Effect of Ultrasound-Assisted Solvent Enzymatic Extraction on Fatty Acid Profiles, Physicochemical Properties, Bioactive Compounds, and Antioxidant Activity of Elaeagnus mollis Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Materials
2.2. Oil Extraction
2.2.1. UASEE
2.2.2. SE
2.2.3. CP
2.3. Physicochemical Characteristics
2.4. Fatty Acid Profiles
2.5. Bioactive Compounds
2.5.1. Tocopherols and Tocotrienols
2.5.2. Squalene
2.5.3. Phytosterols
2.5.4. Total Phenolic Content (TPC)
2.5.5. Phenolic Compounds
2.6. Antioxidant Activity
2.7. Scanning Electron Micrographs (SEM)
2.8. Thermal Stability
2.9. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acid Profiles
3.2. Physicochemical Properties
3.3. Bioactive Compounds
3.3.1. Tocopherols and Tocotrienols
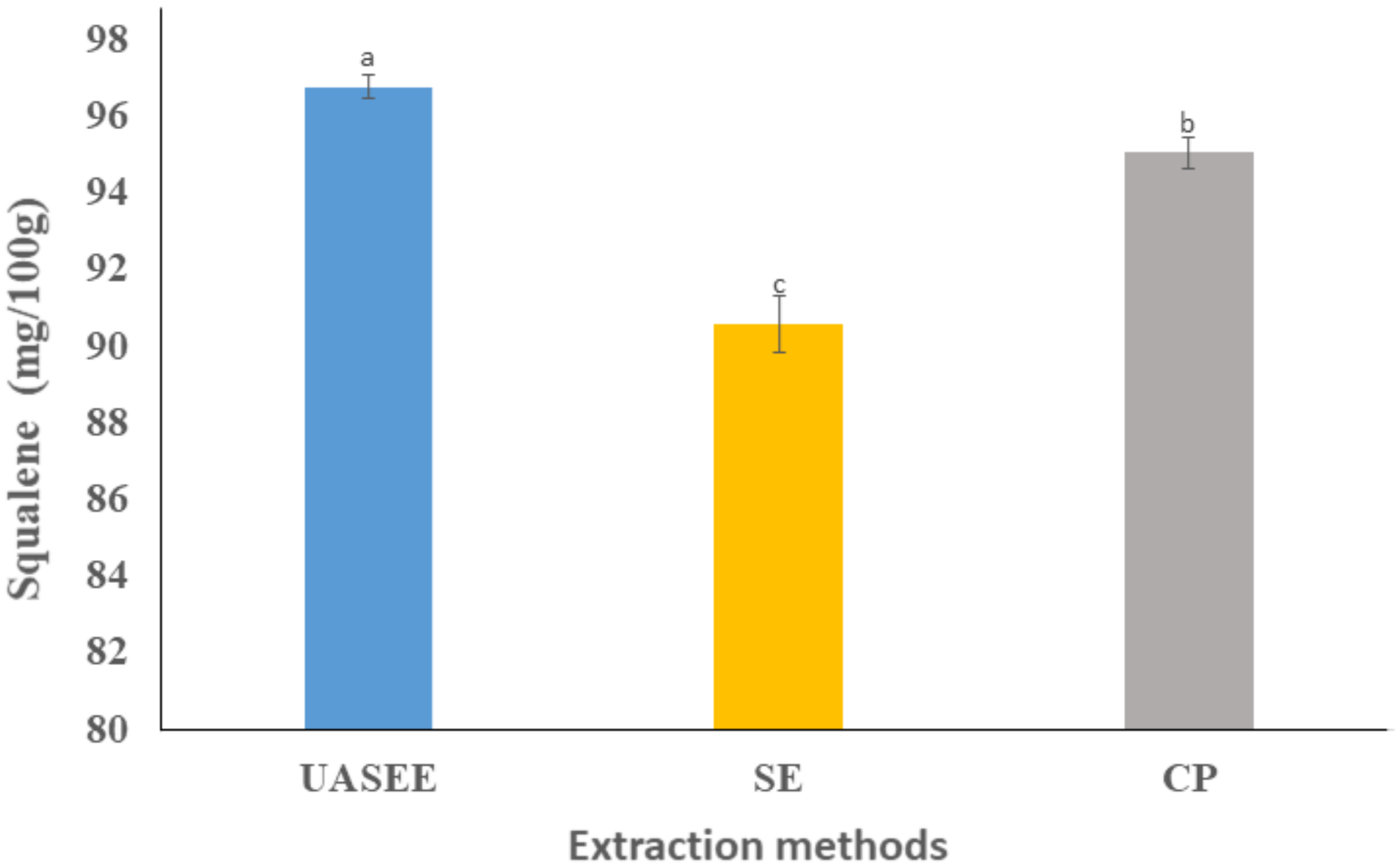
3.3.2. Squalene
3.3.3. Phytosterols
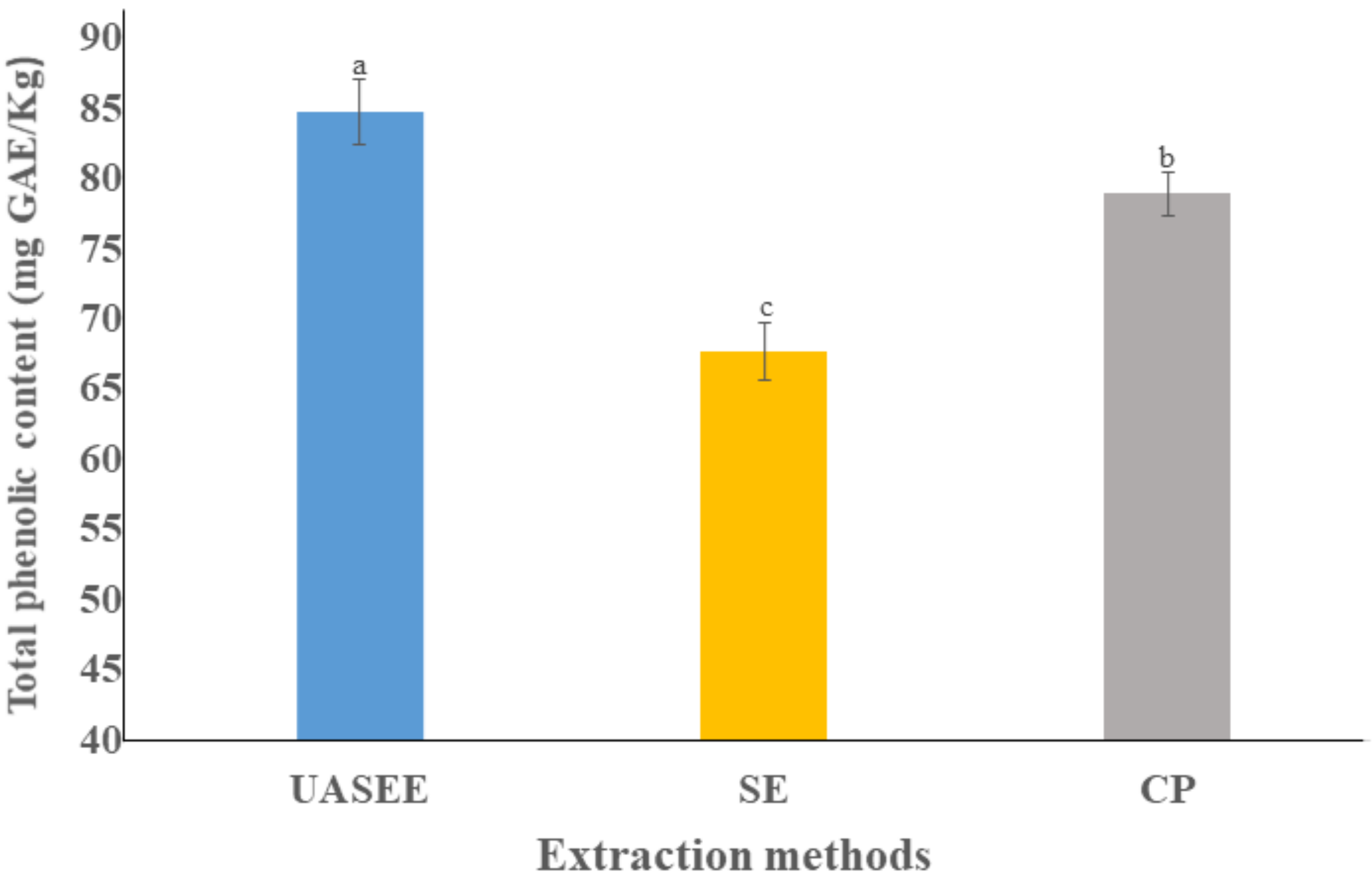
3.3.4. Total Phenolic Content and Profile Analysis
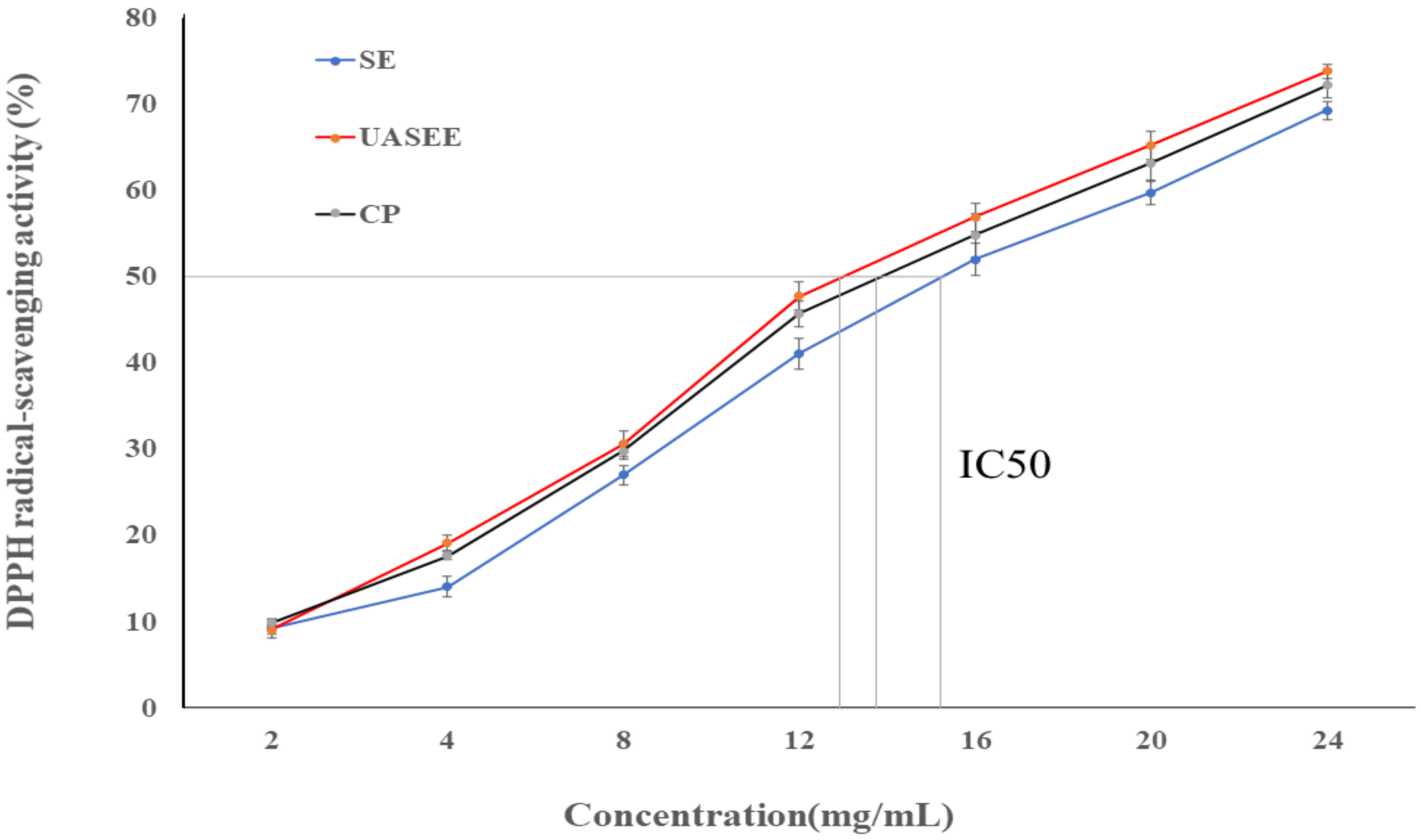
3.4. Antioxidant Activity
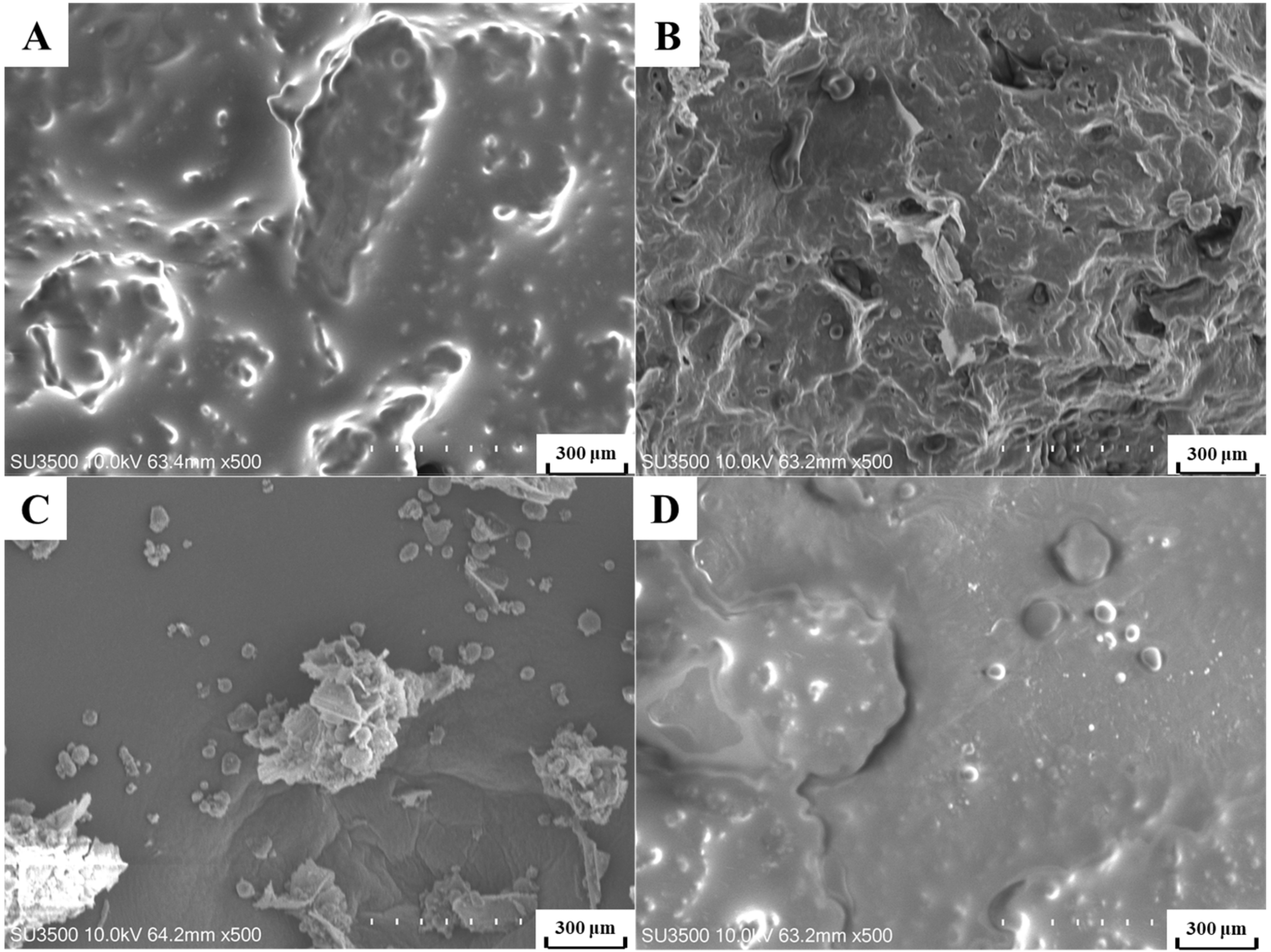
3.5. Microstructural Analysis
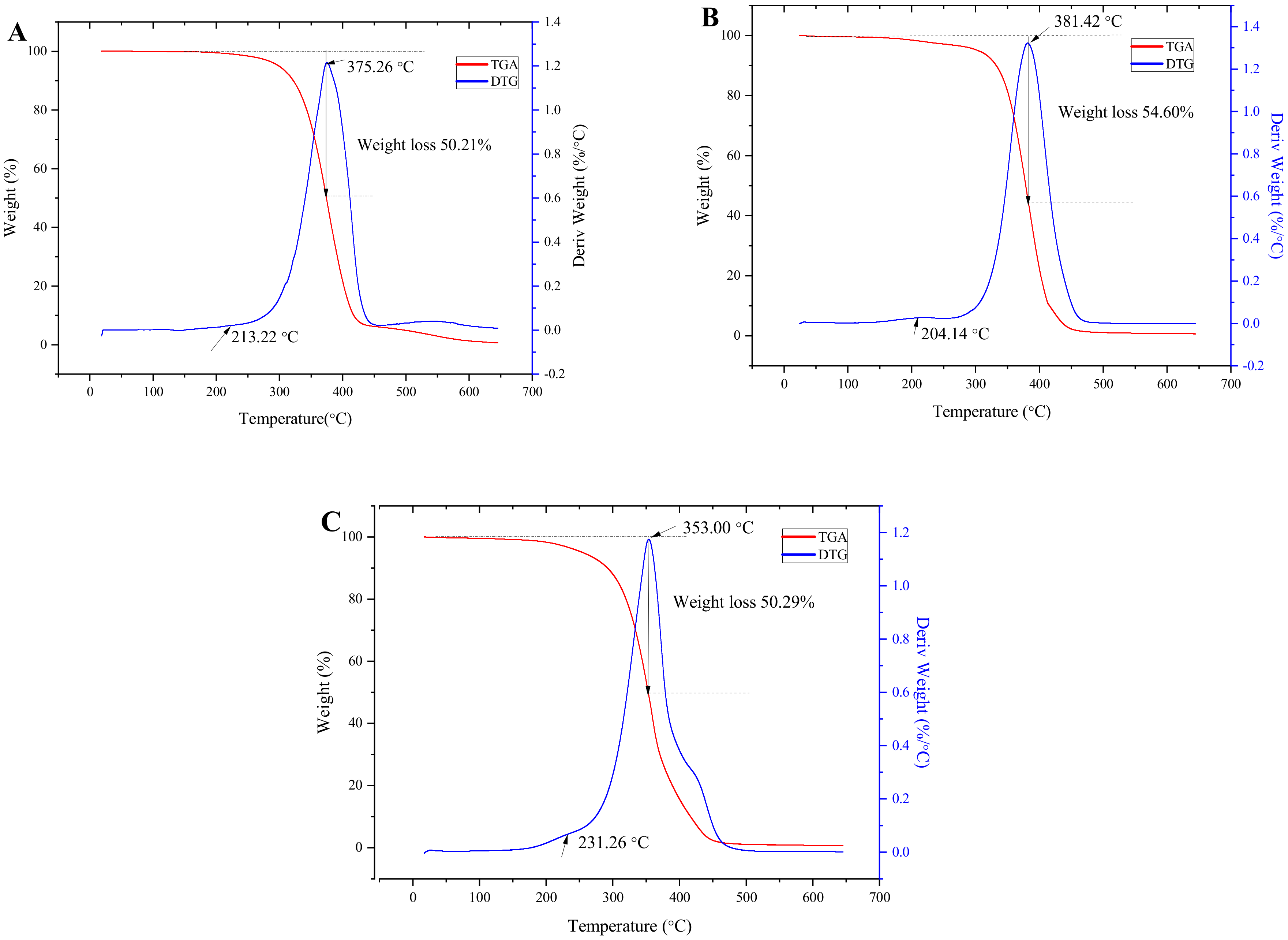
3.6. Thermal Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Zhao, W.; Lai, Y.; Zhang, B.; Zhang, D. Edible Plant Oil: Global Status, Health Issues, and Perspectives. Front. Plant Sci. 2020, 11, 1315. [Google Scholar] [CrossRef] [PubMed]
- Ceole, L.F.; Cardoso, M.D.G.; Soares, M.J. Nerolidol, the main constituent of Piper aduncum essential oil, has anti-Leishmania braziliensis activity. Parasitology 2017, 144, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Jassbi, A.R.; Zare, M.; Jamebozorgi, F.H. Chemical Composition and Biological Activity of the Essential Oil and Solvent Extracts of Scaligeria Nodosa. Open Bioact. Compd. J. 2017, 5, 16–22. [Google Scholar] [CrossRef][Green Version]
- Limaverde, P.W.; Campina, F.E.; da Cunha, F.A.B.; Crispim, F.D.; Figueredo, F.G.; Lima, L.F.; Tintino, C.; de Matos, Y.; Morais-Braga, M.F.B.; Menezes, I.R.A.; et al. Inhibition of the TetK efflux-pump by the essential oil of Chenopodium ambrosioides L. and alpha-terpinene against Staphylococcus aureus IS-58. Food Chem. Toxicol. 2017, 109, 957–961. [Google Scholar] [CrossRef]
- Carneiro, N.S.; Alves, C.C.F.; Alves, J.M.; Egea, M.B.; Martins, C.H.G.; Silva, T.S.; Bretanha, L.C.; Balleste, M.P.; Micke, G.A.; Silveira, E.V.; et al. Chemical composition, antioxidant and antibacterial activities of essential oils from leaves and flowers of Eugenia klotzschiana Berg (Myrtaceae). An. Acad. Bras. Cienc. 2017, 89, 1907–1915. [Google Scholar] [CrossRef]
- Ghasemi, Y.Z.; Dinani, S.T. Optimization of ultrasound-assisted enzymatic extraction of walnut kernel oil using response surface methodology. J. Food Process. Eng. 2018, 41, 18. [Google Scholar] [CrossRef]
- Yuan, W.Q.; Hu, J.Z.; Yin, L.Q.; Lv, Z.L. Comparative Analysis of Essential Bioactive Components of Oils Originating from Three Chinese Loess Plateau Wild Crops. J. Am. Oil Chem. Soc. 2021, 98, 531–540. [Google Scholar] [CrossRef]
- Wang, C.X.; Duan, Z.H.; Fan, L.P.; Li, J.W. Supercritical CO2 Fluid Extraction of Elaeagnus mollis Diels Seed Oil and Its Antioxidant Ability. Molecules 2019, 24, 911. [Google Scholar] [CrossRef]
- Chen, Y. Effects of Elaeagnus mollis Diels. seed oil on antioxidant capacity and lipid metabolism of mice. China Oils Fats 2017, 42, 77–80. [Google Scholar]
- Wang, X.M.; Bai, B.; Li, J.; Zhang, K.; Xie, Y.; Xun, B.N.; Bi, X.P. Development of the Elaeagnus Mollis Oil Submicron-emulsion of Its Anti-fatigue Activity. Food Res. Dev. 2018, 39, 159–165. [Google Scholar]
- Guo, C.X.; Qiao, J.P.; Zhang, S.W.; Li, M.P.; Li, J.; Hatab, S. Elaeagnus mollis Oil Attenuates Non-Alcoholic Fatty Disease in High-Fat Diet Induced Obese Mice via Modifying the Expression of Lipid Metabolism-Related Genes. J. Oleo Sci. 2019, 68, 893–908. [Google Scholar] [CrossRef]
- Lee, K.Y.; Rahman, M.S.; Kim, A.N.; Jeong, E.J.; Kim, B.G.; Lee, M.H.; Kim, H.J.; Choi, S.G. Effect of superheated steam treatment on yield, physicochemical properties and volatile profiles of perilla seed oil. LWT-Food Sci. Technol. 2021, 135, 8. [Google Scholar] [CrossRef]
- Martinez, M.L.; Mattea, M.A.; Maestri, D.M. Pressing and supercritical carbon dioxide extraction of walnut oil. J. Food Eng. 2008, 88, 399–404. [Google Scholar] [CrossRef]
- Singla, M.; Sit, N. Application of ultrasound in combination with other technologies in food processing: A review. Ultrason. Sonochem. 2021, 73, 105506. [Google Scholar] [CrossRef]
- Heidari, S.H.; Dinani, S.T. The Study of Ultrasound-Assisted Enzymatic Extraction of Oil From Peanut Seeds Using Response Surface Methodology. Eur. J. Lipid Sci. Technol. 2018, 120, 13. [Google Scholar] [CrossRef]
- Amigh, S.; Dinani, S.T. Combination of ultrasound-assisted aqueous enzymatic extraction and cooking pretreatment for date seed oil recovery. Heat Mass Transf. 2020, 56, 2345–2354. [Google Scholar] [CrossRef]
- Kan, L.N.; Wang, L.; Ding, Q.Z.; Wu, Y.W.; Ouyang, J. Flash Extraction and Physicochemical Characterization of Oil from Elaeagnus mollis Diels Seeds. J. Oleo Sci. 2017, 66, 345–352. [Google Scholar] [CrossRef]
- Lu, X.R.; Wu, D.; Wang, Y.; Li, D.; Wang, L.J. Evaluation of yield and quality properties of Elaeagnus mollis oil produced by ultrasound-assisted solvent enzymatic extraction. Int. J. Food Eng. 2021, in press. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Naik, M.; Natarajan, V.; Rawson, A.; Rangarajan, J.; Manickam, L. Extraction kinetics and quality evaluation of oil extracted from bitter gourd (Momardica charantia L.) seeds using emergent technologies. LWT-Food Sci. Technol. 2021, 140, 110714. [Google Scholar] [CrossRef]
- Ben Mohamed, H.; Duba, K.S.; Fiori, L.; Abdelgawed, H.; Tlili, I.; Tounekti, T.; Zrig, A. Bioactive compounds and antioxidant activities of different grape (Vitis vinifera L.) seed oils extracted by supercritical CO2 and organic solvent. LWT-Food Sci. Technol. 2016, 74, 557–562. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Determination of Squalene in Vegetable Oil; NY/T 3673-2020; Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2020; pp. 1–40.
- State Administration for Market Regulation of China. Determination of Sterols; GB/T 39995-2021; State Administration for Market Regulation of China: Beijing, China, 2021; pp. 1–40.
- Ribeiro, P.P.C.; Damasceno, K.; de Veras, B.O.; de Oliveira, J.R.S.; Lima, V.L.D.; de Assis, C.R.D.; da Silva, M.V.; de Sousa, F.C.; de Assis, C.F.; Padilha, C.E.D.; et al. Chemical and biological activities of faveleira (Cnidoscolus quercifolius Pohl) seed oil for potential health applications. Food Chem. 2021, 337, 127771. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Sun, W.H.; Li, B.Z.; Shang, N.; Wang, Y.; Lv, W.Q.; Li, D.; Wang, L.J. Value-added application of Platycodon grandiflorus (Jacq.) A.DC. roots (PGR) by ultrasound-assisted extraction (UAE) process to improve physicochemical quality, structural characteristics and functional properties. Food Chem. 2021, 363, 130354. [Google Scholar] [CrossRef]
- Silva, E.K.; Azevedo, V.M.; Cunha, R.L.; Hubinger, M.D.; Meireles, M.A.A. Ultrasound-assisted encapsulation of annatto seed oil: Whey protein isolate versus modified starch. Food Hydrocoll. 2016, 56, 71–83. [Google Scholar] [CrossRef]
- Gharsallah, K.; Rezig, L.; Msaada, K.; Chalh, A.; Soltani, T. Chemical composition and profile characterization of Moringa oleifera seed oil. S. Afr. J. Bot. 2021, 137, 475–482. [Google Scholar] [CrossRef]
- Li, T.; Qu, X.Y.; Zhang, Q.A.; Wang, Z.Z. Ultrasound-assisted extraction and profile characteristics of seed oil from Isatis indigotica Fort. Ind. Crop. Prod. 2012, 35, 98–104. [Google Scholar] [CrossRef]
- Xu, D.X.; Hao, J.; Wang, Z.H.; Liang, D.D.; Wang, J.H.; Ma, Y.S.; Zhang, M. Physicochemical properties, fatty acid compositions, bioactive compounds, antioxidant activity and thermal behavior of rice bran oil obtained with aqueous enzymatic extraction. LWT-Food Sci. Technol. 2021, 149, 111817. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, C.S.; Wang, B.; Yagoub, A.A.; Ma, H.L.; Zhang, X.; Wu, M. Study of ultrasonic cavitation during extraction of the peanut oil at varying frequencies. Ultrason. Sonochem. 2017, 37, 106–113. [Google Scholar] [CrossRef]
- Fu, M.; Qu, Q.; Yang, X.; Zhang, X. Effect of intermittent oven drying on lipid oxidation, fatty acids composition and antioxidant activities of walnut. LWT-Food Sci. Technol. 2016, 65, 1126–1132. [Google Scholar] [CrossRef]
- Ma, W.J.; Qi, B.K.; Li, Y.; Wang, J.; Feng, H.X.; Cao, L.; Sui, X.N.; Jiang, L.Z. Aqueous Enzymatic Extraction Assisted by Ultrasonic of Oil from Evening Primrose Seed. Food Nutr. China 2015, 21, 54–58. [Google Scholar]
- Jiao, J.; Li, Z.G.; Gai, Q.Y.; Li, X.J.; Wei, F.Y.; Fu, Y.J.; Ma, W. Microwave-assisted aqueous enzymatic extraction of oil from pumpkin seeds and evaluation of its physicochemical properties, fatty acid compositions and antioxidant activities. Food Chem. 2014, 147, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.L.; Zhang, Q.; Fei, S.M.; Gu, H.Y.; Yang, L. Optimization of ultrasonic circulating extraction of samara oil from Acer saccharum using combination of Plackett-Burman design and Box-Behnken design. Ultrason. Sonochem. 2017, 35, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Z.; Gui, M.L.; Xu, T.T.; Zhang, L.; Kong, L.T.; Qin, L.; Zou, Z.R. Efficient aqueous enzymatic-ultrasonication extraction of oil from Sapindus mukorossi seed kernels. Ind. Crops Prod. 2019, 134, 124–133. [Google Scholar] [CrossRef]
- Hu, B.; Xi, X.H.; Li, H.C.; Qin, Y.X.; Li, C.; Zhang, Z.Q.; Liu, Y.T.; Zhang, Q.; Liu, A.P.; Liu, S.X.; et al. A comparison of extraction yield, quality and thermal properties from Sapindus mukorossi seed oil between microwave assisted extraction and Soxhlet extraction. Ind. Crops Prod. 2021, 161, 12. [Google Scholar] [CrossRef]
- Liang, S.H.; Yang, R.N.; Dong, C.W.; Yang, Q.P. Physicochemical Properties and Fatty Acid Profiles of Elaeagnus mollis Diels Nut Oils. J. Oleo Sci. 2015, 64, 1267–1272. [Google Scholar] [CrossRef]
- Chiacchierini, E.; Mele, G.; Restuccia, D.; Vinci, G. Impact evaluation of innovative and sustainable extraction technologies on olive oil quality. Trends Food Sci. Technol. 2007, 18, 299–305. [Google Scholar] [CrossRef]
- Nyam, K.L.; Tan, C.P.; Lai, O.M.; Long, K.; Man, Y.B.C. Physicochemical properties and bioactive compounds of selected seed oils. LWT-Food Sci. Technol. 2009, 42, 1396–1403. [Google Scholar] [CrossRef]
- Mathur, P.; Ding, Z.F.; Saldeen, T.; Mehta, J.L. Tocopherols in the Prevention and Treatment of Atherosclerosis and Related Cardiovascular Disease. Clin. Cardiol. 2015, 38, 570–576. [Google Scholar] [CrossRef]
- Konopka, I.; Roszkowska, B.; Czaplicki, S.; Tanska, M. Optimization of Pumpkin Oil Recovery by Using Aqueous Enzymatic Extraction and Comparison of the Quality of the Obtained Oil with the Quality of Cold-Pressed Oil. Food Technol. Biotechnol. 2016, 54, 413–420. [Google Scholar] [CrossRef]
- Fang, X.Z.; Du, M.H.; Luo, F.; Jin, Y.F. Physicochemical Properties and Lipid Composition of Camellia Seed Oil (Camellia oleifera Abel.) Extracted Using Different Methods. Food Sci. Technol. Res. 2015, 21, 779–785. [Google Scholar] [CrossRef]
- Ferrentino, G.; Giampiccolo, S.; Morozova, K.; Haman, N.; Spilimbergo, S.; Scampicchio, M. Supercritical fluid extraction of oils from apple seeds: Process optimization, chemical characterization and comparison with a conventional solvent extraction. Innov. Food Sci. Emerg. Technol. 2020, 64, 102428. [Google Scholar] [CrossRef]
- Basyigit, B.; Daghan, S.; Karaaslan, M. Biochemical, compositional, and spectral analyses of Isot (Urfa pepper) seed oil and evaluation of its functional characteristics. Grasas Y Aceites 2020, 71, 384. [Google Scholar] [CrossRef]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The phenolic compounds of olive oil: Structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef]
- Han, C.P.; Liu, Q.G.; Jing, Y.Q.; Wang, D.; Zhao, Y.; Zhang, H.; Jiang, L.Z. Ultrasound-Assisted Aqueous Enzymatic Extraction of Corn Germ Oil: Analysis of Quality and Antioxidant Activity. J. Oleo Sci. 2018, 67, 745–754. [Google Scholar] [CrossRef]
- Chen, R.Z.; Liu, Z.Q.; Zhao, J.M.; Chen, R.P.; Meng, F.L.; Zhang, M.; Ge, W.C. Antioxidant and immunobiological activity of water-soluble polysaccharide fractions purified from Acanthopanax senticosu. Food Chem. 2011, 127, 434–440. [Google Scholar] [CrossRef]
- Rosenthal, A.; Pyle, D.L.; Niranjan, K. Aqueous and enzymatic processes for edible oil extraction. Enzym. Microb. Technol. 1996, 19, 402–420. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Wang, L.J.; Li, D.; Jiao, S.S.; Chen, X.D.; Mao, Z.H. Ultrasound-assisted extraction of oil from flaxseed. Sep. Purif. Technol. 2008, 62, 192–198. [Google Scholar] [CrossRef]
- Jadhav, A.J.; Holkar, C.R.; Goswami, A.D.; Pandit, A.B.; Pinjari, D.V. Acoustic Cavitation as a Novel Approach for Extraction of Oil from Waste Date Seeds. ACS Sustain. Chem. Eng. 2016, 4, 4256–4263. [Google Scholar] [CrossRef]
- Santos, J.C.O.; dos Santos, I.M.G.; de Souza, A.G.; Prasad, S.; dos Santos, A.V. Thermal stability and kinetic study on thermal decomposition of commercial edible oils by thermogravimetry. J. Food Sci. 2002, 67, 1393–1398. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V.V. Thermal, oxidative and low temperature properties of methyl esters prepared from oils of different fatty acids composition: A comparative study. Thermochim. Acta 2014, 577, 33–40. [Google Scholar] [CrossRef]
| No. | Compounds | Regression Equation | R2 | Linear Range (μg/L) | m/z | RT (min) | LOD (mg/kg) | LOQ (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| 1 | chlorogenic acid | Y = 13025.5X + 9211.17 | 0.9999 | 2–500 | 353.1/191.1 | 1.597 | 1.5 | 5.0 |
| 2 | cinnamic acid | Y = 1833.16X + 5305.62 | 0.9987 | 2–500 | 149.0/131.0 | 2.263 | 1.5 | 5.0 |
| 3 | gallic acid | Y = 422.457X + 142.217 | 0.9998 | 2–200 | 169.0/79.1 | 2.360 | 0.15 | 0.5 |
| 4 | vanillic acid | Y = 150.973X + 1120.40 | 0.9973 | 2–200 | 167.1/108.0 | 2.588 | 0.3 | 1.0 |
| 5 | p-coumaric acid | Y = 9272.58X + 12986.6 | 0.9999 | 2–200 | 163.0/119.1 | 2.555 | 0.3 | 1.0 |
| 6 | sinapic acid | Y = 1819.15X − 4545.19 | 0.9990 | 2–500 | 223.1/164.1 | 2.655 | 1.5 | 5.0 |
| 7 | ferulic acid | Y = 2826.58X − 229.223 | 0.9999 | 2–200 | 193.1/134.1 | 2.871 | 0.3 | 1.0 |
| 8 | ellagic acid | Y = 175.979X − 1233.08 | 0.9990 | 2–200 | 301.2/229.0 | 2.913 | 0.3 | 1.0 |
| 9 | salicylic acid | Y = 18774.9X + 97389.3 | 0.9999 | 2–200 | 137.1/93.1 | 2.943 | 0.3 | 1.0 |
| 10 | 4-hydroxybenzoic acid | Y = 8625.58X + 27775.2 | 0.9997 | 2–200 | 137.1/93.1 | 2.973 | 0.15 | 0.5 |
| 11 | vanillin | Y = 11935.6X + 31234.6 | 0.9999 | 2–200 | 153.2/93.2 | 3.305 | 0.3 | 1.0 |
| 12 | epicatechin | Y = 1594.97X − 4848.19 | 0.9983 | 2–800 | 305.1/125.0 | 3.307 | 5.0 | 20.0 |
| 13 | quercetin | Y = 4288.29X − 7960.59 | 0.9996 | 2–200 | 301.0/151.0 | 3.309 | 0.15 | 0.5 |
| 14 | caffeic acid | Y = 9052.98X + 16558.7 | 0.9999 | 2–200 | 179.1/135.1 | 3.519 | 0.3 | 1.0 |
| Fatty Acid Profiles (%) | Extraction Methods | ||
|---|---|---|---|
| UASEE | SE | CP | |
| Myristic (14:0) | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.02 ± 0.00 a |
| Pentadecanoic (15:0) | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.02 ± 0.00 a |
| Palmitic acid (16:0) | 3.42 ± 0.13 c | 5.72 ± 0.26 a | 4.03 ± 0.13 b |
| Margaric (17:0) | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.05 ± 0.00 a |
| Stearic acid (18:0) | 2.15 ± 0.15 b | 2.91 ± 0.14 a | 2.42 ± 0.20 b |
| Arachidic acid (20:0) | 0.32 ± 0.01 a | 0.35 ± 0.02 a | 0.21 ± 0.01 b |
| Behenic (22:0) | 0.06 ± 0.00 a | 0.06 ± 0.00 a | 0.06 ± 0.00 a |
| Saturated fatty acids (SFA) | 6.04 ± 0.29 c | 9.13 ± 0.41 a | 6.81 ± 0.15 b |
| Oleic acid (18:1) | 33.5 ± 0.21 c | 34.9 ± 0.20 b | 38. 9 ± 0.15 a |
| Eicosenoic acid (20:1) | 0.64 ± 0.01 b | 0.70 ± 0.01 a | 0.64 ± 0.03 b |
| Monounsaturated fatty acids (MUFA) | 34.18 ± 0.21 c | 35.60 ± 0.21 b | 39.57 ± 0.18 a |
| Eicosadienoic (20:2) | 0.10 ± 0.00 a | 0.10 ± 0.01 a | 0.10 ± 0.01 a |
| Linoleic acid (18:2) | 53.1 ± 0.35 a | 48.4 ± 0.67 b | 46.4 ± 0.38 c |
| α-linolenic acid (18:3) | 6.55 ± 0.12 b | 6.84 ± 0.27 b | 7.09 ± 0.13 a |
| Polyunsaturated fatty acids (PUFA) | 59.78 ± 0.44 a | 55.30 ± 0.60 b | 53.62 ± 0.24 b |
| Unsaturated fatty acids (UFA) | 93.96 ± 0.28 a | 90.9 ± 0.35 b | 93.19 ± 0.19 b |
| Physicochemical Properties | Extraction Methods | ||
|---|---|---|---|
| UASEE | SE | CP | |
| Oil yield (%) | 43.35 ± 0.26 a | 43.02 ± 0.77 a | 22.05 ± 0.12 b |
| Specific gravity (20 °C/g/mL) | 0.9150 ± 0.00 a | 0.9149 ± 0.00 a | 0.9149 ± 0.00 a |
| Refractive index (20 °C) | 1.474 ± 0.00 a | 1.474 ± 0.00 a | 1.474 ± 0.00 a |
| Acid value (mg/g) | 1.91 ± 0.02 a | 2.47 ± 0.04 b | 1.87 ± 0.03 a |
| Peroxide value (g/100 g) | 0.14 ± 0.01 a | 0.21 ± 0.03 b | 0.12 ± 0.02 a |
| Iodine value (g/100 g) | 162.96 ± 2.35 a | 151.35 ± 1.96 b | 160.31 ± 1.87 a |
| Saponification value (mg/g) | 174.47 ± 2.60 a | 174.48 ± 2.13 a | 176.77 ± 1.49 a |
| Bioactive Compounds | Extraction Methods | ||
|---|---|---|---|
| UASEE | SE | CP | |
| α-tocopherol (mg/100 g) | 3.58 ± 0.26 a | 2.70 ± 0.15 b | 3.34 ± 0.23 a |
| β-tocopherol (mg/100 g) | ND | ND | ND |
| γ-tocopherol (mg/100 g) | 134.50 ± 2.62 a | 111.75 ± 1.86 b | 132.08 ± 2.39 a |
| δ-tocopherol (mg/100 g) | 1.67 ± 0.05 a | 1.71 ± 0.02 b | 1.69 ± 0.04 a |
| α-tocotrienol (mg/100 g) | 1.13 ± 0.11 b | 1.53± 0.14 a | 1.52 ± 0.06 a |
| β-tocotrienol (mg/100 g) | ND | ND | ND |
| γ-tocotrienol (mg/100 g) | 5.72 ± 0.21 a | 4.97 ± 0.16 b | 5.42 ± 0.12 a |
| δ-tocotrienol (mg/100 g) | 0.71 ± 0.07 a | 0.79 ± 0.05 a | 0.80 ± 0.04 a |
| Total tocopherol and tocotrienol (mg/100 g) | 147.32 ± 2.19 a | 123.45 ± 1.74 b | 144.84 ± 2.24 a |
| β-Sitosterol (mg/100 g) | 183.13 ± 4.62 a | 170.23 ± 5.18 b | 139.03 ± 4.29 c |
| Stigmasterol (mg/100 g) | 6.53 ± 0.26 b | 6.72 ± 0.42 b | 7.58 ± 0.24 a |
| Ergosterol (mg/100 g) | 20.64 ± 1.26 a | 20.35 ± 1.19 a | 16.18 ± 0.83 b |
| Lupeol (mg/100 g) | 42.25 ± 1.37 a | 41.17 ± 0.68 a | 35.78 ± 1.94 b |
| Stigmastanol (mg/100 g) | 9.24 ± 0.20 a | 9.36 ± 0.36 a | 9.09 ± 0.17 a |
| Total Phytosterol (mg/100 g) | 261.78 ± 5.74 a | 247.82 ± 4.38 b | 207.66 ± 5.24 c |
| Salicylic acid (mg/kg) | 0.97 ± 0.03 a | 0.79 ± 0.01 c | 0.86 ± 0.03 b |
| Ferulic acid (mg/kg) | 2.53 ± 0.16 a | 1.43 ± 0.10 c | 2.09 ± 0.12 b |
| Cinnamic acid (mg/kg) | 0.66 ± 0.03 a | 0.63 ± 0.01 a | 0.65 ± 0.02 a |
| p-Coumaric acid (mg/kg) | 1.27 ± 0.06 a | 0.91 ± 0.07 c | 1.09 ± 0.03 b |
| DPPH (IC 50) (mg/mL) | 12.52 ± 0.28 a | 14.87 ± 0.48 b | 13.29 ± 0.30 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Du, H.; Liu, Y.; Wang, Y.; Li, D.; Wang, L. Effect of Ultrasound-Assisted Solvent Enzymatic Extraction on Fatty Acid Profiles, Physicochemical Properties, Bioactive Compounds, and Antioxidant Activity of Elaeagnus mollis Oil. Foods 2022, 11, 359. https://doi.org/10.3390/foods11030359
Lu X, Du H, Liu Y, Wang Y, Li D, Wang L. Effect of Ultrasound-Assisted Solvent Enzymatic Extraction on Fatty Acid Profiles, Physicochemical Properties, Bioactive Compounds, and Antioxidant Activity of Elaeagnus mollis Oil. Foods. 2022; 11(3):359. https://doi.org/10.3390/foods11030359
Chicago/Turabian StyleLu, Xiaorui, Hongmiao Du, Yuanyuan Liu, Yong Wang, Dong Li, and Lijun Wang. 2022. "Effect of Ultrasound-Assisted Solvent Enzymatic Extraction on Fatty Acid Profiles, Physicochemical Properties, Bioactive Compounds, and Antioxidant Activity of Elaeagnus mollis Oil" Foods 11, no. 3: 359. https://doi.org/10.3390/foods11030359
APA StyleLu, X., Du, H., Liu, Y., Wang, Y., Li, D., & Wang, L. (2022). Effect of Ultrasound-Assisted Solvent Enzymatic Extraction on Fatty Acid Profiles, Physicochemical Properties, Bioactive Compounds, and Antioxidant Activity of Elaeagnus mollis Oil. Foods, 11(3), 359. https://doi.org/10.3390/foods11030359




