Enhance Production of γ-Aminobutyric Acid (GABA) and Improve the Function of Fermented Quinoa by Cold Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Screening of GABA-Producing Strains
2.3. Cold Stimulation Treatment and Fermentation Experiment
2.4. Assessment of GABA and Glutamate
2.5. Determination of pH, Total Acidity and Viable Microbial Counts
2.6. Measure of Enzyme Activity
2.7. FTIR
2.8. Crystallinity X-ray Diffraction (XRD)
2.9. Pasting Properties
2.10. Scanning Electron Microscopy
2.11. Water-Soluble Index (WSI), and Water Absorption Index (WAI)
2.12. Statistical Analysis
3. Results and Discussion
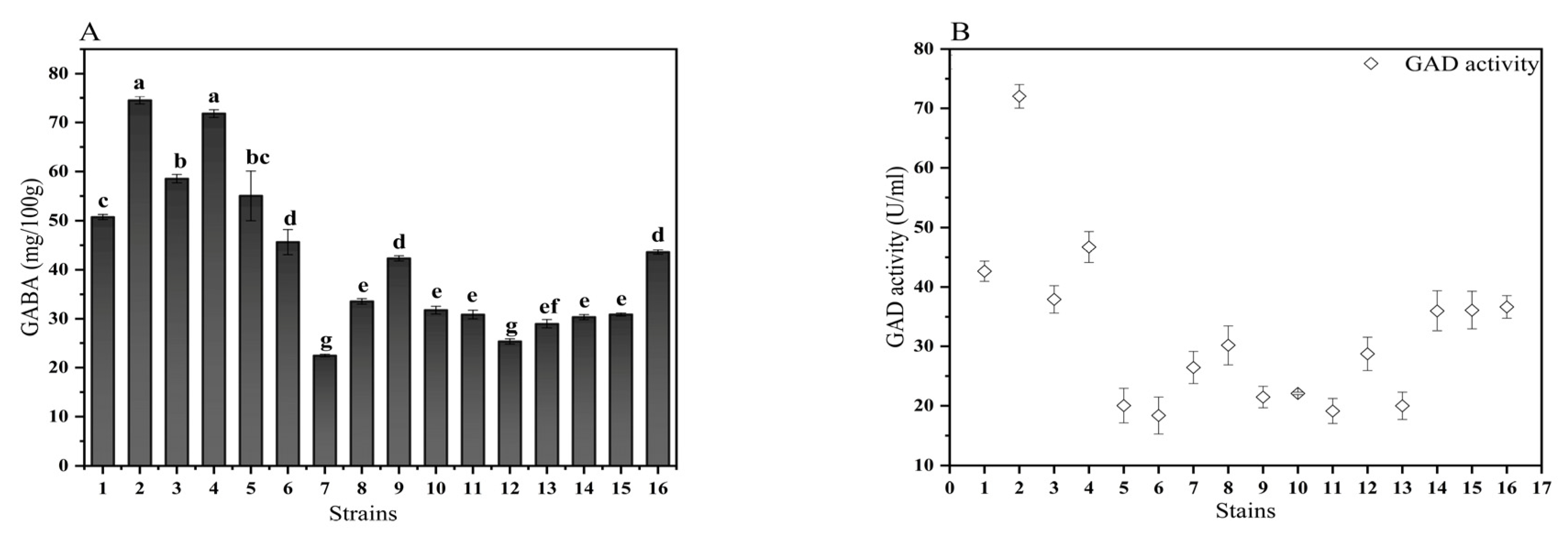
3.1. Screening of GABA-Producing LAB
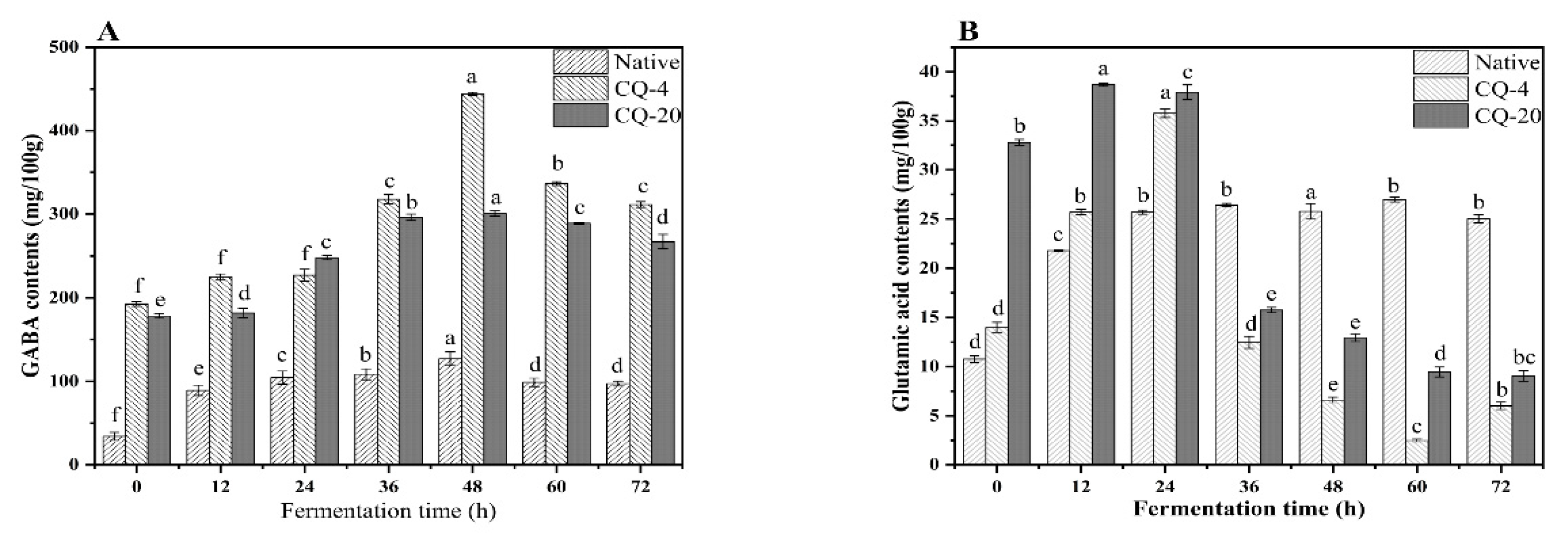
3.2. Impact of Pretreatment and Fermentation on GABA Level of Quinoa
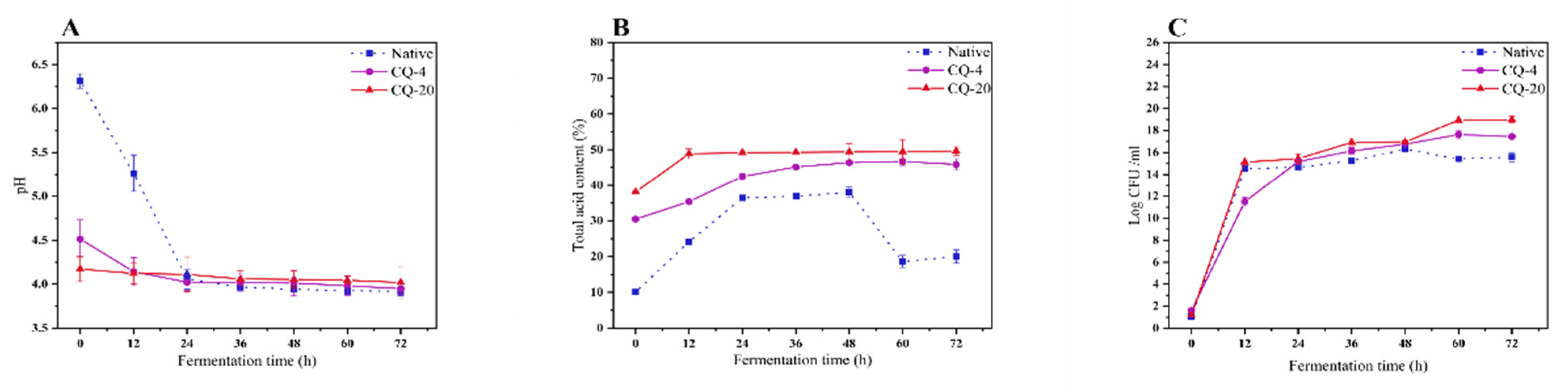
3.3. Changes in pH, Titratable Acidity (TTA) and Number of Viable Microbial
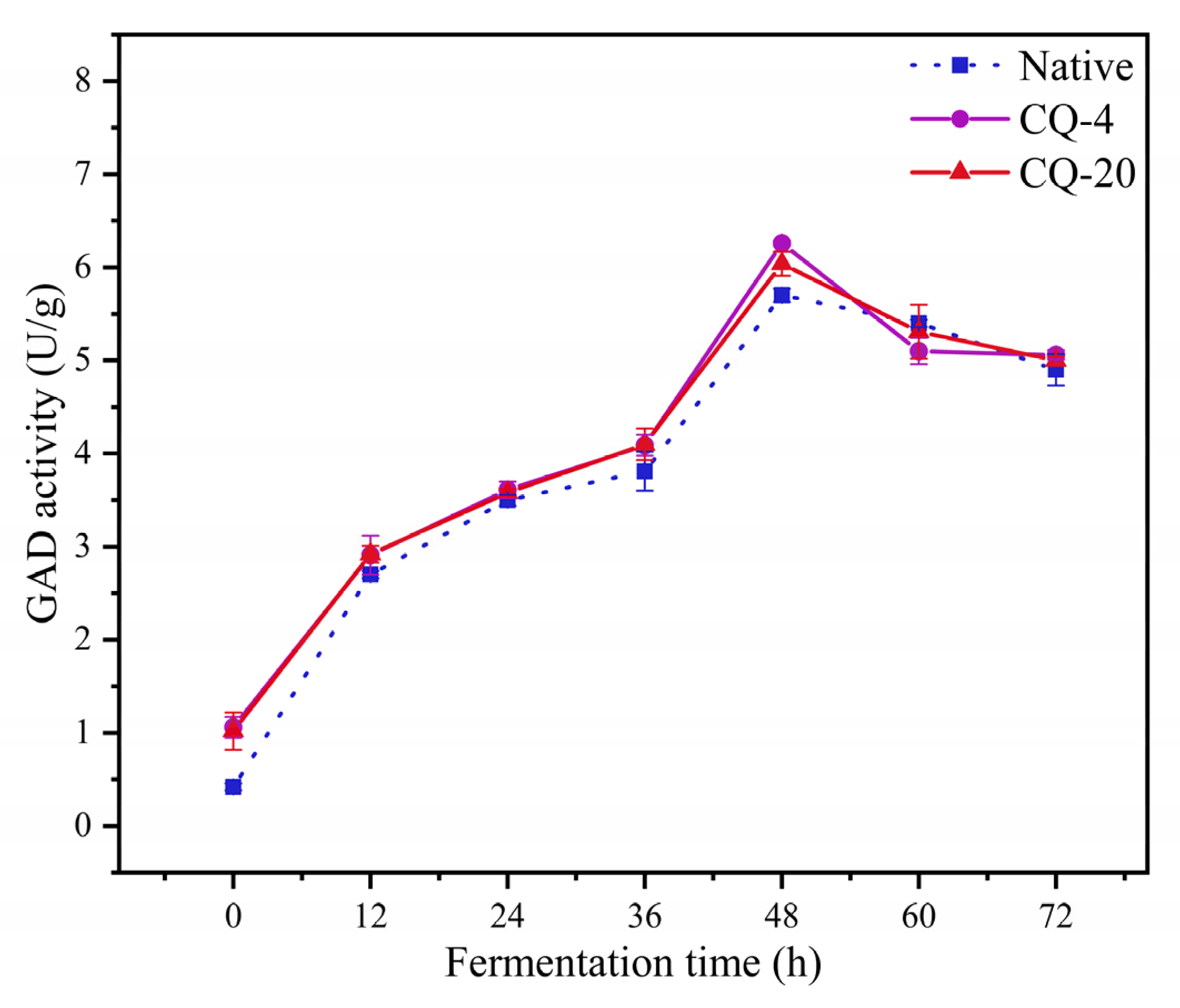
3.4. Enzyme Activity
3.5. Changes in Crystalline Structure
3.6. Changes in Fourier Transform Infrared Spectroscopy (FTIR)
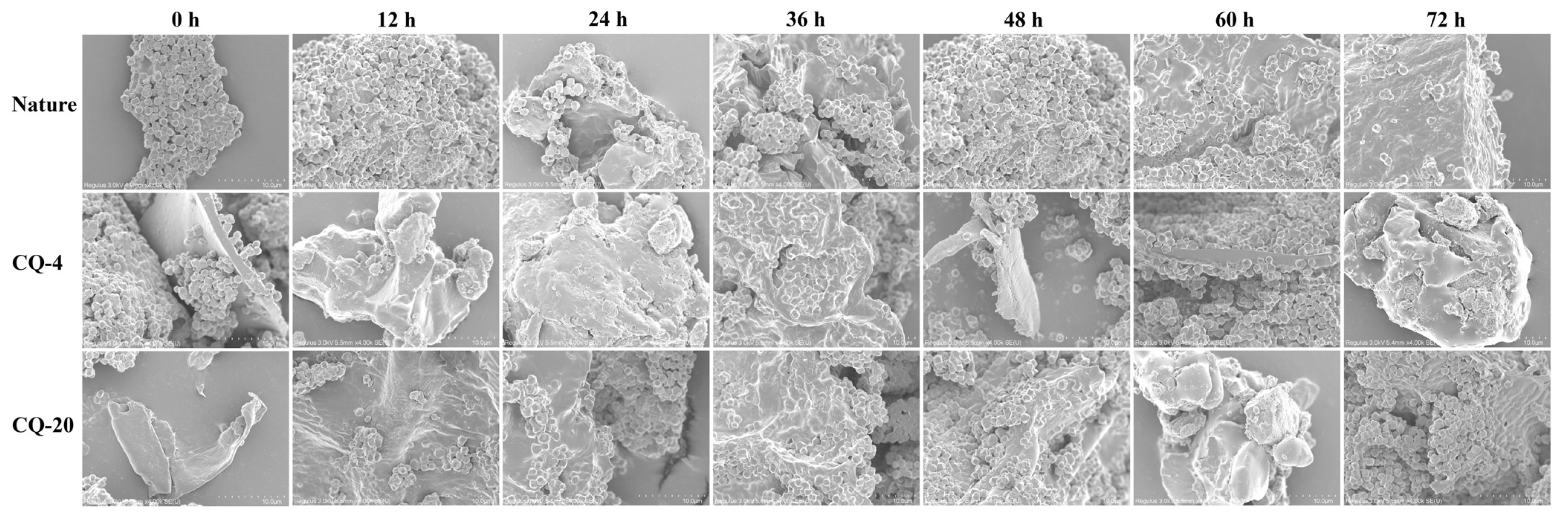
3.7. Scanning Electron Microscopy (SEM)
3.8. WAI and WSI
3.9. Pasting Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nickel, J.; Spanier, L.P.; Botelho, F.T.; Gularte, M.A.; Helbig, E. Effect of different types of processing on the total phenolic compound content, antioxidant capacity, and saponin content of Chenopodium quinoa Willd grains. Food Chem. 2016, 209, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, K.; Gu, Z.; Yang, R. Enhanced gamma-aminobutyric acid accumulation, alleviated componential deterioration and technofunctionality loss of germinated wheat by hypoxia stress. Food Chem. 2018, 269, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Teng, C.; Sun, M.; Zhang, Q.; Zhou, B.; Cui, H.; Ren, G.; Yang, X.; Qin, P. Effect of germination treatment on the structural and physicochemical properties of quinoa starch. Food Hydrocoll. 2021, 115, 106604. [Google Scholar] [CrossRef]
- Adebo, O.A.; Njobeh, P.B.; Kayitesi, E. Fermentation by Lactobacillus fermentum strains (singly and in combination) enhances the properties of ting from two whole grain sorghum types. J. Cereal Sci. 2018, 82, 49–56. [Google Scholar] [CrossRef]
- Limon, R.I.; Penas, E.; Torino, M.I.; Martinez-Villaluenga, C.; Duenas, M.; Frias, J. Fermentation enhances the content of bioactive compounds in kidney bean extracts. Food Chem. 2015, 172, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Li, W.; Yu, Y.; Gu, X.; Zhang, W.; Wang, Z. Dynamic changes of proton populations in potato flours during fermentation and their relationship with digestibility and functional properties. Food Chem. 2020, 313, 126161. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, P.G.; Villegas, J.M.; de Giori, G.S.; Saavedra, L.; Hebert, E.M. Enhancement of gamma-aminobutyric acid (GABA) production by Lactobacillus brevis CRL 2013 based on carbohydrate fermentation. Int. J. Food Microbiol. 2020, 333, 108792. [Google Scholar] [CrossRef]
- Fiorina, P. GABAergic System in Beta-Cells: From Autoimmunity Target to Regeneration Tool; Nephrology Division, Boston Children’s Hospital, Harvard Medical School: Boston, MA, USA, 2013; Volume 62, pp. 3674–3676. [Google Scholar] [CrossRef]
- Bach, B.; Meudec, E.; Lepoutre, J.P.; Rossignol, T.; Blondin, B.; Dequin, S.; Camarasa, C. New Insights into gamma-Aminobutyric Acid Catabolism: Evidence for gamma-Hydroxybutyric Acid and Polyhydroxybutyrate Synthesis in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2009, 75, 4231–4239. [Google Scholar] [CrossRef]
- Wang, Y.S.; Liu, C.Q.; Ma, T.Y.; Zhao, J. Physicochemical and functional properties of gamma-aminobutyric acid-treated soy proteins. Food Chem. 2019, 295, 267–273. [Google Scholar] [CrossRef]
- Li, E.; Luo, X.; Liao, S.; Shen, W.; Li, Q.; Liu, F.; Zou, Y. Accumulation of γ-aminobutyric acid during cold storage in mulberry leaves. Int. J. Food Sci. Technol. 2018, 53, 2664–2672. [Google Scholar] [CrossRef]
- Cholewa, E.; Bown, A.W.; Cholewinski, A.J.; Shelp, B.J.; Snedden, W.A. Cold-shock-stimulated γ-aminobutyric acid synthesis is mediated by an increase. Can. J. Bot. 1997, 75, 375–382. [Google Scholar] [CrossRef]
- Baranzelli, J.; Kringel, D.H.; Colussi, R.; Paiva, F.F.; Aranha, B.C.; Miranda, M.Z.d.; Zavareze, E.d.R.; Dias, A.R.G. Changes in enzymatic activity, technological quality and gamma-aminobutyric acid (GABA) content of wheat flour as affected by germination. LWT 2018, 90, 483–490. [Google Scholar] [CrossRef]
- Mahnoor, A.; Castro-Alba, V.; Lazarte, C.E. Development of an instant-mix probiotic beverage based on fermented quinoa with reduced phytate content. J. Funct. Foods 2021, 87, 104831. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, D.H.; Kang, H.J.; Shin, M.; Yang, S.Y.; Yang, J.; Jung, Y.H. Enhanced production of gamma-aminobutyric acid (GABA) using Lactobacillus plantarum EJ2014 with simple medium composition. LWT 2021, 137, 110443. [Google Scholar] [CrossRef]
- Li, L.L.; Zhang, M.; Bhandari, B. Influence of drying methods on some physicochemical, functional and pasting properties of Chinese yam flour. LWT 2019, 111, 182–189. [Google Scholar] [CrossRef]
- Qi, X.; Cheng, L.; Li, X.; Zhang, D.; Wu, G.; Zhang, H.; Wang, L.; Qian, H.; Wang, Y.N. Effect of cooking methods on solubility and nutrition quality of brown rice powder. Food Chem. 2019, 274, 444–451. [Google Scholar] [CrossRef]
- Li, Y.J.; Chen, X.; Shu, G.W.; Ma, W.C. Screening of Gamma-aminobutyric acid -producing lactic acid bacteria and bacteria and its application in monascus- fermented rice production. Acta Sci. Pol.-Technol. Aliment. 2020, 19, 387–394. [Google Scholar] [CrossRef]
- Wang, D.W.; Wang, Y.; Lan, H.B.; Wang, K.; Zhao, L.; Hu, Z.Y. Enhanced production of gamma-aminobutyric acid in litchi juice fermented by Lactobacillus plantarum HU-C2W. Food Biosci. 2021, 42, 101155. [Google Scholar] [CrossRef]
- Wang, P.; Liu, K.X.; Yang, R.Q.; Gu, Z.X.; Zhou, Q.; Jiang, D. Comparative Study on the Bread Making Quality of Normoxia- and Hypoxia-Germinated Wheat: Evolution of gamma-Aminobutyric Acid, Starch Gelatinization, and Gluten Polymerization during Steamed Bread Making. J. Agric. Food Chem. 2019, 67, 3480–3490. [Google Scholar] [CrossRef]
- Wallace, W.; Secor, J.; Schrader, L.E. Rapid Accumulation of gamma-Aminobutyric Acid and Alanine in Soybean Leaves in Response to an Abrupt Transfer to Lower Temperature, Darkness, or Mechanical Manipulation. Plant Physiol. 1984, 75, 170–175. [Google Scholar] [CrossRef]
- Mohammadi, M.; Aelaei, M.; Saidi, M. Pre-harvest spray of GABA and spermine delays postharvest senescence and alleviates chilling injury of gerbera cut flowers during cold storage. Sci. Rep. 2021, 11, 14166. [Google Scholar] [CrossRef] [PubMed]
- Schindler, S.; Zelena, K.; Krings, U.; Bez, J.; Eisner, P.; Berger, R.G. Improvement of the Aroma of Pea (Pisum sativum) Protein Extracts by Lactic Acid Fermentation. Food Biotechnol. 2012, 26, 58–74. [Google Scholar] [CrossRef]
- Shokri, S.; Terefe, N.S.; Shekarforoush, S.S.; Hosseinzadeh, S. Ultrasound-assisted fermentation for enhancing metabolic and probiotic activities of LactoBacillus brevis. Chem. Eng. Process.-Process Intensif. 2021, 166, 108470. [Google Scholar] [CrossRef]
- Naibaho, J.; Butula, N.; Jonuzi, E.; Korzeniowska, M.; Laaksonen, O.; Föste, M.; Kütt, M.-L.; Yang, B. Potential of brewers’ spent grain in yogurt fermentation and evaluation of its impact in rheological behaviour, consistency, microstructural properties and acidity profile during the refrigerated storage. Food Hydrocoll. 2022, 125, 107412. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lu, F.X.; Lu, Z.X.; Bie, X.M.; Jiao, Y.; Sun, L.J.; Yu, B. Production of gamma-aminobutyric acid by Streptococcus salivarius subsp thermophilus Y2 under submerged fermentation. Amino Acids 2008, 34, 473–478. [Google Scholar] [CrossRef]
- Manful, J.T.; Grimm, C.C.; Gayin, J.; Coker, R.D. Effect of Variable Parboiling on Crystallinity of Rice Samples. Cereal Chem. 2008, 85, 92–95. [Google Scholar] [CrossRef]
- Gao, L.; Wu, Y.; Wan, C.; Wang, P.; Yang, P.; Gao, X.; Eeckhout, M.; Gao, J. Structural and physicochemical properties of pea starch affected by germination treatment. Food Hydrocoll. 2022, 124, 107303. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Maghsoudlou, Y.; Rostamabadi, H.; Rostamabadi, M.M.; Hamedi, H.; Hosseini, S.M.H. Preparation of physically modified oat starch with different sonication treatments. Food Hydrocoll. 2019, 89, 311–320. [Google Scholar] [CrossRef]
- Yurtdas Kirimlioglu, G.; Menceloglu, Y.; Erol, K.; Yazan, Y. In vitro/in vivo evaluation of gamma-aminobutyric acid-loadedN,N-dimethylacrylamide-based pegylated polymeric nanoparticles for brain delivery to treat epilepsy. J. Microencapsul 2016, 33, 625–635. [Google Scholar] [CrossRef]
- Falah, F.; Vasiee, A.; Tabatabaei-Yazdi, F.; Moradi, S.; Sabahi, S. Optimization of gamma-aminobutyric acid (GABA) production by Lactobacillus spp. from agro-food waste. Biomass Convers. Biorefinery 2022, 1–13. [Google Scholar] [CrossRef]
- Garcia-Diaz, S.; Hernandez-Jaimes, C.; Escalona-Buendia, H.B.; Bello-Perez, L.A.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Effects of CaCO3 treatment on the morphology, crystallinity, rheology and hydrolysis of gelatinized maize starch dispersions. Food Chem. 2016, 207, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Johnson, J.; Chu, Y.F.; Feng, H. Enhancement of γ-aminobutyric acid, avenanthramides, and other health-promoting metabolites in germinating oats (Avena sativa L.) treated with and without power ultrasound. Food Chem. 2019, 283, 239–247. [Google Scholar] [CrossRef]
- Megha, S.; Basu, U.; Kav, N.N.V. Metabolic engineering of cold tolerance in plants. Biocatal. Agric. Biotechnol. 2014, 3, 88–95. [Google Scholar] [CrossRef]
- Czarnecka, M.; Czarnecki, J.; Nowak, H.; Roszyk, Z. Effect of lactic fermentation and extrusion of bean and pea seeds on nutritional and functional properties. Nahrung 1998, 42, 4. [Google Scholar] [CrossRef]
- Chauhan, A.; Saxena, D.C.; Singh, S. Total dietary fibre and antioxidant activity of gluten free cookies made from raw and germinated amaranth (Amaranthus spp.) flour. Lwt-Food Sci. Technol. 2015, 63, 939–945. [Google Scholar] [CrossRef]
- Xiao, Y.; Xing, G.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Effect of solid-state fermentation with Cordyceps militaris SN-18 on physicochemical and functional properties of chickpea (Cicer arietinum L.) flour. LWT-Food Sci. Technol. 2015, 63, 1317–1324. [Google Scholar] [CrossRef]
- Su, H.; Tu, J.; Zheng, M.; Deng, K.; Miao, S.; Zeng, S.; Zheng, B.; Lu, X. Effects of oligosaccharides on particle structure, pasting and thermal properties of wheat starch granules under different freezing temperatures. Food Chem. 2020, 315, 126209. [Google Scholar] [CrossRef]
- Gao, S.; Liu, H.; Sun, L.; Liu, N.; Wang, J.; Huang, Y.; Wang, F.; Cao, J.; Fan, R.; Zhang, X.; et al. The effects of dielectric barrier discharge plasma on physicochemical and digestion properties of starch. Int. J. Biol. Macromol. 2019, 138, 819–830. [Google Scholar] [CrossRef]

| Samples | Degree of Crystallinity (%) | Crystal Type | R1047/1022 | Soluble Sugar (%) | |
|---|---|---|---|---|---|
| Groups | Fermentation Time (h) | ||||
| Native | 0 | 38.89 ± 0.78 a | A | 1.31 ± 0.01 b | 4.48 ± 0.02 b |
| 12 | 11.68 ± 0.31 ab | A | 1.51 ± 0.01 bc | 2.41 ± 0.10 b | |
| 24 | 9.58 ± 0.72 b | A | 1.55 ± 0.01 c | 1.79 ± 0.07 d | |
| 36 | 7.48 ± 0.71 b | A | 1.46 ± 0.03 c | 0.76 ± 0.01 d | |
| 48 | 7.03 ± 0.41 a | A | 1.46 ± 0.04 a | 0.25 ± 0.03 a | |
| 60 | 6.97 ± 0.38 bc | A | 1.43 ± 0.02 cd | 0.24 ± 0.02 c | |
| 72 | 6.71 ± 0.19 c | A | 1.42 ± 0.01 c | 0.23 ± 0.02 c | |
| CQ−4 | 0 | 10.6 ± 0.51 a | A | 1.45 ± 0.01 ab | 6.47 ± 0.04 d |
| 12 | 9.06 ± 0.81 bc | A | 1.44 ± 0.01 b | 4.73 ± 0.09 c | |
| 24 | 8.94 ± 0.79 c | A | 1.41 ± 0.04 b | 3.56 ± 0.07 c | |
| 36 | 7.02 ± 0.01 d | A | 1.39 ± 0.03 a | 3.01 ± 0.02 b | |
| 48 | 6.83 ± 0.84 ab | A | 1.36 ± 0.01 a | 1.25 ± 0.03 b | |
| 60 | 6.82 ± 0.57 c | A | 1.35 ± 0.02 b | 0.79 ± 0.02 a | |
| 72 | 6.15 ± 0.67 b | A | 1.36 ± 0.01 c | 0.33 ± 0.04 cd | |
| CQ−20 | 0 | 8.61 ± 0.01 a | A | 1.52 ± 0.01 d | 6.33 ± 0.06 c |
| 12 | 7.95 ± 1.00 b | A | 1.48 ± 0.02 b | 4.26 ± 0.05 c | |
| 24 | 7.59 ± 0.83 bc | A | 1.46 ± 0.01 c | 3.79 ± 0.03 d | |
| 36 | 7.23 ± 0.62 a | A | 1.43 ± 0.01 b | 2.05 ± 0.04 b | |
| 48 | 6.83 ± 0.21 a | A | 1.43 ± 0.01 ab | 1.31 ± 0.02 a | |
| 60 | 6.80 ± 0.01 c | A | 1.43 ± 0.04 c | 0.31 ± 0.06 a | |
| 72 | 6.34 ± 0.39 b | A | 1.45 ± 0.02 d | 0.29 ± 0.09 b | |
| Fermentation Times (h) | WSI (g/g) | WAI (g/100g) | ||||
|---|---|---|---|---|---|---|
| Native | CQ−4 | CQ−20 | Native | CQ−4 | CQ−20 | |
| 0 | 16.33 ± 0.01 d | 21.03 ± 0.01 d | 27.85 ± 0.02 ab | 2.13 ± 0.35 b | 2.54 ± 0.22 d | 2.40 ± 0.05 b |
| 12 | 19.66 ± 0.01 c | 22.21 ± 0.03 d | 28.20 ± 0.02 ab | 2.50 ± 0.22 a | 2.59 ± 0.17 d | 2.46 ± 0.02 b |
| 24 | 22.66 ± 0.02 a | 23.34 ± 0.01 d | 29.94 ± 0.03 a | 2.50 ± 0.20 a | 2.59 ± 0.15 d | 2.58 ± 0.03 ab |
| 36 | 22.92 ± 0.05 a | 23.99 ± 0.01 d | 28.62 ± 0.02 ab | 2.51 ± 0.26 a | 2.63 ± 0.33 c | 2.65 ± 0.02 ab |
| 48 | 23.32 ± 0.01 b | 27.48 ± 0.05 b | 30.59 ± 0.01 a | 2.58 ± 0.15 a | 2.65 ± 0.13 bc | 2.73 ± 0.07 a |
| 60 | 20.71 ± 0.02 b | 25.94 ± 0.03 bc | 26.28 ± 0.03 bc | 2.51 ± 0.09 a | 2.59 ± 0.04 d | 2.57 ± 0.04 ab |
| 72 | 20.08 ± 0.01 b | 25.40 ± 0.02 bc | 21.81 ± 0.09 c | 2.51 ± 0.19 a | 2.52 ± 0.10 d | 2.35 ± 0.27 b |
| Varieties | Fermentation Time (h) | PV (cP) | BV (cP) | TV (cP) | SV (cP) | FV (cP) | PT (°C) |
|---|---|---|---|---|---|---|---|
| Native | 0 | 774 ± 4.9 d | 107 ± 15.8 c | 667 ± 9.1 a | 129 ± 10.6 bc | 796 ± 12.2 b | 94.90 ± 13.7 d |
| 12 | 683 ± 5.1 a | 85 ± 14.6 a | 598 ± 5.9 b | 217 ± 14.0 a | 815 ± 13.1 b | 94.80 ± 15.7 a | |
| 24 | 606 ± 9.1 a | 65 ± 14.1 a | 567 ± 13.4 c | 190 ± 3.9 a | 757 ± 5.6 a | 51.20 ± 13.4 a | |
| 36 | 604 ± 14.7 a | 37 ± 14.5 ab | 541 ± 2.6 d | 190 ± 10.7 a | 731 ± 21.3 b | 50.55 ± 12.5 a | |
| 48 | 761 ± 2.9 a | 73 ± 24.6 c | 688 ± 21.0 bc | 212 ± 9.0 b | 900 ± 14.6 b | 56.30 ± 10.9 a | |
| 60 | 674 ± 8.7 a | 46 ± 12.0 b | 628 ± 7.0 a | 168 ± 19.4 b | 796 ± 9.1 c | 51.10 ± 9.8 c | |
| 72 | 719 ± 11.5 b | 103 ± 17.9 ab | 616 ± 12.1 b | 213 ± 10.0 e | 829 ± 6.7 d | 59.05 ± 6.9 c | |
| CQ−4 | 0 | 617 ± 8.8 b | 154 ± 11.6 b | 463 ± 15.7 b | 254 ± 24.7 a | 717 ± 21.7 d | 95.35 ± 9.3 a |
| 12 | 546 ± 8.5 a | 106 ± 20.6 a | 432 ± 21.5 a | 178 ± 10.5 bc | 618 ± 12.3 d | 50.35 ± 10.3 a | |
| 24 | 544 ± 9.3 a | 101 ± 20.1 b | 431 ± 24.1 a | 169 ± 5.1 b | 600 ± 21.0 c | 50.05 ± 13.7 c | |
| 36 | 524 ± 7.9 c | 93 ± 19.5 a | 440 ± 15.8 c | 149 ± 6.0 a | 581 ± 16.9 a | 50.00 ± 19.1 b | |
| 48 | 608 ± 11.5 a | 143 ± 10.5 a | 465 ± 18.0 a | 241 ± 13.0 b | 706 ± 14.2 ab | 50.40 ± 6.9 b | |
| 60 | 564 ± 10.6 a | 123 ± 10.4 b | 441 ± 8.0 c | 179 ± 21.7 c | 620 ± 14.0 d | 50.10 ± 13.0 b | |
| 72 | 500 ± 20.8 a | 109 ± 9.9 c | 391 ± 13.7 b | 133 ± 12.9 d | 524 ± 15.7 c | 51.10 ± 7.3 d | |
| CQ−20 | 0 | 494 ± 23.7 b | 81 ± 12.6 c | 434 ± 25.6 a | 157 ± 25.1 e | 591 ± 24.6 e | 95.35 ± 2.9 c |
| 12 | 486 ± 23.4 b | 60 ± 10.5 b | 405 ± 17.9 a | 130 ± 18.9 b | 535 ± 26.9 b | 50.10 ± 3.9 a | |
| 24 | 423 ± 8.3 b | 42 ± 11.5 bc | 387 ± 15.1 a | 135 ± 16.4 a | 522 ± 14.9 c | 50.05 ± 11.0 a | |
| 36 | 418 ± 5.9 a | 36 ± 5.9 a | 356 ± 22.3 a | 111 ± 16.7 c | 487 ± 21.5 c | 50.05 ± 5.7 a | |
| 48 | 454 ± 25.6 b | 63 ± 4.6 b | 391 ± 15.3 b | 146 ± 21.0 c | 537 ± 23.3 b | 55.10 ± 16.7 a | |
| 60 | 413 ± 27.9 b | 58 ± 20.5 b | 352 ± 9.8 b | 114 ± 12.5 b | 440 ± 21.0 a | 50.15 ± 20.9 c | |
| 72 | 408 ± 30.1 a | 56 ± 3.3 b | 347 ± 11.3 b | 993 ± 19.8 b | 466 ± 19.8 b | 50.40 ± 25.1 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, M.; Li, T.; Zhang, X.; Wang, L. Enhance Production of γ-Aminobutyric Acid (GABA) and Improve the Function of Fermented Quinoa by Cold Stress. Foods 2022, 11, 3908. https://doi.org/10.3390/foods11233908
Zhang Y, Zhang M, Li T, Zhang X, Wang L. Enhance Production of γ-Aminobutyric Acid (GABA) and Improve the Function of Fermented Quinoa by Cold Stress. Foods. 2022; 11(23):3908. https://doi.org/10.3390/foods11233908
Chicago/Turabian StyleZhang, Yucui, Ming Zhang, Ting Li, Xinxia Zhang, and Li Wang. 2022. "Enhance Production of γ-Aminobutyric Acid (GABA) and Improve the Function of Fermented Quinoa by Cold Stress" Foods 11, no. 23: 3908. https://doi.org/10.3390/foods11233908
APA StyleZhang, Y., Zhang, M., Li, T., Zhang, X., & Wang, L. (2022). Enhance Production of γ-Aminobutyric Acid (GABA) and Improve the Function of Fermented Quinoa by Cold Stress. Foods, 11(23), 3908. https://doi.org/10.3390/foods11233908






