Low Temperatures Affect the Physiological Status and Phytochemical Content of Flat Leaf Kale (Brassica oleracea var. acephala) Sprouts
Abstract
1. Introduction
2. Materials and Methods
2.1. Germination, Growth Conditions and Low Temperature Tretments of Plants
2.2. Yield and Length of Sprouts
2.3. Sprouts Storage, Draying and Homogenization
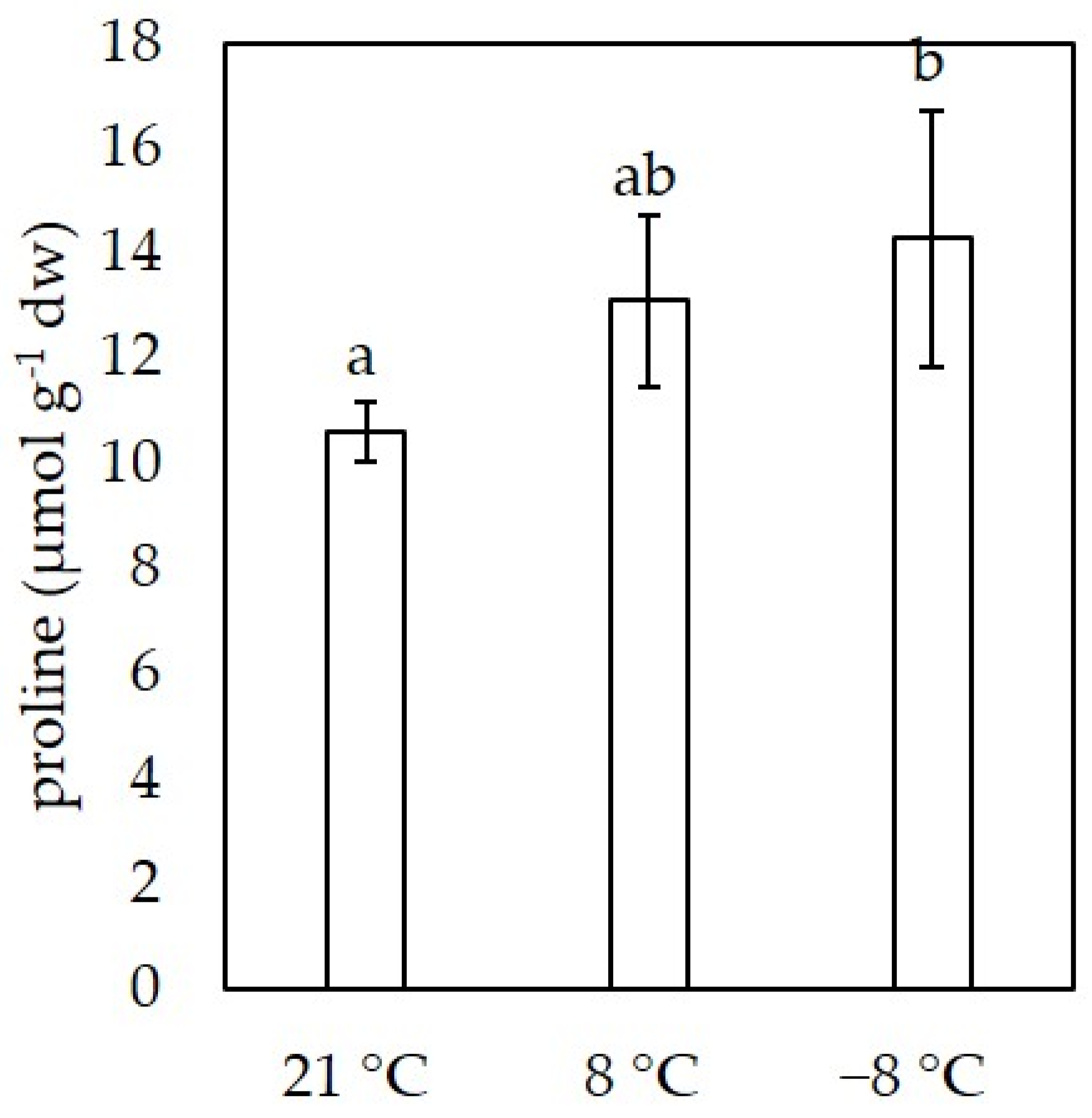
2.4. Determination of Proline Content
2.5. Determination of Pigments
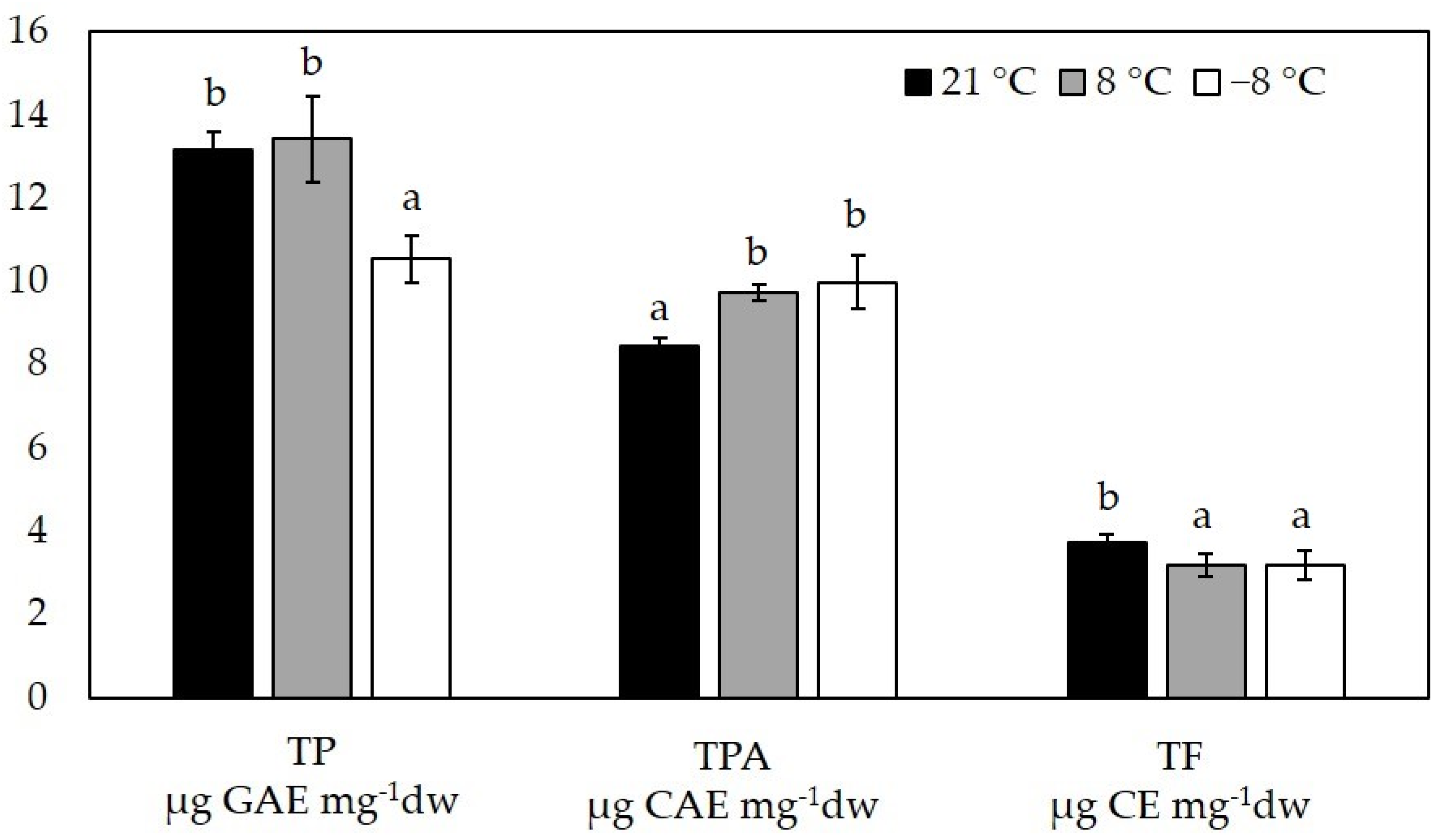
2.6. Determination of Polyphenolic Compounds
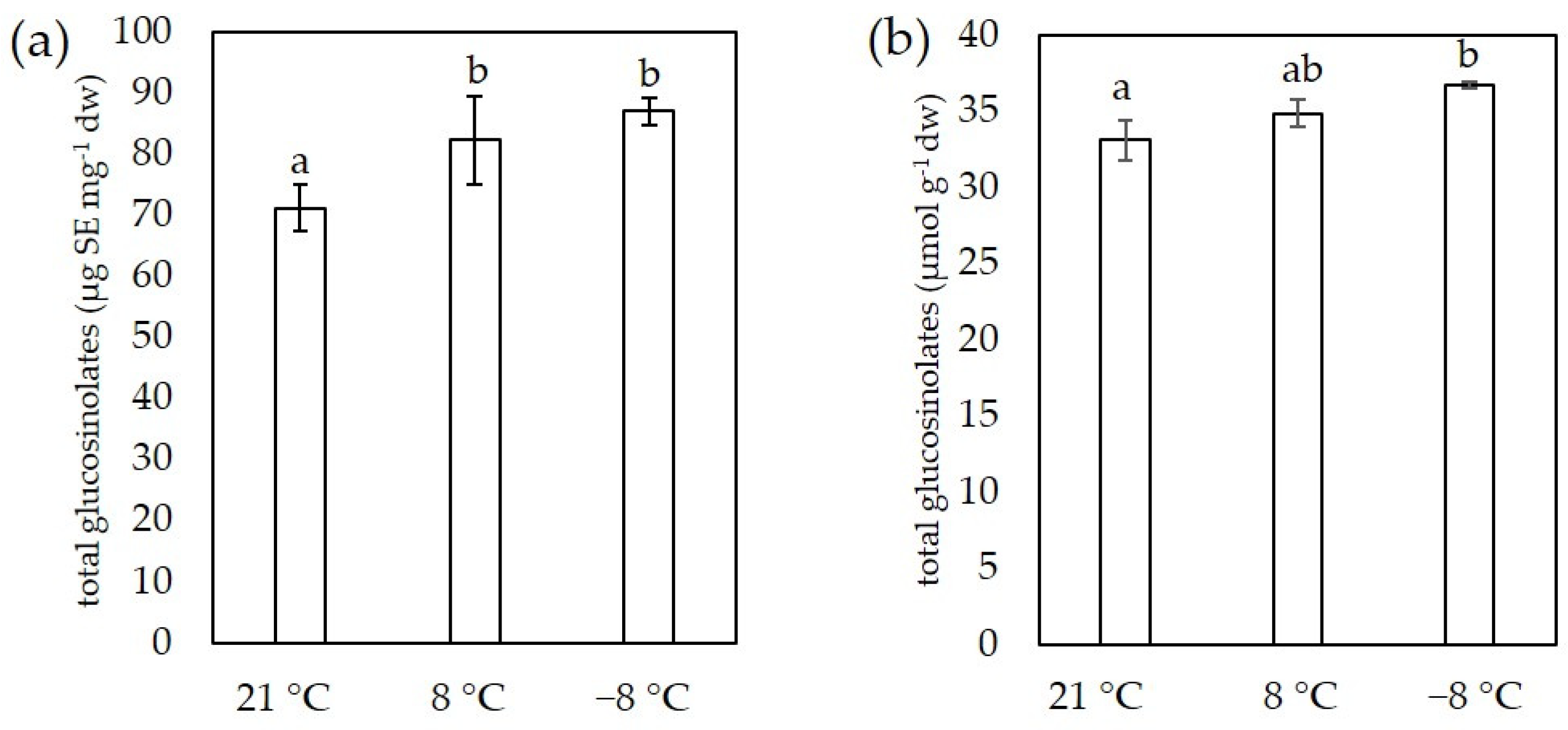
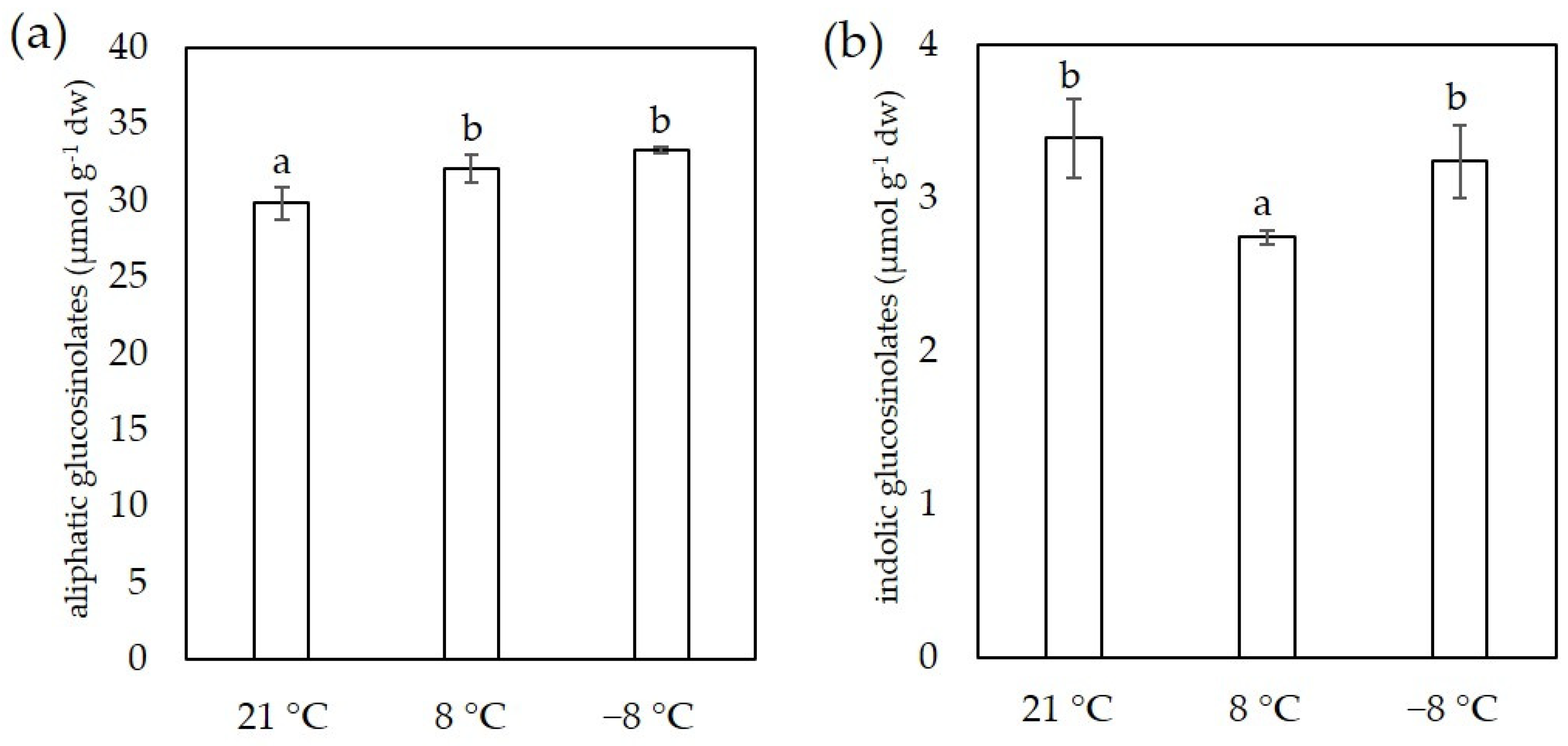
2.7. Determination of Glucosinolates
2.8. Statistical Analysis
3. Results
3.1. Influence of Low Temperature on Sprouts Length and Yield
3.2. Proline Content
3.3. Pigments Content
3.4. Polyphenol Content
3.5. Glucosinolates Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teng, J.; Liao, P.; Wang, M. The role of emerging micro-scale vegetables in human diet and health benefits—An updated review based on microgreens. Food Funct. 2021, 12, 1914–1932. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Pavlović, I.; Radojčić Redovniković, I.; Salopek-Sondi, B. Comparative analysis of phytochemicals and activity of endogenous enzymes associated with their stability, bioavailability and food quality in five Brassicaceae sprouts. Food Chem. 2018, 269, 96–102. [Google Scholar] [CrossRef]
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Datti, A.; Benincasa, P. Sprouts and microgreens: Trends, opportunities, and horizons for novel research. Agronomy 2020, 10, 1424. [Google Scholar] [CrossRef]
- Aloo, S.O.; Ofosu, F.K.; Kilonzi, S.M.; Shabbir, U.; Oh, D.H. Edible plant sprouts: Health benefits, trends, and opportunities for novel exploration. Nutrients 2021, 13, 2882. [Google Scholar] [CrossRef]
- Le, T.N.; Chiu, C.-H.; Hsieh, P.-C. Bioactive compounds and bioactivities of Brassica oleracea L. var. italica sprouts and microgreens: An updated overview from a nutraceutical perspective. Plants 2020, 9, 946. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, P.R.; Barnes, D.M. Phytochemical composition and biological activity of 8 varieties of radish (Raphanus sativus L.) sprouts and mature taproots. J. Food Sci. 2011, 76, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Paśko, P.; Tyszka-Czochara, M.G.; Gdula-Argasińska, J.; Żmudzki, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S. Comparative study of predominant phytochemical compounds and proapoptotic potential of broccoli sprouts and florets. Plant Foods Hum. Nutr. 2018, 73, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Zarrelli, A.; Soteriou, G.A.; Kyratzis, A.; Antoniou, C.; Pizzolongo, F.; Romano, R.; et al. Ontogenetic variation in the mineral, phytochemical and yield attributes of brassicaceous microgreens. Foods 2021, 10, 1032. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Salopek-Sondi, B. Cruciferous (Brassicaceae) vegetables. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Sanches Silva, T., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 195–202. [Google Scholar]
- Šamec, D.; Kruk, V.; Ivanišević, P. Influence of seed origin on morphological characteristics and phytochemicals levels in Brassica oleracea var. acephala. Agronomy 2019, 9, 502. [Google Scholar] [CrossRef]
- Šamec, D.; Urlić, B.; Salopek-Sondi, B. Kale (Brassica oleracea var. acephala) as a superfood: Review of the scientific evidence behind the statement. Crit. Rev. Food Sci. Nutr. 2019, 59, 2411–2422. [Google Scholar] [CrossRef]
- Giorgetti, L.; Giorgi, G.; Cherubini, E.; Gervasi, P.G.; Della Croce, C.M.; Longo, V.; Bellani, L. Screening and identification of major phytochemical compounds in seeds, sprouts and leaves of Tuscan black kale Brassica oleracea (L.) ssp. acephala (DC) var. sabellica L. Nat. Prod. Res. 2017, 32, 1617–1626. [Google Scholar] [CrossRef]
- Drozdowska, M.; Leszczyńska, T.; Koronowicz, A.; Piasna-Słupecka, E.; Domagała, D.; Kusznierewicz, B. Young shoots of red cabbage are a better source of selected nutrients and glucosinolates in comparison to the vegetable at full maturity. Eur. Food Res. Technol. 2020, 246, 2505–2515. [Google Scholar] [CrossRef]
- Bella, M.C.D.; Niklas, A.; Toscano, S.; Picchi, V.; Romano, D.; Scalzo, R.L.; Branca, F. Morphometric characteristics, polyphenols and ascorbic acid variation in brassica oleracea l. novel foods: Sprouts, microgreens and baby leaves. Agronomy 2020, 10, 782. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Granica, S.; Kołodziejczyk-Czepas, J.; Magiera, A.; Czerwińska, M.E.; Nowak, P.; Rutkowska, M.; Wasiński, P.; Owczarek, A. Variability of sinapic acid derivatives during germination and their contribution to antioxidant and anti-inflammatory effects of broccoli sprouts in human plasma and human peripheral blood mononuclear cells. Food Funct. 2020, 11, 7231–7244. [Google Scholar] [CrossRef]
- Fuente, B.; López-García, G.; Máñez, V.; Alegría, A.; Reyes Barberá, R.; Cilla, A. Antiproliferative effect of bioaccessible fractions of four brassicaceae microgreens on human colon cancer cells linked to their phytochemical composition. Antioxidants 2020, 9, 368. [Google Scholar] [CrossRef]
- Kim, M.J.; Yang, H.J.; Lee, H.Y.; Park, Y.M.; Shin, D.Y.; Lee, Y.H.; Kang, Y.G.; Kim, T.S.; Lee, S.P.; Park, K.-H. Improving effects of Brassica oleracea L. var. italica sprout extract on alcoholic liver dysfunction. Korean J. Plant Res. 2020, 33, 163–169. [Google Scholar]
- Abellán, Á.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Sorting out the value of cruciferous sprouts as sources of bioactive compounds for nutrition and health. Nutrients 2019, 11, 429. [Google Scholar] [CrossRef]
- Podda, A.; Pollastri, S.; Bartolini, P.; Pisuttu, C.; Pellegrini, E.; Nali, C.; Cencetti, G.; Michelozzi, M.; Frassinetti, S.; Giorgetti, L.; et al. Drought stress modulates secondary metabolites in Brassica oleracea L. convar. acephala (DC) Alef, var. sabellica L. J. Sci. Food Agric. 2019, 99, 5533–5540. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, M.C.; Toscano, S.; Arena, D.; Moreno, D.A.; Romano, D.; Branca, F. Effects of growing cycle and genotype on the morphometric properties and glucosinolates amount and profile of sprouts, microgreens and baby leaves of broccoli (Brassica oleracea L. var. italica Plenck) and Kale (B. oleracea L. var. acephala DC.). Agronomy 2021, 11, 1685. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Sprouts vs. microgreens as novel functional foods: Variation of nutritional and phytochemical profiles and their in vitro bioactive properties. Molecules 2020, 25, 4648. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.; Kiraga, S.; Islam, M.N.; Ali, M.; Reza, M.N.; Lee, W.-H.; Chung, S.-O. Effects of temperature, relative humidity, and carbon dioxide concentration on growth and glucosinolate content of kale grown in a plant factory. Foods 2021, 10, 1524. [Google Scholar] [CrossRef]
- Almuhayawi, S.M.; Almuhayawi, M.S.; Al Jaouni, S.K.; Selim, S.; Hassan, A.H.A. Effect of Laser Light on Growth, Physiology, Accumulation of Phytochemicals, and Biological Activities of Sprouts of Three Brassica Cultivars. J. Agric. Food Chem. 2021, 69, 6240–6250. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Tian, L.; Gu, Z.; Yang, R. Low salinity promotes the growth of broccoli sprouts by regulating hormonal homeostasis and photosynthesis. Hortic. Environ. Biotechnol. 2019, 60, 19–30. [Google Scholar] [CrossRef]
- Amer, M.A.; Mohamed, T.R.; Abdel Rahman, R.A.; Ali, M.; Badr, A. Studies on exogenous elicitors promotion of sulforaphane content in broccoli sprouts and its effect on the MDA-MB-231 breast cancer cell line. Ann. Agric. Sci. 2021, 66, 46–52. [Google Scholar] [CrossRef]
- Limwiwattana, D.; Tongkhao, K.; Na Jom, K. Effect of sprouting temperature and air relative humidity on metabolic profiles of sprouting black gram (Vigna mungo L.). J. Food Process. Preserv. 2015, 40, 306–315. [Google Scholar] [CrossRef]
- Ampofo, J.; Ngadi, M.; Ramaswamy, H.S. The impact of temperature treatments on elicitation of the phenylpropanoid pathway, phenolic accumulations and antioxidative capacities of common bean (Phaseolus vulgaris) sprouts. Food Bioproc. Tech. 2020, 13, 1544–1555. [Google Scholar] [CrossRef]
- Carillo, P.; Mastrolonardo, G.; Nacca, F.; Parisi, D.; Verlotta, A.; Fuggi, A. Nitrogen metabolism in durum wheat under salinity: Accumulation of proline and glycine betaine. Funct. Plant Biol. 2008, 35, 412–426. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV–VIS spectroscopy. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; pp. F4.3.1–F4.3.8. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic—Phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents inmulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 4th ed.; Council of Europe: Strasbourg, France, 2004; pp. 2377–2378.
- Aghajanzadeh, T.A.; Hawkesford, M.J.; De Kok, L.J. The significance of glucosinolates for sulfur storage in Brassicaceae seedlings. Front. Plant Sci. 2014, 5, 704. [Google Scholar] [CrossRef]
- Jakovljević, T.; Cvjetko, M.; Sedak, M.; Đokić, M.; Bilandžić, N.; Vorkapić-Furač, J.; Radojčić-Redovniković, I. Balance of glucosinolates content under Cd stress n two Brassica species. Plant Physiol. Biochem. 2013, 63, 99–106. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, A.A.T.; David, A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Dangi, A.K.; Sharma, B.; Khangwal, I.; Shukla, P. Combinatorial interactions of biotic and abiotic stresses in plants and their molecular mechanisms: Systems biology approach. Mol. Biotechnol. 2018, 60, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.M.V.; Rosa, E.; Fahey, J.W.; Stephenson, K.K.; Carvalho, R.; Aires, A. Influence of temperature and ontogeny on the levels of glucosinolates in broccoli (Brassica oleracea Var. italica) sprouts and their effect on the induction of mammalian phase 2 enzymes. J. Agric. Food Chem. 2002, 50, 6239–6244. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, F.; Yang, P.; Li, J.; Yan, G.; Hu, L. Responses of canola (Brassica napus L.) cultivars under contrasting temperature regimes during early seedling growth stage as revealed by multiple physiological criteria. Acta Physiol. Plant. 2012, 37, 7. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, Q.; Ding, C.; Huang, Y.; Liao, J.; Chen, T.; Feng, S.; Zhou, L.; Zhang, Z.; Chen, Y.; et al. Effect of low temperature on chlorophyll biosynthesis and chloroplast biogenesis of rice seedlings during greening. Int. J. Mol. Sci. 2020, 21, 1390. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, K.; Gu, Y.; Zhang, L.; Li, W.; Li, Z. Effects of low-temperature stress and brassinolide application on the photosynthesis and leaf structure of tung tree seedlings. Front. Plant Sci. 2020, 10, 1767. [Google Scholar] [CrossRef]
- Hou, W.; Sun, A.H.; Chen, H.L.; Yang, F.S.; Pan, J.L.; Guan, M.Y. Effects of chilling and high temperatures on photosynthesis and chlorophyll fluorescence in leaves of watermelon seedlings. Biol. Plant. 2016, 60, 148–154. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Žnidarčič, D.; Ban, D.; Šircelj, H. Carotenoid and chlorophyll composition of commonly consumed leafy vegetables in Mediterranean countries. Food Chem. 2011, 129, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Ljubej, V.; Radojčić Redovniković, I.; Salopek-Sondi, B.; Smolko, A.; Roje, S.; Šamec, D. Chilling and freezing temperature stress differently influence glucosinolates content in Brassica oleracea var. acephala. Plants 2021, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Ljubej, V.; Karalija, E.; Salopek-Sondi, B.; Šamec, D. Effects of short-term exposure to low temperatures on proline, pigments, and phytochemicals level in kale (Brassica oleracea var. acephala). Horticulturae 2021, 7, 341. [Google Scholar] [CrossRef]
- Yang, Y.-Z.; Li, T.; Teng, R.-M.; Han, M.-H.; Zhuang, J. Low temperature effects on carotenoids biosynthesis in the leaves of green and albino tea plant (Camellia sinensis (L.) O. Kuntze). Sci. Hortic. 2021, 285, 110164. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Kim, S.J.; Cho, M.H. Melatonin and polyphenol contents in some edible sprouts (alfalfa, chicory, rape, red kale and sunflower). J. Food. Sci. Nutr. 2011, 16, 184–188. [Google Scholar] [CrossRef][Green Version]
- Świeca, M.; Baraniak, B. Nutritional and antioxidant potential of lentil sprouts affected by elicitation with temperature stress. J. Agric. Food Chem. 2014, 62, 3306–3313. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Maina, S.; Misinzo, G.; Bakari, G.; Kim, H.-Y. Human, animal and plant health benefits of glucosinolates and strategies for enhanced bioactivity: A systematic review. Molecules 2020, 25, 3682. [Google Scholar] [CrossRef]
- Miękus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Świergiel, A.H. Health benefits of plant-derived sulfur compounds, glucosinolates, and organosulfur compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Oloyede, O.O.; Lignou, S.; Wagstaff, C.; Methven, L. Taste and flavor perceptions of glucosinolates, isothiocyanates, and related compounds. Mol. Nutr. Food Res. 2018, 62, e1700990. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Kim, J.K.; Kim, H.; Kim, Y.J.; Park, Y.J.; Kim, S.J.; Kim, C.; Park, S.U. Transcriptome analysis and metabolic profiling of green and red kale (Brassica oleracea var. acephala) seedlings. Food Chem 2018, 241, 7–13. [Google Scholar] [CrossRef]
- Zeng, W.; Tao, H.; Li, Y.; Wang, J.; Xia, C.; Li, S.; Wang, M.; Wang, Q.; Miao, H. The flavor of Chinese kale sprouts is affected by genotypic variation of glucosinolates and their breakdown products. Food Chem. 2021, 359, 129824. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Marhuenda, J.; García-Viguera, C.; Zafrilla, P.; Moreno, D.A. Influence of cooking methods on glucosinolates and isothiocyanates content in novel cruciferous foods. Foods 2019, 8, 257. [Google Scholar] [CrossRef] [PubMed]

| 21 °C | 8 °C | |
|---|---|---|
 | ||
| Mass 1 seedling (mg) | 26.83 ± 3.90 | 23.40 ± 5.31 * |
| Root length (mm) | 54.95 ± 10.75 | 35.62 ± 8.82 * |
| Shoots length (mm) | 6.95 ± 1.91 | 6.19 ± 2.03 |
| 21 °C | 8 °C | −8 °C | |
|---|---|---|---|
| Chlorophyll a (µg mg−1 dw) | 1.98 ± 0.32 b | 1.43 ± 0.15 a | 1.47 ± 0.35 a |
| Chlorophyll b (µg mg−1 dw) | 0.81 ± 0.09 b | 0.58 ± 0.06 a | 0.59 ± 0.12 a |
| Total chlorophylls (µg mg−1 dw) | 2.79 ± 0.38 b | 2.02 ± 0.20 a | 2.06 ± 0.47 a |
| Total carotenoids (µg mg−1 dw) | 0.64 ± 0.06 b | 0.51 ± 0.04 a | 0.51 ± 0.06 a |
| Chlorophyll a/Chlorophyll b | 2.46 ± 0.28 a | 2.45 ± 0.17 a | 2.50 ± 0.15 a |
| Chlorophylls/carotenoids | 4.34 ± 0.33 a | 3.95 ± 0.16 a | 3.97 ± 0.50 a |
| μmol g−1 dw | 21 °C | 8 °C | −8 °C |
|---|---|---|---|
| Glucoiberin | 3.94 ± 0.17 b | 3.13 ± 0.09 a | 3.78 ± 0.04 b |
| Progoitrin | 4.69 ± 0.10 a | 5.85 ± 0.20 b | 4.92 ± 0.35 a |
| Sinigrin | 17.95 ± 0.80 a | 16.82 ± 0.96 a | 20.55 ± 0.49 b |
| Glucoraphanin | nd | 2.76 ± 0.15 | nd |
| Gluconapin | 0.69 ± 0.07 a | 0.66 ± 0.04 a | 0.86 ± 0.01 b |
| 4-hydroxyglucobrassicin | 1.73 ± 0.13 a | 1.65 ± 0.03 a | 1.98 ± 0.19 a |
| Glucobrassicanapin | 0.66 ± 0.05 b | 0.50 ± 0.02 a | 0.67 ± 0.05 b |
| 4-methoxyglucobrassicin | 0.14 ± 0.01 b | 0.09 ± 0.01 a | 0.13 ± 0.02 b |
| Neoglucobrasscin | 0.71 ± 0.05 b | 0.51 ± 0.06 a | 0.61 ± 0.06 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šamec, D.; Ljubej, V.; Redovniković, I.R.; Fistanić, S.; Salopek-Sondi, B. Low Temperatures Affect the Physiological Status and Phytochemical Content of Flat Leaf Kale (Brassica oleracea var. acephala) Sprouts. Foods 2022, 11, 264. https://doi.org/10.3390/foods11030264
Šamec D, Ljubej V, Redovniković IR, Fistanić S, Salopek-Sondi B. Low Temperatures Affect the Physiological Status and Phytochemical Content of Flat Leaf Kale (Brassica oleracea var. acephala) Sprouts. Foods. 2022; 11(3):264. https://doi.org/10.3390/foods11030264
Chicago/Turabian StyleŠamec, Dunja, Valentina Ljubej, Ivana Radojčić Redovniković, Stjepana Fistanić, and Branka Salopek-Sondi. 2022. "Low Temperatures Affect the Physiological Status and Phytochemical Content of Flat Leaf Kale (Brassica oleracea var. acephala) Sprouts" Foods 11, no. 3: 264. https://doi.org/10.3390/foods11030264
APA StyleŠamec, D., Ljubej, V., Redovniković, I. R., Fistanić, S., & Salopek-Sondi, B. (2022). Low Temperatures Affect the Physiological Status and Phytochemical Content of Flat Leaf Kale (Brassica oleracea var. acephala) Sprouts. Foods, 11(3), 264. https://doi.org/10.3390/foods11030264








