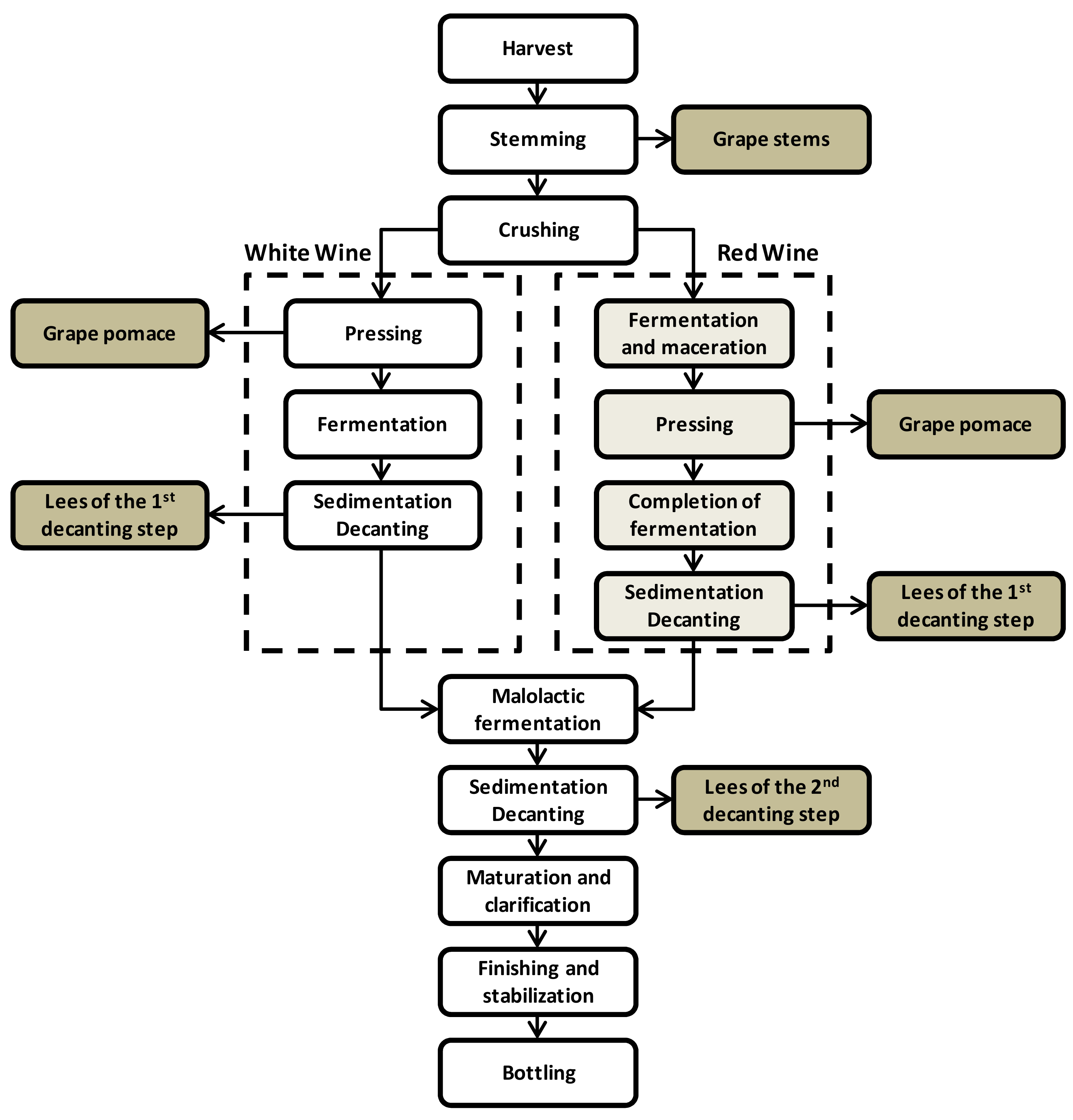
3. Polyphenols from Wine Production Wastes: Source and Applications
Applications of Polyphenols from the Wine Industry
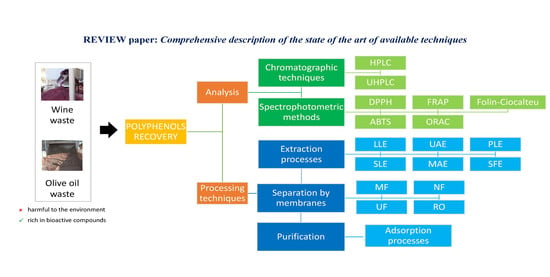
4. Polyphenol Analysis: From Quantification to Antioxidant Capacity
4.1. Chromatographic Techniques
4.2. Spectrophotometric Methods
4.2.1. Folin-Ciocalteu Method
4.2.2. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
4.2.3. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-Sulfonic Acid) Assay
4.2.4. Ferric Reducing Antioxidant Power (FRAP)
4.2.5. Oxygen Radical Absorbance Capacity (ORAC)
5. From Agri-Food Wastes to Polyphenols: Processing Techniques
5.1. Extraction Processes
5.2. Conditioning of Polyphenol Extracts: From Particulate Matter and Colloidal Removal to Volume Size Reduction and Concentration
5.3. Recovery of Polyphenols by Adsorption Technologies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roselló-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: Non-conventional methods for the recovery of high-added value compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and their potential role to fight viral diseases: An overview. Sci. Total Environ. 2021, 801, 149719. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Cassano, A.; De Luca, G.; Conidi, C.; Drioli, E. Effect of polyphenols-membrane interactions on the performance of membrane-based processes. A review. Coord. Chem. Rev. 2017, 351, 45–75. [Google Scholar] [CrossRef]
- Berbel, J.; Posadillo, A. Review and analysis of alternatives for the valorisation of agro-industrial olive oil by-products. Sustainability 2018, 10, 237. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Yáñez-Fernández, J.; Fíla, V. Phenolic compounds recovered from agro-food by-products using membrane technologies: An overview. Food Chem. 2016, 213, 753–762. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef]
- López Carreras, N.; Miguel, M.; Aleixandre, A. Propiedades beneficiosas de los terpenos iridoides sobre la salud. Nutr. Clin. Y Diet. Hosp. 2012, 32, 81–91. [Google Scholar]
- Ricciutelli, M.; Marconi, S.; Boarelli, M.C.; Caprioli, G.; Sagratini, G.; Ballini, R.; Fiorini, D. Olive oil polyphenols: A quantitative method by high-performance liquid-chromatography-diode-array detection for their determination and the assessment of the related health claim. J. Chromatogr. A 2017, 1481, 53–63. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Araújo, M.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Phenolic compounds from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211. [Google Scholar] [CrossRef]
- Čepo, D.V.; Radić, K.; Jurmanović, S.; Jug, M.; Rajković, M.G.; Pedisić, S.; Moslavac, T.; Albahari, P. Valorization of olive pomace-based nutraceuticals as antioxidants in chemical, food, and biological models. Molecules 2018, 23, 2070. [Google Scholar] [CrossRef]
- Tamasi, G.; Baratto, M.C.; Bonechi, C.; Byelyakova, A.; Pardini, A.; Donati, A.; Leone, G.; Consumi, M.; Lamponi, S.; Magnani, A.; et al. Chemical characterization and antioxidant properties of products and by-products from Olea europaea L. Food Sci. Nutr. 2019, 7, 2907–2920. [Google Scholar] [CrossRef]
- Yakhlef, W.; Arhab, R.; Romero, C.; Brenes, M.; de Castro, A.; Medina, E. Phenolic composition and antimicrobial activity of Algerian olive products and by-products. LWT Food Sci. Technol. 2018, 93, 323–328. [Google Scholar] [CrossRef]
- Ruiz, E.; Romero-García, J.M.; Romero, I.; Manzanares, P.; María José Negro, E.C. Olive-derived biomass as a source of energy and chemicals. Biofuels Bioprod. Biorefin. 2017, 11, 1077–1094. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Galanakis, C.M.; Castro-Muñoz, R. Recovery of polyphenols from olive mill wastewaters by membrane operations. Membr. Technol. Biorefin. 2016, 163–187. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Kiai, H.; Hafidi, A. Phenolic profile and antioxidant activities of olive mill wastewater. Food Chem. 2012, 132, 406–412. [Google Scholar] [CrossRef]
- Pérez, M.; López-Yerena, A.; Lozano-Castellón, J.; Olmo-Cunillera, A.; Lamuela-Raventós, R.M.; Martin-Belloso, O.; Vallverdú-Queralt, A. Impact of novel technologies on virgin olive oil processing, consumer acceptance, and the valorization of olive mill wastes. Antioxidants 2021, 10, 417. [Google Scholar] [CrossRef]
- Ruiz-Moreno, M.J.; Raposo, R.; Moreno-Rojas, J.M.; Zafrilla, P.; Cayuela, J.M.; Mulero, J.; Puertas, B.; Guerrero, R.F.; Piñeiro, Z.; Giron, F.; et al. Efficacy of olive oil mill extract in replacing sulfur dioxide in wine model. LWT Food Sci. Technol. 2015, 61, 117–123. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Di Michele, A.; Pannucci, E.; Botticella, E.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R. Nanostructured starch combined with hydroxytyrosol in poly(vinyl alcohol) based ternary films as active packaging system. Carbohydr. Polym. 2018, 193, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández Ponce, M.T.; Mantell Serrano, C.; Martínez de la Ossa-Fernández, E.J. Characterization of olive leaf extract polyphenols loaded by supercritical solvent impregnation into PET/PP food packaging films. J. Supercrit. Fluids 2018, 140, 196–206. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tsatalas, P.; Galanakis, I.M. Implementation of phenols recovered from olive mill wastewater as UV booster in cosmetics. Ind. Crops Prod. 2018, 111, 30–37. [Google Scholar] [CrossRef]
- Pérez-Bibbins, B.; Torrado-Agrasar, A.; Salgado, J.M.; Oliveira, R.P.d.S.; Domínguez, J.M. Potential of lees from wine, beer, and cider manufacturing as a source of economic nutrients: An overview. Waste Manag. 2015, 40, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crops Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.L.; Haroutounian, S.A. Grape stem extracts: Polyphenolic content and assessment of their in vitro antioxidant properties. LWT Food Sci. Technol. 2012, 48, 316–322. [Google Scholar] [CrossRef]
- Kopsahelis, N.; Dimou, C.; Papadaki, A.; Xenopoulos, E.; Kyraleou, M.; Kallithraka, S.; Kotseridis, Y.; Papanikolaou, S.; Koutinas, A.A. Refining of wine lees and cheese whey for the production of microbial oil, polyphenol-rich extracts and value-added co-products. J. Chem. Technol. Biotechnol. 2018, 93, 257–268. [Google Scholar] [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef]
- Câmara, J.S.; Lourenço, S.; Silva, C.; Lopes, A.; Andrade, C.; Perestrelo, R. Exploring the potential of wine industry by-products as source of additives to improve the quality of aquafeed. Microchem. J. 2020, 155, 104758. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.L.; Barral, M.T.; Cruz, J.M.; Moldes, A.B. Valorization of winery waste vs. the costs of not recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef]
- Zacharof, M.P. Grape Winery Waste as Feedstock for Bioconversions: Applying the Biorefinery Concept. Waste Biomass Valorization 2017, 8, 1011–1025. [Google Scholar] [CrossRef]
- Ene, S.A.; Teodosiu, C.; Robu, B.; Volf, I. Water footprint assessment in the winemaking industry: A case study for a Romanian medium size production plant. J. Clean. Prod. 2013, 43, 122–135. [Google Scholar] [CrossRef]
- Ioannou, L.A.; Puma, G.L.; Fatta-Kassinos, D. Treatment of winery wastewater by physicochemical, biological and advanced processes: A review. J. Hazard. Mater. 2015, 286, 343–368. [Google Scholar] [CrossRef]
- Alves, V.L.C.D.; Rico, B.P.M.; Cruz, R.M.S.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Preparation and characterization of a chitosan film with grape seed extract-carvacrol microcapsules and its effect on the shelf-life of refrigerated Salmon (Salmo salar). LWT Food Sci. Technol. 2018, 89, 525–534. [Google Scholar] [CrossRef]
- Charradi, K.; Mahmoudi, M.; Bedhiafi, T.; Jebari, K.; El May, M.V.; Limam, F.; Aouani, E. Safety evaluation, anti-oxidative and anti-inflammatory effects of subchronically dietary supplemented high dosing grape seed powder (GSP) to healthy rat. Biomed. Pharmacother. 2018, 107, 534–546. [Google Scholar] [CrossRef]
- Matos, M.S.; Romero-Díez, R.; Álvarez, A.; Bronze, M.R.; Rodríguez-Rojo, S.; Mato, R.B.; Cocero, M.J.; Matias, A.A. Polyphenol-Rich Extracts Obtained from Winemaking Waste Streams as Natural Ingredients with Cosmeceutical Potential. Antioxidants 2019, 8, 355. [Google Scholar] [CrossRef]
- Hamza, A.A.; Heeba, G.H.; Elwy, H.M.; Murali, C.; El-Awady, R.; Amin, A. Molecular characterization of the grape seeds extract’s effect against chemically induced liver cancer: In vivo and in vitro analyses. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Li, L.; Qiu, R.L.; Lin, Y.; Cai, Y.; Bian, Y.; Fan, Y.; Gao, X.J. Resveratrol suppresses human cervical carcinoma cell proliferation and elevates apoptosis via the mitochondrial and p53 signaling pathways. Oncol. Lett. 2018, 15, 9845–9851. [Google Scholar] [CrossRef] [PubMed]
- Averilla, J.N.; Oh, J.; Kim, H.J.; Kim, J.S.; Kim, J.-S. Potential health benefits of phenolic compounds in grape processing by-products. Food Sci. Biotechnol. 2019, 28. [Google Scholar] [CrossRef] [PubMed]
- Fabra, M.J.; Falcó, I.; Randazzo, W.; Sánchez, G.; López-Rubio, A. Antiviral and antioxidant properties of active alginate edible films containing phenolic extracts. Food Hydrocoll. 2018, 81, 96–103. [Google Scholar] [CrossRef]
- D’Antuono, I.; Kontogianni, V.G.; Kotsiou, K.; Linsalata, V.; Logrieco, A.F.; Tasioula-Margari, M.; Cardinali, A. Polyphenolic characterization of olive mill wastewaters, coming from Italian and Greek olive cultivars, after membrane technology. Food Res. Int. 2014, 65, 301–310. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Alli, I.; Ereifej, K.; Alhamad, M.; Al-Tawaha, A.R.; Rababah, T. Optimisation, characterisation and quantification of phenolic compounds in olive cake. Food Chem. 2010, 123, 117–122. [Google Scholar] [CrossRef]
- López-fernández, O.; Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Rocchetti, G.; Lorenzo, J.M. Determination of polyphenols using liquid chromatography–tandem mass spectrometry technique (LC–MS/MS): A review. Antioxidants 2020, 9, 479. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razek, A.G.; Badr, A.N.; Shehata, M.G. Characterization of Olive Oil By-products: Antioxidant Activity, Its Ability to Reduce Aflatoxigenic Fungi Hazard and Its Aflatoxins. Annu. Res. Rev. Biol. 2017, 1435065, 2347–2565. [Google Scholar] [CrossRef]
- Romero, C.; Medina, E.; Mateo, M.A.; Brenes, M. New by-products rich in bioactive substances from the olive oil mill processing. J. Sci. Food Agric. 2018, 98, 225–230. [Google Scholar] [CrossRef]
- La Scalia, G.; Micale, R.; Cannizzaro, L.; Marra, F.P. A sustainable phenolic compound extraction system from olive oil mill wastewater. J. Clean. Prod. 2017, 142, 3782–3788. [Google Scholar] [CrossRef]
- Aissa, I.; Kharrat, N.; Aloui, F.; Sellami, M.; Bouaziz, M.; Gargouri, Y. Valorization of antioxidants extracted from olive mill wastewater. Biotechnol. Appl. Biochem. 2017, 64, 579–589. [Google Scholar] [CrossRef]
- Ioannou-Ttofa, L.; Michael-Kordatou, I.; Fattas, S.C.; Eusebio, A.; Ribeiro, B.; Rusan, M.; Amer, A.R.B.; Zuraiqi, S.; Waismand, M.; Linder, C.; et al. Treatment efficiency and economic feasibility of biological oxidation, membrane filtration and separation processes, and advanced oxidation for the purification and valorization of olive mill wastewater. Water Res. 2017, 114, 1–13. [Google Scholar] [CrossRef]
- Kaleh, Z.; Geißen, S.U. Selective isolation of valuable biophenols from olive mill wastewater. J. Environ. Chem. Eng. 2016, 4, 373–384. [Google Scholar] [CrossRef]
- Lozano-Sánchez, J.; Castro-Puyana, M.; Mendiola, J.A.; Segura-Carretero, A.; Cifuentes, A.; Ibáñez, E. Recovering bioactive compounds from olive oil filter cake by advanced extraction techniques. Int. J. Mol. Sci. 2014, 15, 16270–16283. [Google Scholar] [CrossRef]
- Fernández, M.d.l.Á.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; De Faveri, D.; Fiori, L.; Perego, P. Antioxidants from winemaking wastes: A study on extraction parameters using response surface methodology. J. Food Biochem. 2012, 36, 28–37. [Google Scholar] [CrossRef]
- Caldas, T.W.; Mazza, K.E.L.; Teles, A.S.C.; Mattos, G.N.; Brígida, A.I.S.; Conte-Junior, C.A.; Borguini, R.G.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.V. Phenolic compounds recovery from grape skin using conventional and non-conventional extraction methods. Ind. Crops Prod. 2018, 111, 86–91. [Google Scholar] [CrossRef]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Antoniolli, A.; Fontana, A.R.; Piccoli, P.; Bottini, R. Characterization of polyphenols and evaluation of antioxidant capacity in grape pomace of the cv. Malbec. Food Chem. 2015, 178, 172–178. [Google Scholar] [CrossRef]
- Mohd Maidin, N.; Michael, N.; Oruna-Concha, M.J.; Jauregi, P. Polyphenols extracted from red grape pomace by a surfactant based method show enhanced collagenase and elastase inhibitory activity. J. Chem. Technol. Biotechnol. 2018, 93, 1916–1924. [Google Scholar] [CrossRef]
- Jurčević, I.L.; Dora, M.; Guberovic, I.; Petras, M.; Brncic, S.R.; Dikic, D. Polyphenols from wine lees as a novel functional bioactive compound in the protection against oxidative stress and hyperlipidaemia. Food Technol. Biotechnol. 2017, 55, 109–116. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; González, J.; Domínguez, H. A membrane process for the recovery of a concentrated phenolic product from white vinasses. Chem. Eng. J. 2017, 327, 210–217. [Google Scholar] [CrossRef]
- Aydoğan, C. Recent advances and applications in LC-HRMS for food and plant natural products: A critical review. Anal. Bioanal. Chem. 2020, 412, 1973–1991. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, E.R.; Chiţescu, C.L.; Geană, E.I.; Gird, C.E.; Socoteanu, R.P.; Boscencu, R. Advanced analytical approaches for the analysis of polyphenols in plants matrices—A review. Separations 2021, 8, 65. [Google Scholar] [CrossRef]
- Piovesana, S.; Cavaliere, C.; Cerrato, A.; Montone, C.M.; Laganà, A.; Capriotti, A.L. Developments and pitfalls in the characterization of phenolic compounds in food: From targeted analysis to metabolomics-based approaches. TrAC Trends Anal. Chem. 2020, 133. [Google Scholar] [CrossRef]
- Giacobbo, A.; Dias, B.B.; Onorevoli, B.; Bernardes, A.M.; de Pinho, M.N.; Caramão, E.B.; Rodrigues, E.; Jacques, R.A. Wine lees from the 1st and 2nd rackings: Valuable by-products. J. Food Sci. Technol. 2019, 56, 1559–1566. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Method. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteau, V. Tyrosine and Tryptophane in Proteins. J. Biol. Chem. 1927, 73, 627–648. [Google Scholar] [CrossRef]
- Alcalde, B.; Granados, M.; Saurina, J. Exploring the antioxidant features of polyphenols by spectroscopic and electrochemical methods. Antioxidants 2019, 8, 523. [Google Scholar] [CrossRef]
- Elkacmi, R.; Kamil, N.; Bennajah, M. Separation and purification of high purity products from three different olive mill wastewater samples. J. Environ. Chem. Eng. 2017, 5, 829–837. [Google Scholar] [CrossRef]
- Poveda, J.M.; Loarce, L.; Alarcón, M.; Díaz-Maroto, M.C.; Alañón, M.E. Revalorization of winery by-products as source of natural preservatives obtained by means of green extraction techniques. Ind. Crops Prod. 2018, 112, 617–625. [Google Scholar] [CrossRef]
- Dimou, C.; Vlysidis, A.; Kopsahelis, N.; Papanikolaou, S.; Koutinas, A.A.; Kookos, I.K. Techno-economic evaluation of wine lees refining for the production of value-added products. Biochem. Eng. J. 2016, 116, 157–165. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Neguelescu, G.P. Methods for Total Antioxidant Activity Determination. A Review. Biochem. Anal. Biochem. 2011, 1. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles, and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Tirzitis, G.; Bartosz, G. Determination of antiradical and antioxidant activity: Basic principles and new insights. Acta Biochim. Pol. 2010, 57, 139–142. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Tournour, H.H.; Segundo, M.A.; Magalhães, L.M.; Barreiros, L.; Queiroz, J.; Cunha, L.M. Valorization of grape pomace: Extraction of bioactive phenolics with antioxidant properties. Ind. Crops Prod. 2015, 74, 397–406. [Google Scholar] [CrossRef]
- Gupta, D. Methods for determination of antioxidant capacity: A review. Int. J. Pharm. Sci. Res. 2015, 6, 546–566. [Google Scholar] [CrossRef]
- Pasten, A.; Uribe, E.; Stucken, K.; Rodríguez, A.; Vega-Gálvez, A. Influence of Drying on the Recoverable High-Value Products from Olive (cv. Arbequina) Waste Cake. Waste Biomass Valorization 2019, 10, 1627–1638. [Google Scholar] [CrossRef]
- Hoyos-Arbeláez, J.; Vázquez, M.; Contreras-Calderón, J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef]
- Elhachem, M.; Cayot, P.; Abboud, M.; Louka, N.; Maroun, R.G.; Bou-Maroun, E. The importance of developing electrochemical sensors based on molecularly imprinted polymers for a rapid detection of antioxidants. Antioxidants 2021, 10, 382. [Google Scholar] [CrossRef]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Recovery of Added-Value Compounds from Orange and Spinach Processing Residues: Green Extraction of Phenolic Compounds and Evaluation of Antioxidant Activity. Antioxidants 2021, 10, 1800. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Alvarino, T.; Cortina, J.L.; Saurina, J.; Granados, M. Olive Mill and Winery Wastes as Viable Sources of Bioactive Compounds: A Study on Polyphenols Recovery. Antioxidants 2020, 9, 1074. [Google Scholar] [CrossRef]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Fruit and vegetable processing wastes as natural sources of antioxidant-rich extracts: Evaluation of advanced extraction technologies by surface response methodology. J. Environ. Chem. Eng. 2021, 9. [Google Scholar] [CrossRef]
- Štambuk, P.; Tomašković, D.; Tomaz, I.; Maslov, L.; Stupić, D.; Karoglan Kontić, J. Application of pectinases for recovery of grape seeds phenolics. 3 Biotechnology 2016, 6, 224. [Google Scholar] [CrossRef]
- El Darra, N.; Grimi, N.; Vorobiev, E.; Louka, N.; Maroun, R. Extraction of Polyphenols from Red Grape Pomace Assisted by Pulsed Ohmic Heating. Food Bioprocess Technol. 2013, 6, 1281–1289. [Google Scholar] [CrossRef]
- Pappas, V.M.; Lakka, A.; Palaiogiannis, D.; Athanasiadis, V.; Bozinou, E.; Ntourtoglou, G.; Makris, D.P.; Dourtoglou, V.G.; Lalas, S.I. Optimization of pulsed electric field as standalone “green” extraction procedure for the recovery of high value-added compounds from fresh olive leaves. Antioxidants 2021, 10, 1554. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Enzyme-assisted extraction of bioactive non-extractable polyphenols from sweet cherry (Prunus avium L.) pomace. Food Chem. 2021, 339, 128086. [Google Scholar] [CrossRef]
- Maroun, R.G.; Rajha, H.N.; El Darra, N.; El Kantar, S.; Chacar, S.; Debs, E.; Vorobiev, E.; Louka, N. Emerging technologies for the extraction of polyphenols from natural sources. In Polyphenols: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 265–293. [Google Scholar]
- Elkacmi, R.; Boulmal, N.; Kamil, N.; Bennajah, M. Techno-economical evaluation of a new technique for olive mill wastewater treatment. Sustain. Prod. Consum. 2017, 10, 38–49. [Google Scholar] [CrossRef]
- Leouifoudi, I.; Harnafi, H.; Zyad, A. Olive Mill Waste Extracts: Polyphenols Content, Antioxidant, and Antimicrobial Activities. Adv. Pharmacol. Sci. 2015, 2015. [Google Scholar] [CrossRef]
- Kalogerakis, N.; Politi, M.; Foteinis, S.; Chatzisymeon, E.; Mantzavinos, D. Recovery of antioxidants from olive mill wastewaters: A viable solution that promotes their overall sustainable management. J. Environ. Manage. 2013, 128, 749–758. [Google Scholar] [CrossRef]
- Lafka, T.I.; Lazou, A.E.; Sinanoglou, V.J.; Lazos, E.S. Phenolic and antioxidant potential of olive oil mill wastes. Food Chem. 2011, 125, 92–98. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Casazza, A.A.; Perego, P. Valorization of olive oil solid waste using high pressure-high temperature reactor. Food Chem. 2011, 128, 704–710. [Google Scholar] [CrossRef]
- Ramos, P.; Santos, S.A.O.; Guerra, Â.R.; Guerreiro, O.; Felício, L.; Jerónimo, E.; Silvestre, A.J.D.; Neto, C.P.; Duarte, M. Valorization of olive mill residues: Antioxidant and breast cancer antiproliferative activities of hydroxytyrosol-rich extracts derived from olive oil by-products. Ind. Crops Prod. 2013, 46, 359–368. [Google Scholar] [CrossRef]
- De Bruno, A.; Romeo, R.; Fedele, F.L.; Sicari, A.; Piscopo, A.; Poiana, M. Antioxidant activity shown by olive pomace extracts. J. Environ. Sci. Heal. Part B 2018, 53, 526–533. [Google Scholar] [CrossRef]
- Da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Comparison of microwave, ultrasonic and conventional techniques for extraction of bioactive compounds from olive leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 58, 102234. [Google Scholar] [CrossRef]
- Xie, P.; Huang, L.; Zhang, C.; Deng, Y.; Wang, X.; Cheng, J. Enhanced extraction of hydroxytyrosol, maslinic acid and oleanolic acid from olive pomace: Process parameters, kinetics and thermodynamics, and greenness assessment. Food Chem. 2019, 276, 662–674. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Ultrasound increases the aqueous extraction of phenolic compounds with high antioxidant activity from olive pomace. LWT Food Sci. Technol. 2018, 89, 284–290. [Google Scholar] [CrossRef]
- Putnik, P.; Barba, F.J.; Španić, I.; Zorić, Z.; Dragović-Uzelac, V.; Bursać Kovačević, D. Green extraction approach for the recovery of polyphenols from Croatian olive leaves (Olea europea). Food Bioprod. Process. 2017, 106, 19–28. [Google Scholar] [CrossRef]
- Casagrande, M.; Zanela, J.; Pereira, D.; de Lima, V.A.; Oldoni, T.L.C.; Carpes, S.T. Optimization of the extraction of antioxidant phenolic compounds from grape pomace using response surface methodology. J. Food Meas. Charact. 2019, 13, 1120–1129. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A.; Decorti, D. The combined extraction of polyphenols from grape marc: Ultrasound assisted extraction followed by supercritical CO2 extraction of ultrasound-raffinate. LWT Food Sci. Technol. 2015, 61, 98–104. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, D.; Zhang, Q.A.; Sun, D.W. Ultrasound-assisted extraction of phenolics from wine lees: Modeling, optimization and stability of extracts during storage. Ultrason. Sonochem. 2014, 21, 706–715. [Google Scholar] [CrossRef]
- Ferri, M.; Vannini, M.; Ehrnell, M.; Eliasson, L.; Xanthakis, E.; Monari, S.; Sisti, L.; Marchese, P.; Celli, A.; Tassoni, A. From winery waste to bioactive compounds and new polymeric biocomposites: A contribution to the circular economy concept. J. Adv. Res. 2020, 24, 1–11. [Google Scholar] [CrossRef]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. TrAC Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
- Bazinet, L.; Doyen, A. Antioxidants, mechanisms, and recovery by membrane processes. Crit. Rev. Food Sci. Nutr. 2015, 57, 677–700. [Google Scholar] [CrossRef]
- Antónia Nunes, M.; Pawlowski, S.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P.; Velizarov, S. Valorization of olive pomace by a green integrated approach applying sustainable extraction and membrane-assisted concentration. Sci. Total Environ. 2019, 652, 40–47. [Google Scholar] [CrossRef]
- Cassano, A.; Bentivenga, A.; Conidi, C.; Galiano, F.; Saoncella, O.; Figoli, A. Membrane-Based Clarification and Fractionation of Red Wine Lees Aqueous Extracts. Polymers 2019, 11, 1089. [Google Scholar] [CrossRef]
- Giacobbo, A.; Meneguzzi, A.; Bernardes, A.M.; de Pinho, M.N. Pressure-driven membrane processes for the recovery of antioxidant compounds from winery effluents. J. Clean. Prod. 2017, 155, 172–178. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Comite, A.; Jezowska, A.; Pagliero, M.; Costa, C.; Firpo, R. Treatment of olive mill wastewater through integrated pressure-driven membrane processes. Membranes 2020, 10, 334. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Paraskeva, C.A. Purification of grape marc phenolic compounds through solvent extraction, membrane filtration and resin adsorption/desorption. Sep. Purif. Technol. 2015, 156, 328–335. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Ruby-Figueroa, R.; Castro-Muñoz, R. Nanofiltration and tight ultrafiltration membranes for the recovery of polyphenols from agro-food by-products. Int. J. Mol. Sci. 2018, 19, 351. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Paraskeva, C.A. Isolation of organic compounds with high added values from agro-industrial solid wastes. J. Environ. Manage. 2018, 216, 183–191. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Paraskeva, C.A. Preliminary design of a phenol’s purification plant. J. Chem. Technol. Biotechnol. 2020, 95, 373–383. [Google Scholar] [CrossRef]
- Savarese, M.; De Marco, E.; Falco, S.; D’Antuoni, I.; Sacchi, R. Biophenol extracts from olive oil mill wastewaters by membrane separation and adsorption resin. Int. J. Food Sci. Technol. 2016, 51, 2386–2395. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Vavouraki, A.I.; Kornaros, M.E.; Paraskeva, C.A. Purification of olive mill wastewater phenols through membrane filtration and resin adsorption/desorption. J. Hazard. Mater. 2015, 285, 69–76. [Google Scholar] [CrossRef]
- Mudimu, O.A.; Peters, M.; Brauner, F.; Braun, G. Overview of membrane processes for the recovery of polyphenols from olive mill wastewater olive mill wastewater. Am. J. Environ. Sci. 2012, 8, 195–201. [Google Scholar] [CrossRef][Green Version]
- Hamza, M.; Sayadi, S. Valorisation of olive mill wastewater by enhancement of natural hydroxytyrosol recovery. Int. J. Food Sci. Technol. 2015, 50, 826–833. [Google Scholar] [CrossRef]
- Alfano, A.; Corsuto, L.; Finamore, R.; Savarese, M.; Ferrara, F.; Falco, S.; Santabarbara, G.; De Rosa, M.; Schiraldi, C. Valorization of Olive Mill Wastewater by Membrane Processes to Recover Natural Antioxidant Compounds for Cosmeceutical and Nutraceutical Applications or Functional Foods. Antioxidants 2018, 7, 72. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Martínez-Férez, A. About the recovery of the phenolic fraction from olive mill wastewater by micro and ultracentrifugation membranes. Chem. Eng. Trans. 2017, 60, 271–276. [Google Scholar] [CrossRef]
- Kontos, S.S.; Katrivesis, F.K.; Constantinou, T.C.; Zoga, C.A.; Ioannou, I.S.; Koutsoukos, P.G.; Paraskeva, C.A. Implementation of membrane filtration and melt crystallization for the effective treatment and valorization of olive mill wastewaters. Sep. Purif. Technol. 2018, 193, 103–111. [Google Scholar] [CrossRef]
- Kontos, S.S.; Iakovides, I.C.; Koutsoukos, P.G.; Paraskeva, C.A. Isolation of Purified High Added Value Products from Olive Mill Wastewater Streams through the Implementation of Membrane Technology and Cooling Crystallization Process. Int. Conf. Nanotechnol. Based Innov. Appl. Environ. 2016, 47, 337–342. [Google Scholar] [CrossRef]
- Giacobbo, A.; Do Prado, J.M.; Meneguzzi, A.; Bernardes, A.M.; De Pinho, M.N. Microfiltration for the recovery of polyphenols from winery effluents. Sep. Purif. Technol. 2015, 143, 12–18. [Google Scholar] [CrossRef]
- Giacobbo, A.; Bernardes, A.M.; de Pinho, M.N. Sequential pressure-driven membrane operations to recover and fractionate polyphenols and polysaccharides from second racking wine lees. Sep. Purif. Technol. 2017, 173, 49–54. [Google Scholar] [CrossRef]
- Kontogiannopoulos, K.N.; Patsios, S.I.; Mitrouli, S.T.; Karabelas, A.J. Tartaric acid and polyphenols recovery from winery waste lees using membrane separation processes. J. Chem. Technol. Biotechnol. 2017, 92, 2934–2943. [Google Scholar] [CrossRef]
- Giacobbo, A.; Bernardes, A.M.; Rosa, M.J.F.; De Pinho, M.N. Concentration polarization in ultrafiltration/nanofiltration for the recovery of polyphenols from winery wastewaters. Membranes 2018, 8, 46. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Ieri, F.; Bernini, R. Sustainability, innovation, and green chemistry in the production and valorization of phenolic extracts from Olea europaea L. Sustainability 2016, 8, 1002. [Google Scholar] [CrossRef]
- Pérez-Larrán, P.; Díaz-Reinoso, B.; Moure, A.; Alonso, J.L.; Domínguez, H. Adsorption technologies to recover and concentrate food polyphenols. Curr. Opin. Food Sci. 2018, 23, 165–172. [Google Scholar] [CrossRef]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J.C. Recovery, concentration, and purification of phenolic compounds by adsorption: A review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Víctor-Ortega, M.D.; Airado-Rodríguez, D. Revalorization of agro-industrial effluents based on gallic acid recovery through a novel anionic resin. Process Saf. Environ. Prot. 2018, 115, 17–26. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Huang, S.; Zhang, L.; Ge, Z.; Sun, L.; Zong, W. Purification of polyphenols from distiller’s grains by macroporous resin and analysis of the polyphenolic components. Molecules 2019, 24, 1284. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Amirian, P.; Mahvi, A.H.; Ansari-Moghaddam, A. Application of adsorption process for phenolic compounds removal from aqueous environments: A systematic review. Glob. Nest J. 2016, 18, 146–163. [Google Scholar]
- Frascari, D.; Rubertelli, G.; Arous, F.; Ragini, A.; Bresciani, L.; Arzu, A.; Pinelli, D. Valorisation of olive mill wastewater by phenolic compounds adsorption: Development and application of a procedure for adsorbent selection. Chem. Eng. J. 2019, 360, 124–138. [Google Scholar] [CrossRef]
- Pinelli, D.; Molina Bacca, A.E.; Kaushik, A.; Basu, S.; Nocentini, M.; Bertin, L.; Frascari, D. Batch and Continuous Flow Adsorption of Phenolic Compounds from Olive Mill Wastewater: A Comparison between Nonionic and Ion Exchange Resins. Int. J. Chem. Eng. 2016, 2016. [Google Scholar] [CrossRef]
- Víctor-Ortega, M.D.; Ochando-Pulido, J.M.; Martínez-Ferez, A. Performance and modeling of continuous ion exchange processes for phenols recovery from olive mill wastewater. Process Saf. Environ. Prot. 2016, 100, 242–251. [Google Scholar] [CrossRef]
- Frascari, D.; Bacca, A.E.M.; Zama, F.; Bertin, L.; Fava, F.; Pinelli, D. Olive mill wastewater valorisation through phenolic compounds adsorption in a continuous flow column. Chem. Eng. J. 2016, 283, 293–303. [Google Scholar] [CrossRef]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J.C. Batch and fixed bed column studies on phenolic adsorption from wine vinasses by polymeric resins. J. Food Eng. 2017, 209, 52–60. [Google Scholar] [CrossRef]

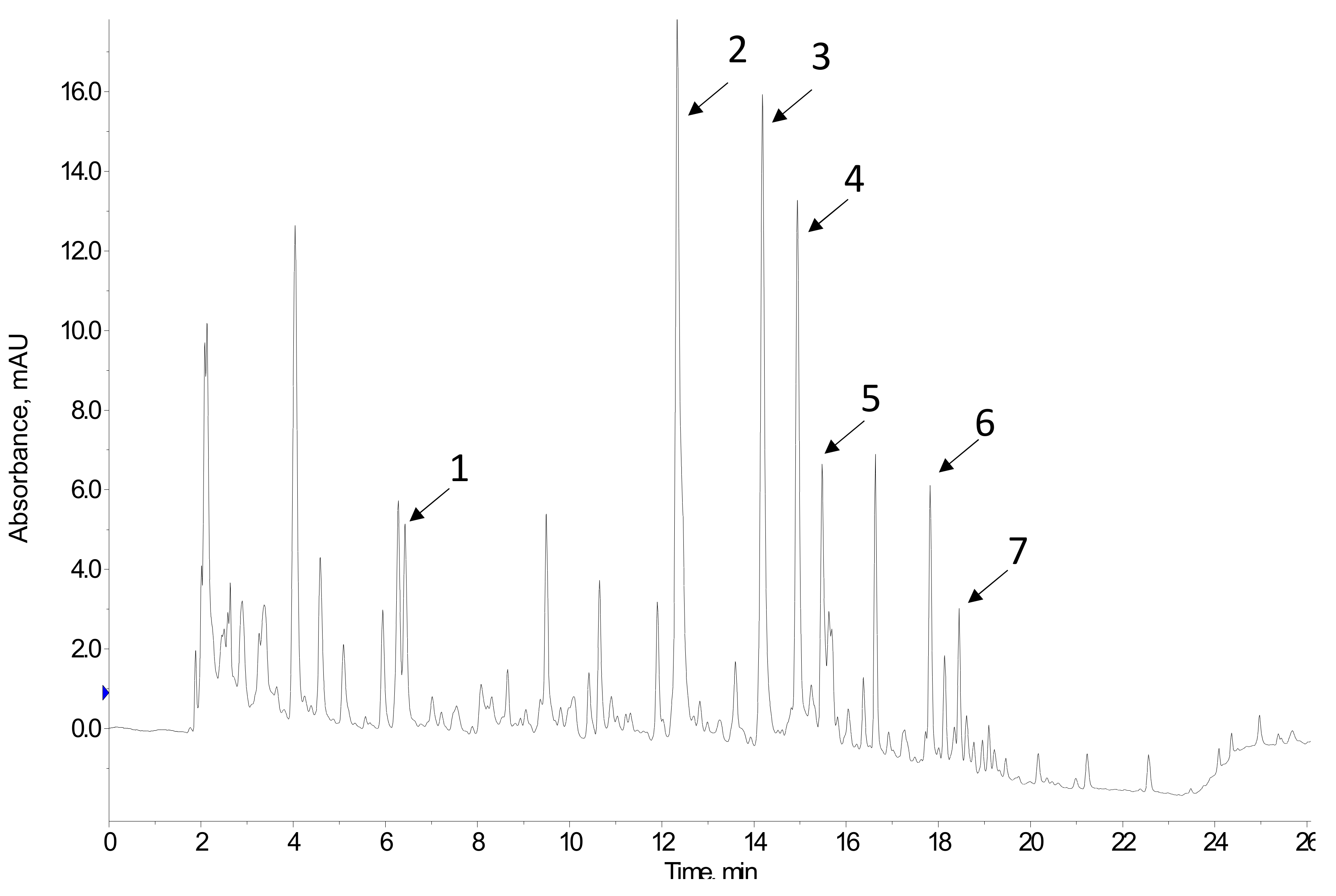
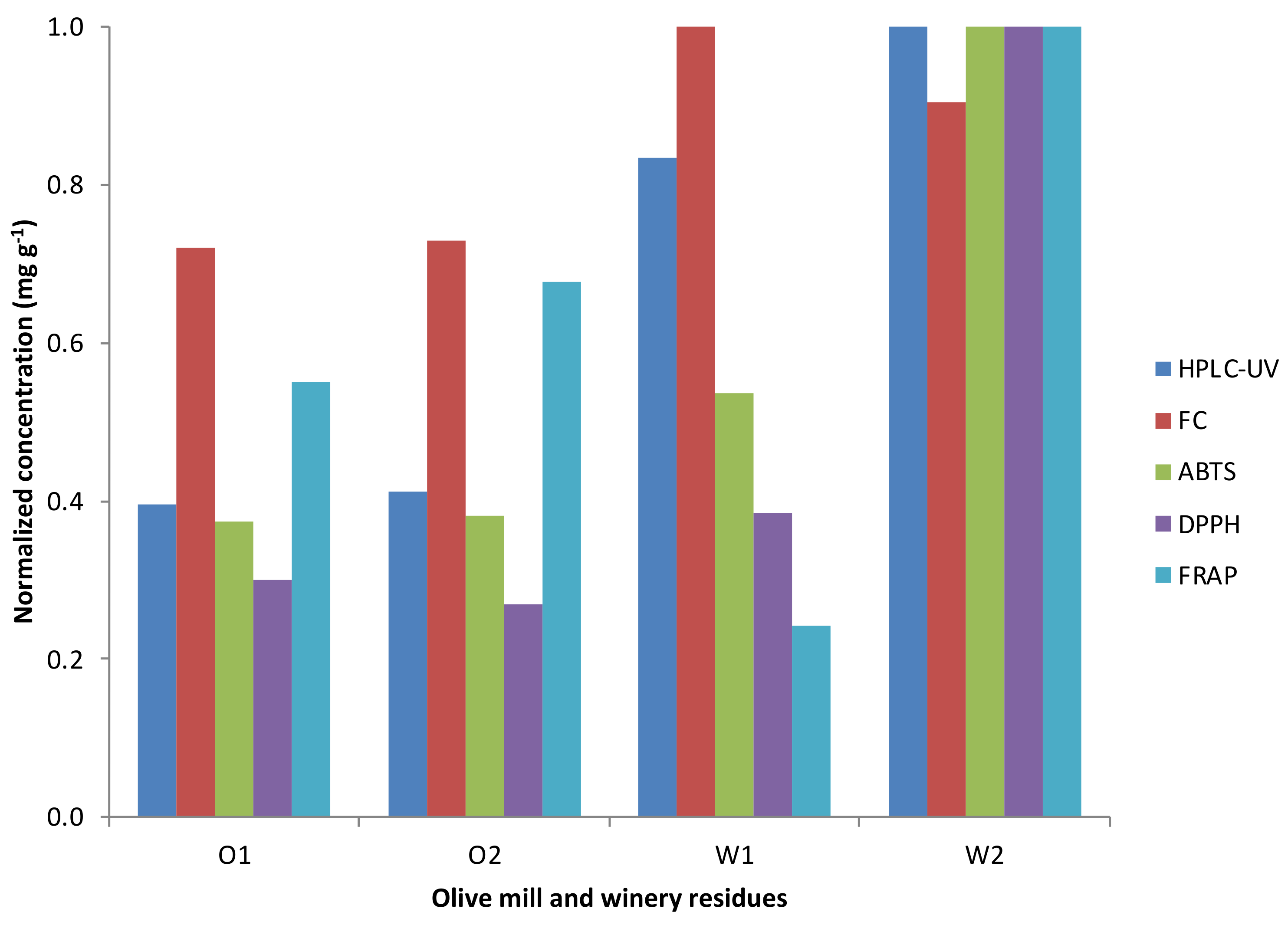
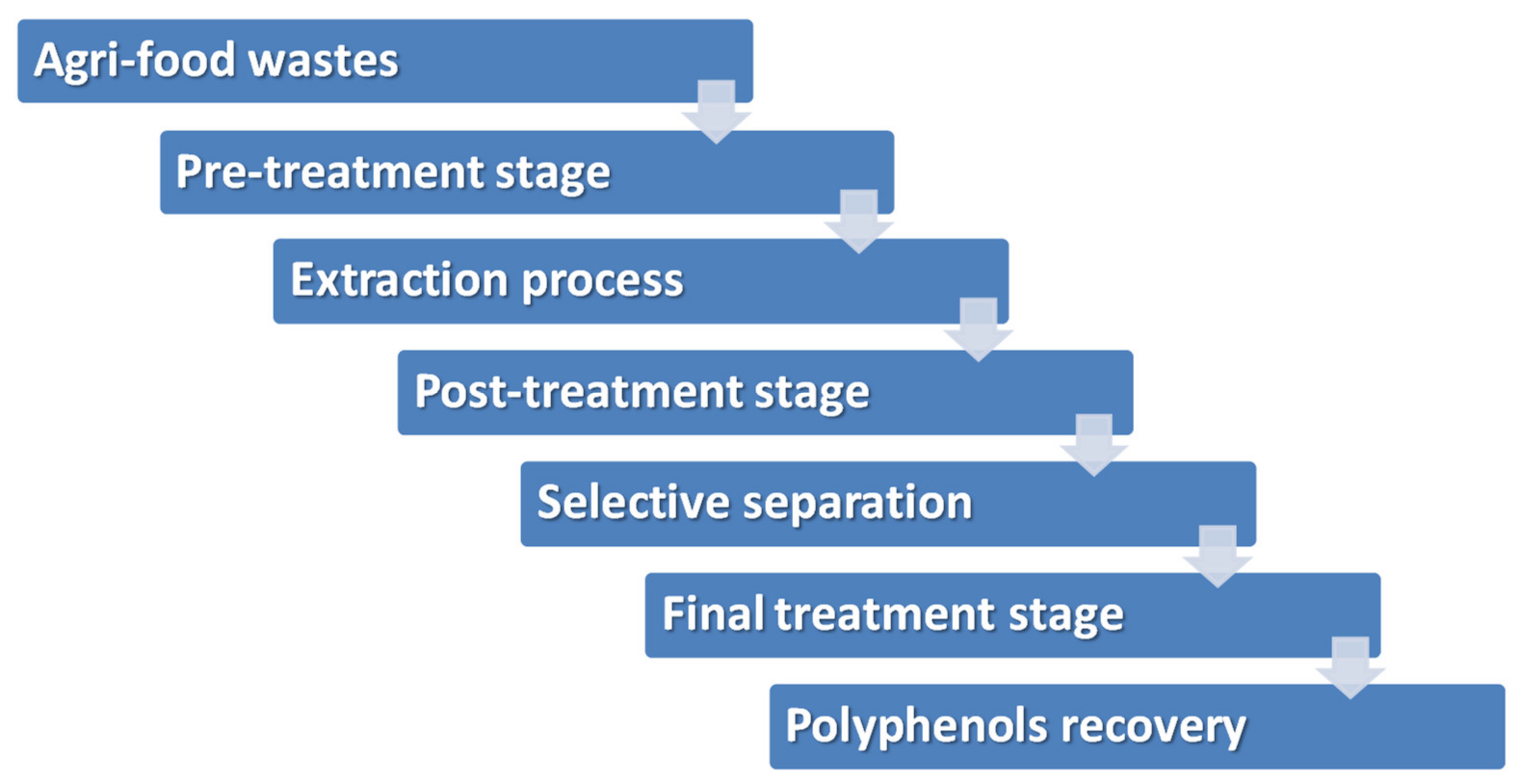

| Sample | Identified Phenolic Compounds | Concentration | Reference |
|---|---|---|---|
| Olive pomaces | Hydroxytyrosol | 5.3–512.6 mg kg−1 dw | [14,15] |
| Tyrosol | 886.7 mg kg−1 dw | ||
| Oleuropein | <0.5–162.9 mg kg−1 dw | ||
| 3,4-dihydroxybenzoic acid | 37.2 mg kg−1 dw | ||
| Vanillic acid | 21.6 mg kg−1 dw | ||
| Homovanillic acid | 12.5 mg kg−1 dw | ||
| p-hydroxybenzoic acid | 3.3 mg kg−1 dw | ||
| Luteolin | 32.9–410.9 mg kg−1 dw | ||
| Rutin | 1.3–354.2 mg kg−1 dw | ||
| Caffeic acid | 0.7–876.2 mg kg−1 dw | ||
| Chlorogenic acid | 9.7–47.7 mg kg−1 dw | ||
| Ferulic acid | 6.1–34.6 mg kg−1 dw | ||
| p-coumaric acid | 8.0–67.1 mg kg−1 dw | ||
| Quercetin | 0.5–36.6 mg kg−1 dw | ||
| Naringenin | 0.9–3.3 mg kg−1 dw | ||
| Olive mill wastewaters | Hydroxytyrosol | 102–1409 mg L−1 | [16] |
| Tyrosol | 14–425 mg L−1 | ||
| Caffeic acid | 1–4 mg L−1 | ||
| Elenolic acid | 87–1884 mg L−1 | ||
| Salidroside | 33–265 mg L−1 | ||
| Comselogoside | 1–2 mg L−1 | ||
| Hydroxytyrosol 4-O-Glucoside | 54–3150 mg L−1 | ||
| Hydroxytyrosol 1-O-Glucoside | 23–27 mg L−1 | ||
| Hydroxytyrosol Glycol | 132–325 mg L−1 | ||
| Ester of caffeic | 1 mg L−1 |
| Sample | Identified Phenolic Compounds | Concentration | Reference |
|---|---|---|---|
| Grape pomaces | p-hydroxybenzoic acid | 6.03–50.9 mg kg−1 dw | [28] |
| Protocatechuic acid | 4.34–57.4 mg kg−1 dw | ||
| Gallic acid | 149–987 mg kg−1 dw | ||
| Ellagic acid | 196–1040 mg kg−1 dw | ||
| Vanillic acid | 97.5–302 mg kg−1 dw | ||
| Syringic acid | 24.1–660 mg kg−1 dw | ||
| p-coumaric acid | 2.85–77.4 mg kg−1 dw | ||
| Chlorogenic acid | 4.51–102 mg kg−1 dw | ||
| Caffeic acid | 75.1–82.8 mg kg−1 dw | ||
| Resveratrol | 6.10–78.0 mg kg−1 dw | ||
| Quercetin | 547–848 mg kg−1 dw | ||
| Rutin | 8.11–569 mg kg−1 dw | ||
| Kaemferol | 454–553 mg kg−1 dw | ||
| Catechin | 403–3711 mg kg−1 dw | ||
| Grape stems | Gallic acid | 70.4–469 mg kg−1 dw | [29] |
| (+)-Catechin | 385–1858 mg kg−1 dw | ||
| (+)-Epicatechin | 12.3–189 mg kg−1 dw | ||
| Procyanidin B3 | 138–993 mg kg−1 dw | ||
| Procyanidin B2 | 36.0–165 mg kg−1 dw | ||
| Epicatechin gallate | 34.2–130 mg kg−1 dw | ||
| trans-Caftaric acid | 5.1–274 mg kg−1 dw | ||
| trans-Resveratrol | 74.0–266 mg kg−1 dw | ||
| 3-Viniferin | 167–499 mg kg−1 dw | ||
| Wine lees | (+)-Catechin | 43.1–50.1 mg L−1 | [30] |
| (-)-Epicatechin | 7.7–517.1 mg L−1 | ||
| Procyanidin B1 | 15.3–46.8 mg L−1 | ||
| Procyanidin B2 | 19.4–29.7 mg L−1 | ||
| Myricetin | 1.3–1.8 mg L−1 | ||
| Quercetin | 4.2 mg L−1 | ||
| Gallic acid | 8.1–35.9 mg L−1 | ||
| trans-caftaric acid | 21.2–23.3 mg L−1 | ||
| trans-coutaric acid | 1.3–9.6 mg L−1 | ||
| Caffeic acid | 0.7 mg L−1 | ||
| p-coumaric acid | 0.6–0.9 mg L−1 | ||
| Ferulic acid | 0.2–0.9 mg L−1 |
| Technique | Sample | Solvent | Experimental Conditions | Polyphenols Concentration | Reference |
|---|---|---|---|---|---|
| Olive Mill Residues | |||||
| Liquid–liquid extraction | Olive mill wastewater | Ethyl acetate | 25 °C, four extraction cycles | 6.490 ± 0.063 g GAE L−1 | [50] |
| Liquid–liquid extraction | Olive mill wastewater | Ethyl acetate | 25 °C, 20 min, four extraction cycles | 1407 mg GAE L−1 | [89] |
| Liquid–liquid extraction | Olive mill wastewater | Ethyl acetate | 1:1 v/v, 25 °C, two extraction cycles | 8.90 ± 0.728 mg GAE L−1 | [90] |
| Liquid–liquid extraction | Olive mill wastewater | Ethyl acetate | 25 °C, three extraction cycles | 9.8 g tyrosol equivalents L−1 | [21] |
| Liquid–liquid extraction | Olive mill wastewater | Ethyl acetate | 1:2 v/v, 27 °C, 30 min | 3440 mg GAE L−1 | [91] |
| SLE | Olive pomace | Methanol | 1:25 w/v, 70 °C, 12 h | 4.37 mg GAE g−1 | [45] |
| SLE | Olive pomace | Ethanol | 1:5 w/v, 25 °C, 180 min, pH 2 | 1.23 ± 0.21 caffeic acid equivalents (CAE) | [92] |
| SLE | Olive pomace | Methanol | 1:10, w/v, 180 °C, 90 min | 45.2 mg CAE g−1 | [93] |
| SLE | Dry olive mill residue | Water | 1:15 w/v, 25 °C, 40 min | 25 mg GAE g−1 | [94] |
| SLE | Olive pomace | Dimethyl sulfoxide | 1:3 w/v, 25 °C, 30 min | 1.3 g kg−1 | [48] |
| SLE | Olive pomace | Ethanol:water 80:20 v/v | 1:2 w/v, 25 °C, 120 min | 171 ± 4 mg of gallic acid 100 g−1 | [95] |
| SLE | Olive leaves | Dimethyl sulfoxide | 1:15 w/v, 25 °C, 30 min | 50 g kg−1 | [48] |
| MAE | Olive leaves | Water | 86 °C, 3 min | 104.22 ± 0.61 mg GAE g−1 | [96] |
| UAE | Olive pomace | Isopropanol:water 1:1 v/v | 1:5 w/v, 25 °C, 40 min | 69.66 mg GAE g−1 | [47] |
| UAE | Olive pomace | Ethanol:water 90:10, v/v | 1:30, w/v, 50 °C, 5 min | 55.1 mg g−1 hydroxytyrosol, 381.2 mg g−1 maslinic acid and 29.8 mg g−1 oleanolic acid | [97] |
| UAE | Olive pomace | Water | 1:50 w/v, 30 °C, 75 min | 19.71 ± 1.41 mg GAE g−1 | [98] |
| PLE | Olive pomace | Ethanol:water 50:50 v/v | 120 °C, 20 min | 5.8% extraction yield (8 gr) | [53] |
| PLE | Olive leaves | Ethanol:water1234550:50 v/v | 80 °C, 5 min | 53.15 mg GAE g−1 | [99] |
| SFE | Olive pomace | Carbon dioxide | 40 °C, 350 bar, 60 min | 0.76 ± 0.15 CAE | [92] |
| PEF | Olive leaves | Ethanol:water (25% EtOH) | Pulse duration: 10 µs, pulse period: 1000 µs, electric field 1 kV cm−1, time: 30 min | 20.75 mg GAE g−1 | [86] |
| Winery residues | |||||
| SLE | Grape pomace | Ethanol:water 50:50 v/v | 1:25 w/v, 60 °C, 2 h | 196.2 ± 22.7 mg GAE g−1 | [58] |
| SLE | Grape pomace | Ethyl acetate | 1:10 w/v, 25 °C, 6 h | 70.5 ± 0.03 mg GAE g−1 | [28] |
| SLE | Grape skins | Ethanol | 0.10:1 w/v, 25 °C, 19 h | 3.22 mg GAE g−1 | [55] |
| SLE | Grape pomace | Acetone | 1:12.5 w/v, 60 °C, 45 min | 31.25 mg GAE g−1 | [100] |
| SLE | Wine lees | Methanol/2% HCl 95:5 v/v | 1:5 w/v, 25 °C, 60 min, three extraction cycles | 2316.6 ± 37.9 mg GAE 100 g−1 | [60] |
| UAE | Grape skins | Ethanol:water 50:50 v/v | 1:10 w/v, 28 °C, 9 min | 80 mg GAE g−1 | [56] |
| UAE combined with SFE | Grape pomace | UAE: Ethanol:water (ethanol concentration 449.73 g L−1), SFE: Carbon dioxide | UAE: 1:4 w/v, 80 °C, 4 min SFE: 8 MPa, 40 °C, 30 min | 3493 mg GAE 100 g−1 | [101] |
| UAE | Grape pomace | Ethanol:water 1:1 v/v | 1:70 w/v, 20 °C, 60 min | 438984 ± 4034 ppm GAE | [57] |
| UAE | Grape seeds, pomace, and stems | Ethanol:water (44% of ethanol) | 1:4 w/v, ˂ 50°C, 3 min, two extraction cycles | 188, 89.15, 63.46 mg GAE g−1 for grape seeds, pomace, and stems, respectively | [70] |
| UAE | Wine lees | Ethanol 43.9% | 1:60 w/v, 60 °C, 25 min | 58.76 mg GAE g−1 | [102] |
| PLE | Grape pomace | Ethanol:water 50:50 v/v | 80 °C, 50 min, 100 bar | 79 g GAE kg−1 | [103] |
| EAE | Grape seeds | Water, pH 3.55, Lallzyme EX-V | Enzyme dosage 20 mg g−1, 48 °C, 2.60 h | Flavan-3-ols 21.41 ± 21 mg kg−1 Gallic acid 227.04 ± 0.35 mg kg−1 | [84] |
| OH | Grape pomace | Ethanol:water (30% ethanol) | 400 V cm−1, 50 °C, 60 min | 620 mg GAE 100 g−1 | [85] |
| Technique | Sample | Membrane | Polyphenols Concentration | Reference |
|---|---|---|---|---|
| Olive Mill Residues | ||||
| MF, UF and NF | Olive mill wastewater | Permapore EOV 1046 (MF), Permapore DGU 1812 BS EM (UF) and PERMAPORE AEN 1812 BS (NF) | 2456 to 5284 μg mL−1 (MF) 1404 to 3065 μg mL−1 (UF) 373 to 1583 μg mL−1 (NF) | [44] |
| MF and UF | Olive mill wastewater | Microlab 130 S (MF) and 18 PCI (UF) | 7.2 g L−1 of hydroxytyrosol | [117] |
| MF, UF and NF | Olive mill wastewater | Becopad P550 (MF) and PES spiral membrane from 100 kDa and 3–5 Da MWCO | 250, 250 and 430 mg in the NF retentate of the first, second, and third treatment, respectively | [118] |
| NF and RO | Olive pomace | NF270 (NF), NF90 (NF) and BW30 (RO) | 1063.9, 1069.4 and 1234.3 mg GAE L−1 for NF270, NF90 and BW30, respectively | [106] |
| MF, UF, NF and RO | Green leaves, dried leaves, and pitted olive pulp | Tubular ceramic membranes in titanium oxide (MF) and spiral wound module membranes in PES (UF, NF and RO) | 244.15, 57.63 and 289.93 mg g−1 for green leaves, dried leaves, and pitted olive pulp, respectively | [126] |
| UF, NF and RO | Olive mill wastewater, grape marc, and olive leaves | Tubular ceramic zirconia membrane (UF) and spiral wound polymeric membrane (NF and RO) | 378, 98, and 190 g GAE L−1 for olive mill wastewater, olive leaf extract and grape marc extract, respectively | [113] |
| MF and RO | Olive mill wastewater | Membralox EP19-40 (MF) and SW30HR (RO) | 1070 mg L−1 | [109] |
| Winery residues | ||||
| MF, UF and NF | Vinasses | Iberlact (MF and NF) and Tami (UF) membranes | 0.45 g GAE g−1 | [61] |
| MF | Wine lees | V0.2 and MFP5, flat-sheet membranes, and plasma membrane-associated membranes (PAM) hollow fiber | 26.1 mg GAE L−1 | [122] |
| MF | Wine lees | PVDF flat-sheet membrane with 0.2 mm pore size and polyimide hollow fiber membrane with 0.4 mm pore size | 1 g GAE L−1 | [108] |
| UF and NF | Wine lees | M-U2540 (UF), ESP04 (UF), HYDRACoRe 70pHT (NF), NF270 (NF), NF90 (NF), and HFW1000 (NF) | 2.65 g GAE L−1 | [124] |
| MF and NF | Wine lees | Polyvinylidenefluoride (PVDF) hollow fiber membranes (MF), NP010 (NF), NP030 (NF) and MPF36 (NF) | 982.1 mg GAE L−1 | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods 2022, 11, 362. https://doi.org/10.3390/foods11030362
Tapia-Quirós P, Montenegro-Landívar MF, Reig M, Vecino X, Cortina JL, Saurina J, Granados M. Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods. 2022; 11(3):362. https://doi.org/10.3390/foods11030362
Chicago/Turabian StyleTapia-Quirós, Paulina, María Fernanda Montenegro-Landívar, Mònica Reig, Xanel Vecino, José Luis Cortina, Javier Saurina, and Mercè Granados. 2022. "Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases" Foods 11, no. 3: 362. https://doi.org/10.3390/foods11030362
APA StyleTapia-Quirós, P., Montenegro-Landívar, M. F., Reig, M., Vecino, X., Cortina, J. L., Saurina, J., & Granados, M. (2022). Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods, 11(3), 362. https://doi.org/10.3390/foods11030362