In-Country Method Validation of a Paper-Based, Smartphone-Assisted Iron Sensor for Corn Flour Fortification Programs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Characterization of Mexican Corn Flour Samples
2.2. Replication Experiment (Determination of RE)
2.3. Comparison of Methods Experiment (Determination of SyE)
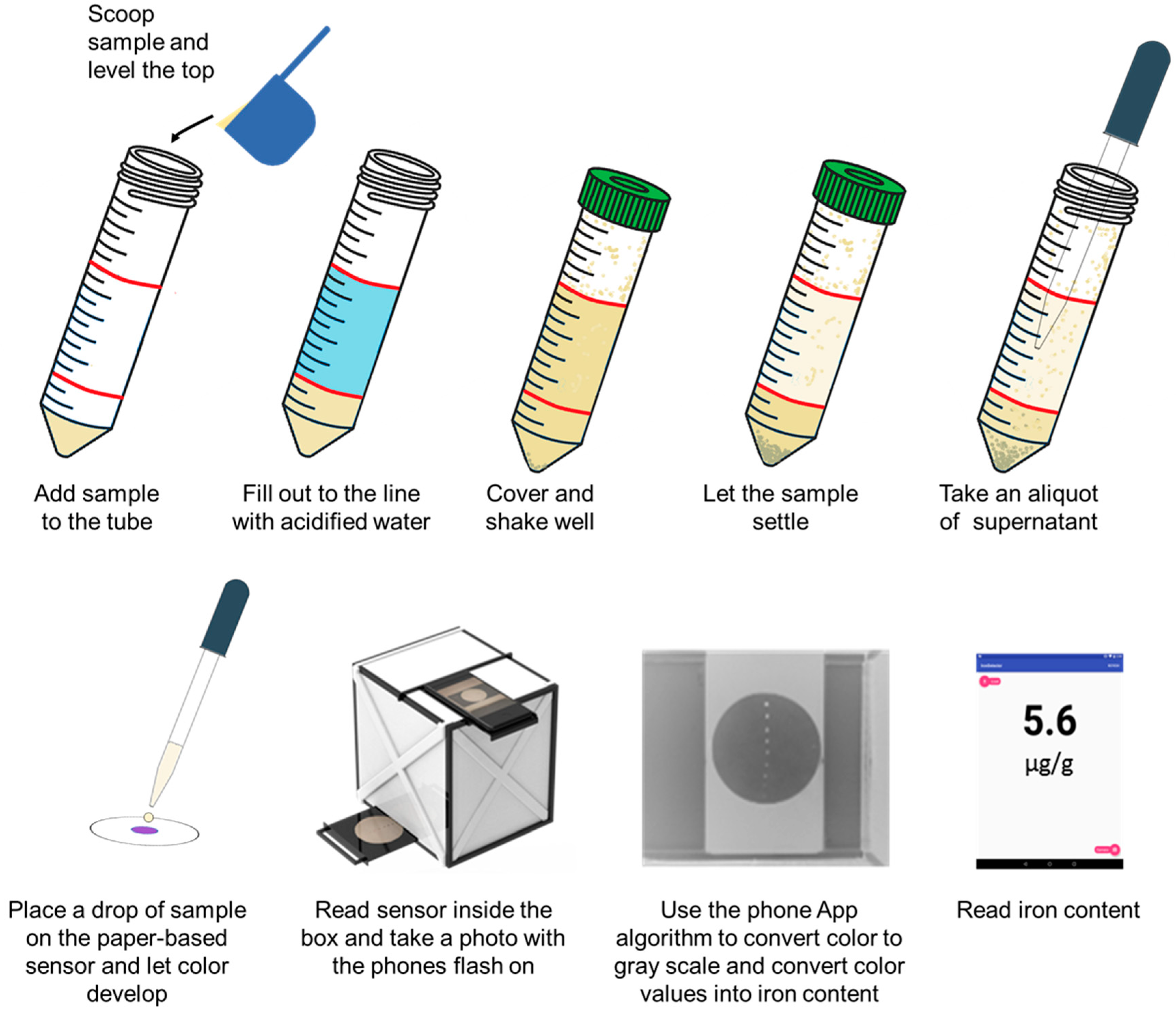
2.4. Development of an ASSURED-Designed Sampling Preparation Kit
2.5. Statistical Analysis
3. Results
3.1. Characterization of Mexican Corn Flours
3.2. Replication Experiment
3.3. Comparison of Methods Experiment
3.4. Development of a Sample Preparation Kit
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bailey, R.L.; West, K.P.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Mkambula, P.; Mbuya, M.N.N.; Rowe, L.A.; Sablah, M.; Friesen, V.M.; Chadha, M.; Osei, A.K.; Ringholz, C.; Vasta, F.C.; Gorstein, J. The unfinished agenda for food fortification in low-and middle-income countries: Quantifying progress, gaps and potential opportunities. Nutrients 2020, 12, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luthringer, C.L.; Rowe, L.A.; Vossenaar, M.; Garrett, G.S. Regulatory monitoring of fortified foods: Identifying barriers and good practices. Glob. Health Sci. Pract. 2015, 3, 446–461. [Google Scholar] [CrossRef] [Green Version]
- Uauy, R.; Hertrampf, E.; Reddy, M. Iron fortification of foods: Overcoming technical and practical barriers. J. Nutr. 2002, 132, 849S–852S. [Google Scholar] [CrossRef] [Green Version]
- Waller, A.W.; Lotton, J.L.; Gaur, S.; Andrade, J.M.; Andrade, J.E. Evaluation of micronutrient sensors for food matrices in resource-limited settings: A systematic narrative review. J. Food Sci. 2018, 83, 1792–1804. [Google Scholar] [CrossRef] [Green Version]
- Khansili, N.; Rattu, G.; Krishna, P. Label-free optical biosensors for food and biological sensor applications. Sens. Actuators B Chem. 2018, 265, 35–49. [Google Scholar] [CrossRef]
- Ruiz-Garcia, L.; Lunadei, L.; Barreiro, P.; Robla, J.I. A review of wireless sensor technologies and applications in agriculture and food industry: State of the art and current trends. Sensors 2009, 9, 4728–4750. [Google Scholar] [CrossRef] [Green Version]
- Peeling, R.W.; Holmes, K.K.; Mabey, D.; Ronald, A. Rapid tests for sexually transmitted infections (stis): The way forward. Sex Transm. Infect. 2006, 82, 1–6. [Google Scholar] [CrossRef]
- Li, F.; Bao, Y.; Wang, D.; Wang, W.; Niu, L. Smartphones for sensing. Sci. Bull. 2016, 61, 190–201. [Google Scholar] [CrossRef]
- Mahato, K.; Srivastava, A.; Chandra, P. Paper based diagnostics for personalized health care: Emerging technologies and commercial aspects. Biosens. Bioelectron. 2017, 96, 246–259. [Google Scholar] [CrossRef]
- Cha, S.S.; Seo, B.K. Smartphone use and smartphone addiction in middle school students in Korea: Prevalence, social networking service, and game use. Health Psychol. Open 2018, 5, 1–14. [Google Scholar] [CrossRef]
- Rateni, G.; Dario, P.; Cavallo, F. Smartphone-based food diagnostic technologies: A review. Sensors 2017, 17, 1453. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.Z.; Li, S.; Wang, S.; Lu, X. Detecting chemical hazards in foods using microfluidic paper-based analytical devices (μpads): The real-world application. Micromachines 2018, 9, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busa, L.S.A.; Mohammadi, S.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Advances in microfluidic paper-based analytical devices for food and water analysis. Micromachines 2016, 7, 86. [Google Scholar] [CrossRef]
- Trofimchuk, E.; Nilghaz, A.; Sun, S.; Lu, X. Determination of norfloxacin residues in foods by exploiting the coffee-ring effect and paper-based microfluidics device coupling with smartphone-based detection. J. Food Sci. 2020, 85, 736–743. [Google Scholar] [CrossRef]
- Alahmad, W.; Varanusupakul, P. Recent developments and applications of microfluidic paper-based analytical devices for the detection of biological and chemical hazards in foods: A critical review. Crit. Rev. Anal. Chem. 2021, 1–20. [Google Scholar] [CrossRef]
- Waller, A.W.; Toc, M.; Rigsby, D.J.; Gaytán-Martínez, M.; Andrade, J.E. Development of a paper-based sensor compatible with a mobile phone for the detection of common iron formulas used in fortified foods within resource-limited settings. Nutrients 2019, 11, 1673. [Google Scholar] [CrossRef] [Green Version]
- Chun, H.J.; Park, Y.M.; Han, Y.D.; Jang, Y.H.; Yoon, H.C. Paper-based glucose biosensing system utilizing a smartphone as a signal reader. BioChip J. 2014, 8, 218–226. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Bao, X.; Han, J.; Xia, J.; Tian, X.; Ni, L. A smartphone-based colorimetric reader coupled with a remote server for rapid on-site catechols analysis. Talanta 2016, 160, 194–204. [Google Scholar] [CrossRef]
- Martinez, A.; Phillips, S.; Carrilho, E.; Thomas, S.; Sindi, H.; Whitesides, G. Simple telemedicine for developing regions: Camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal. Chem. 2008, 80, 3699–3707. [Google Scholar] [CrossRef] [Green Version]
- Mentele, M.; Cunningham, J.; Koehler, K.; Volckens, J.; Henry, C. Microfluidic paper-based analytical device for particulate metals. Anal. Chem. 2012, 84, 4474–4480. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, V.; Pezzotti, G.; Pezzotti, I.; Cano, J.; Buonasera, K.; Giannini, D.; Giardi, M. Biosensors for effective environmental and agrifood protection and commercialization: From research to market. Microchim. Acta 2010, 170, 215–225. [Google Scholar] [CrossRef]
- Luong, J.; Male, K.; Glennon, J. Biosensor technology: Technology push versus market pull. Biotechnol. Adv. 2008, 26, 492–500. [Google Scholar] [CrossRef] [Green Version]
- Westgard, J.O.; Barry, P.L.; Quam, E.F.; Ehrmeyer, S.S. Basic Method Validation: Training in Analytical Quality Management for Healthcare Laboratories; Westgard Quality Corporation: Madison, WI, USA, 1999. [Google Scholar]
- Poitevin, E.; Nicolas, M.; Graveleau, L.; Richoz, J.; Andrey, D.; Monard, F. Improvement of aoac official method 984.27 for the determination of nine nutritional elements in food products by inductively coupled plasma-atomic emission spectroscopy after microwave digestion: Single-laboratory validation and ring trial. J. AOAC Int. 2009, 92, 1484–1518. [Google Scholar] [CrossRef] [Green Version]
- NOM-247-SSA1-2008. Norma Oficial Mexicana. Productos y Servicios. Cereales y sus Productos. Cereales, Harinas de Cereales, Sémolas o Semolinas. Alimentos a Base de: Cereales, Semillas Comestibles, de Harinas, Sémolas o Semolinas o sus Mezclas. Productos de Panificación. Disposiciones y Especificaciones Sanitarias y Nutrimentales. Métodos de Prueba; Diario Oficial de la Federación, Secretaria de Salud, Estados Unidos Mexicanos: Mexico City, Mexico, 2008.
- Trullols, E.; Ruisanchez, I.; Rius, F.X. Validation of qualitative analytical methods. TrAC Trends Anal. Chem. 2004, 23, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Pulido, A.; Ruisánchez, I.; Boqué, R.; Rius, F. Uncertainty of results in routine qualitative analysis. TrAC Trends Anal. Chem. 2003, 22, 647–654. [Google Scholar] [CrossRef]
- Parikh, R.; Mathai, A.; Parikh, S.; Sekhar, G.C.; Thomas, R. Understanding and using sensitivity, specificity and predictive values. Indian J. Ophthalmol. 2008, 56, 45. [Google Scholar] [CrossRef] [PubMed]
- Abras, C.; Maloney-Krichmar, D.; Preece, J. User-Centered Design. In Encyclopedia of Human-Computer Interaction; Bainbridge, W., Ed.; Sage Publications: Thousand Oaks, CA, USA, 2004; pp. 445–456. [Google Scholar]
- Kangas, E.; Kinnunen, T. Applying user-centered design to mobile application development. Commun. ACM 2005, 48, 55–59. [Google Scholar] [CrossRef]
- IBM. IBM SPSS Statistics for Windows, Version 24.0; IBM: Chicago, IL, USA, 2016. [Google Scholar]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Paredes López, O.; Guevara Lara, F.; Bello Pérez, L.A. La nixtamalización y el valor nutritivo del maíz. Ciencias 2009, 92, 263–270. [Google Scholar]
- Pappa, M.R.; de Palomo, P.P.; Bressani, R. Effect of lime and wood ash on the nixtamalization of maize and tortilla chemical and nutritional characteristics. Plant Foods Hum. Nutr. 2010, 65, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.M.M.; Figueroa, J.; Santiago-Ramos, D.; Villa, G.A.; Sandoval, S.J.; Rayas-Duarte, P.; Véles-Medina, J.J.; Flores, H.E.M. The effect of different nixtamalisation processes on some physicochemical properties, nutritional composition and glycemic index. J. Cereal Sci. 2015, 65, 140–146. [Google Scholar] [CrossRef]
- Allen, L.H.; De Benoist, B.; Dary, O.; Hurrell, R. Guidelines on Food Fortification with Micronutrients; WHO: Geneva, Switzerland, 2006.
- Feldsine, P.; Abeyta, C.; Andrews, W.H. Aoac international methods committee guidelines for validation of qualitative and quantitative food microbiological official methods of analysis. J. AOAC Int. 2002, 85, 1187–1200. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R. Food Fraud and Economically Motivated Adulteration of Food and Food Ingredients; Congressional Research Service: Washington, DC, USA, 2014.
- Barcaccia, G.; Lucchin, M.; Cassandro, M. Dna barcoding as a molecular tool to track down mislabeling and food piracy. Diversity 2016, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Mejia, L.A.; Kuo, W.Y.; Beltran-Velazquez, F. Provision of micronutrients in coexisting public health programs and risk of excessive intake: Regulatory considerations. Ann. N. Y. Acad. Sci. 2019, 1446, 66–80. [Google Scholar] [CrossRef]
- Morales, C.; Bosco, F.; González Santamaría, A.L. Evaluación del Contenido de Tiamina en Cuatro Marcas de Harina de Maíz Nixtamalizado, Fortificadas y Comercializadas en el Salvador; Universidad de El Salvador: San Salvador, El Salvador, 2009. [Google Scholar]
- Rohner, F.; Kangambega, M.O.; Khan, N.; Kargougou, R.; Garnier, D.; Sanou, I.; Ouaro, B.D.; Petry, N.; Wirth, J.P.; Jooste, P. Comparative validation of five quantitative rapid test kits for the analysis of salt iodine content: Laboratory performance, user-and field-friendliness. PLoS ONE 2015, 10, e0138530. [Google Scholar] [CrossRef]
- Laillou, A.; Icard-Vernière, C.; Rochette, I.; Picq, C.; Berger, J.; Sambath, P.; Mouquet-Rivier, C. Rapid quantification of iron content in fish sauce and soy sauce: A promising tool for monitoring fortification programs. Food Nutr. Bull. 2013, 34, S124–S132. [Google Scholar] [CrossRef] [Green Version]
- Piety, N.Z.; Yang, X.; Kanter, J.; Vignes, S.M.; George, A.; Shevkoplyas, S.S. Validation of a low-cost paper-based screening test for sickle cell anemia. PLoS ONE 2016, 11, e0144901. [Google Scholar] [CrossRef] [Green Version]
- Kanakasabapathy, M.K.; Sadasivam, M.; Singh, A.; Preston, C.; Thirumalaraju, P.; Venkataraman, M.; Bormann, C.L.; Draz, M.S.; Petrozza, J.C.; Shafiee, H. An automated smartphone-based diagnostic assay for point-of-care semen analysis. Sci. Transl. Med. 2017, 9, 382. [Google Scholar] [CrossRef] [Green Version]
- AACC. Approved Methods of Analysis: Method 40–40, 11th ed.; AACC: Washington, DC, USA, 1999. [Google Scholar]
- Appel, M.; Kuper, C.; Woutersen, R. Disposition, accumulation and toxicity of iron fed as iron (ii) sulfate or as sodium iron edta in rats. Food Chem. Toxicol. 2001, 39, 261–269. [Google Scholar] [CrossRef]
- Hund, E.; Massart, D.L.; Smeyers-Verbeke, J. Inter-laboratory studies in analytical chemistry. Anal. Chim. Acta 2000, 423, 145–165. [Google Scholar] [CrossRef]


| Company | Sample ID | N (%) | P (%) | Mg (%) | K (%) | Ca (%) | S (%) | B (ppm) | Mn (ppm) | Cu (ppm) | Zn (ppm) | Al (ppm) | Na (ppm) | Fe (ppm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 1 | 1.27 | 0.242 | 0.081 | 0.30 | 0.21 | 0.089 | 1.2 | 4.6 | 1.0 | 55.1 | 2.7 | 113 | 53.7 |
| 2 | 1.38 | 0.245 | 0.089 | 0.30 | 0.21 | 0.100 | 1.2 | 4.0 | 1.1 | 51.0 | 4.4 | 79.5 | 50.4 | |
| B | 3 | 1.25 | 0.265 | 0.091 | 0.31 | 0.08 | 0.091 | 1.2 | 4.9 | 1.0 | 50.1 | 14.8 | 89.4 | 78.6 |
| 4 | 1.28 | 0.271 | 0.094 | 0.32 | 0.06 | 0.090 | 1.0 | 4.4 | 0.8 | 57.7 | 1.3 | 20.4 | 83.3 | |
| 5 | 1.35 | 0.303 | 0.098 | 0.32 | 0.08 | 0.100 | 0.9 | 4.5 | 0.8 | 52.7 | 1.6 | 15.1 | 75.0 | |
| 6 | 1.40 | 0.266 | 0.095 | 0.32 | 0.08 | 0.091 | 1.0 | 4.5 | 0.9 | 68.4 | 5.1 | 44.0 | 119.0 | |
| 7 | 1.22 | 0.272 | 0.101 | 0.31 | 0.07 | 0.09 | 0.9 | 4.7 | 1.1 | 79.1 | 3.5 | 48.4 | 120.0 | |
| 8 | 1.19 | 0.286 | 0.104 | 0.34 | 0.07 | 0.094 | 1.3 | 4.8 | 1.0 | 89.0 | 1.2 | 42.2 | 157.0 | |
| 9 | 1.34 | 0.276 | 0.091 | 0.32 | 0.08 | 0.091 | 1.3 | 4.7 | 1.1 | 47.9 | 5.0 | 98.1 | 76.1 | |
| 10 | 1.32 | 0.299 | 0.105 | 0.33 | 0.09 | 0.097 | 1.1 | 4.8 | 1.1 | 76.0 | 3.0 | 32.1 | 118.0 | |
| 11 | 1.25 | 0.256 | 0.092 | 0.30 | 0.06 | 0.087 | 1.0 | 3.8 | 1.0 | 56.8 | 3.3 | 42.5 | 90.1 | |
| 12 | 1.27 | 0.267 | 0.092 | 0.31 | 0.07 | 0.092 | 1.4 | 4.3 | 1.0 | 43.7 | 3.9 | 98.1 | 67.0 | |
| 13 | 1.33 | 0.273 | 0.096 | 0.31 | 0.06 | 0.086 | 1.0 | 4.5 | 0.9 | 64.1 | 1.0 | 39.1 | 104.0 | |
| 14 | 1.26 | 0.249 | 0.085 | 0.28 | 0.08 | 0.086 | 1.3 | 4.2 | 1.0 | 30.9 | 3.4 | 80.6 | 43.5 | |
| 15 | 1.27 | 0.245 | 0.088 | 0.29 | 0.06 | 0.084 | 1.0 | 3.8 | 0.9 | 79.4 | 1.7 | 29.7 | 135.0 | |
| 16 | 1.26 | 0.249 | 0.091 | 0.29 | 0.06 | 0.087 | 1.0 | 4.0 | 0.9 | 72.5 | 3.7 | 44.2 | 119.0 | |
| C | 17 | 1.25 | 0.251 | 0.087 | 0.30 | 0.13 | 0.088 | 1.5 | 3.2 | 0.6 | 20.0 | 1.5 | 30.0 | 19.9 |
| D | 18 | 1.29 | 0.266 | 0.101 | 0.31 | 0.06 | 0.091 | 1.1 | 4.5 | 1.0 | 69.2 | 2.9 | 55.9 | 114.0 |
| E | 19 | 1.20 | 0.265 | 0.096 | 0.36 | 0.28 | 0.083 | 1.4 | 4.5 | 0.8 | 53.2 | 6.9 | 66.8 | 47.9 |
| 20 | 1.20 | 0.281 | 0.099 | 0.35 | 0.34 | 0.086 | 1.4 | 4.8 | 1.2 | 54.7 | 8.0 | 41.6 | 68.7 | |
| 21 | 1.24 | 0.288 | 0.095 | 0.35 | 0.37 | 0.085 | 1.5 | 4.6 | 0.7 | 55.0 | 7.6 | 48.1 | 77.2 | |
| 22 | 1.24 | 0.249 | 0.090 | 0.33 | 0.26 | 0.082 | 1.2 | 4.2 | 0.6 | 15.5 | 4.8 | 60.1 | 18.4 | |
| 23 | 1.28 | 0.262 | 0.093 | 0.34 | 0.29 | 0.091 | 1.6 | 4.6 | 1.0 | 49.5 | 9.1 | 61.7 | 73.0 | |
| 24 | 1.24 | 0.273 | 0.098 | 0.35 | 0.30 | 0.087 | 1.3 | 4.7 | 0.9 | 58.2 | 6.6 | 54.5 | 74.7 | |
| 25 | 1.28 | 0.255 | 0.091 | 0.35 | 0.31 | 0.094 | 1.5 | 4.3 | 0.8 | 47.5 | 7.3 | 49.8 | 56.2 | |
| 26 | 1.23 | 0.238 | 0.087 | 0.33 | 0.32 | 0.089 | 1.3 | 4.2 | 0.8 | 44.8 | 4.4 | 45.0 | 44.5 | |
| 27 | 1.24 | 0.287 | 0.101 | 0.35 | 0.30 | 0.089 | 1.5 | 4.8 | 0.8 | 108 | 4.3 | 36.6 | 69.2 | |
| F | 28 | 1.32 | 0.345 | 0.097 | 0.32 | 0.14 | 0.095 | 1.7 | 7.0 | 0.8 | 44.9 | 3.1 | 13.1 | 47.7 |
| 29 | 1.29 | 0.328 | 0.094 | 0.31 | 0.14 | 0.092 | 1.5 | 7.0 | 0.9 | 41.9 | 2.8 | 13.7 | 48.3 |
| n Days | ||||||
|---|---|---|---|---|---|---|
| 3 | 3 | 2 | 2 | 2 | Average RE | |
| CV% | 11% | 19% | 14% | 4% | 11% | 12% |
| AES Classification | Total | |||
|---|---|---|---|---|
| Pass 1 | Reject 2 | |||
| Nu3Px Classification | Pass | 26 | 3 | 29 |
| Reject | 5 | 11 | 16 | |
| Total | 31 | 14 | 45 | |
| Step in Sample Preparation | Matrix Tested | Sample Kit Tool | CV% (n = 5) | Laboratory Precision Tool | CV% (n = 5) |
|---|---|---|---|---|---|
| Deposition | Water | Eyedropper | 7.24 | Microliter Pipette | 0 |
| Deposition | Water | Plastic pipette | 2.75 | - | - |
| Deposition | Water | Glass pipette | 6.48 | - | - |
| Dilution | Water | Conical tube | 1.03 | Volumetric Pipette | 0.55 |
| Type of Analytical Error | Preliminary Error Evaluation | Final Error Evaluation |
|---|---|---|
| Random Error | 15.9% | 12.0% |
| Systematic Error (Constant) | 1.01 μg Fe/g flour | 1.79 ± 9.99 μg Fe/g flour |
| Systematic Error (Proportional) | 13.1% |
| ID | AES (μg/g Flour) | AES (Theoretical Policy) | AES (Actual Policy) | Nu3px (μg/g Flour) | Nu3Px (Theoretical Policy) | Nu3Px (Actual Policy) | Sensitivity Based on Theoretical Limits | Sensitivity Based on Actual Policy |
|---|---|---|---|---|---|---|---|---|
| 1A | 53.7 | High | Good | 50.9 | Good | Good | No match | Match |
| 2A | 50.4 | Good | Good | 56.2 | High | Good | No match | Match |
| 3B | 78.6 | High | Good | 66.9 | High | Good | Match | Match |
| 4B | 83.3 | High | Good | 90.6 | High | Good | Match | Match |
| 5B | 75 | High | Good | 77.7 | High | Good | Match | Match |
| 6B | 119 | High | Good | 120.4 | High | Good | Match | Match |
| 7B | 120 | High | Good | 120.9 | High | Good | Match | Match |
| 8B | 157 | High | Good | 152.4 | High | Good | Match | Match |
| 9B | 76.1 | High | Good | 95.9 | High | Good | Match | Match |
| 10B | 118 | High | Good | 108.9 | High | Good | Match | Match |
| 11B | 90.1 | High | Good | 88.6 | High | Good | Match | Match |
| 12B | 67 | High | Good | 69.7 | High | Good | Match | Match |
| 13B | 104 | High | Good | 95.9 | High | Good | Match | Match |
| 14B | 43.5 | Good | Good | 57.5 | High | Good | No match | Match |
| 15B | 135 | High | Good | 133.0 | High | Good | Match | Match |
| 16B | 119 | High | Good | 112.6 | High | Good | Match | Match |
| 17C | 19.9 | Low | Low | 32.1 | Good | Low | No match | Match |
| 18D | 114 | High | Good | 89.9 | High | Good | Match | Match |
| 22E | 18.4 | Low | Low | 10.3 | Low | Low | Match | Match |
| 23E | 73 | High | Good | 78.2 | High | Good | Match | Match |
| 24E | 74.7 | High | Good | 100.0 | High | Good | Match | Match |
| 25E | 56.2 | High | Good | 78.0 | High | Good | Match | Match |
| 26E | 44.5 | Good | Good | 55.9 | High | Good | No match | Match |
| 27E | 69.2 | High | Good | 76.7 | High | Good | Match | Match |
| 28F | 47.7 | Good | Good | 60.2 | High | Good | No match | Match |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waller, A.W.; Gaytán-Martínez, M.; Andrade Laborde, J.E. In-Country Method Validation of a Paper-Based, Smartphone-Assisted Iron Sensor for Corn Flour Fortification Programs. Foods 2022, 11, 276. https://doi.org/10.3390/foods11030276
Waller AW, Gaytán-Martínez M, Andrade Laborde JE. In-Country Method Validation of a Paper-Based, Smartphone-Assisted Iron Sensor for Corn Flour Fortification Programs. Foods. 2022; 11(3):276. https://doi.org/10.3390/foods11030276
Chicago/Turabian StyleWaller, Anna W., Marcela Gaytán-Martínez, and Juan E. Andrade Laborde. 2022. "In-Country Method Validation of a Paper-Based, Smartphone-Assisted Iron Sensor for Corn Flour Fortification Programs" Foods 11, no. 3: 276. https://doi.org/10.3390/foods11030276
APA StyleWaller, A. W., Gaytán-Martínez, M., & Andrade Laborde, J. E. (2022). In-Country Method Validation of a Paper-Based, Smartphone-Assisted Iron Sensor for Corn Flour Fortification Programs. Foods, 11(3), 276. https://doi.org/10.3390/foods11030276







