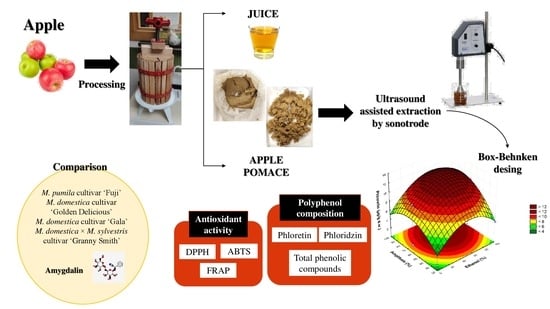
Establishment of a Sonotrode Ultrasound-Assisted Extraction of Phenolic Compounds from Apple Pomace
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Experimental Design
2.4. Ultrasound-Assisted Extraction by Sonotrode
2.5. Antioxidant Assays
2.6. Determination of Phenolic Compounds by HPLC–ESI–TOF–MS Analysis
3. Results and Discussion
3.1. Identification of Phenolic Compounds by HPLC–ESI–TOF–MS
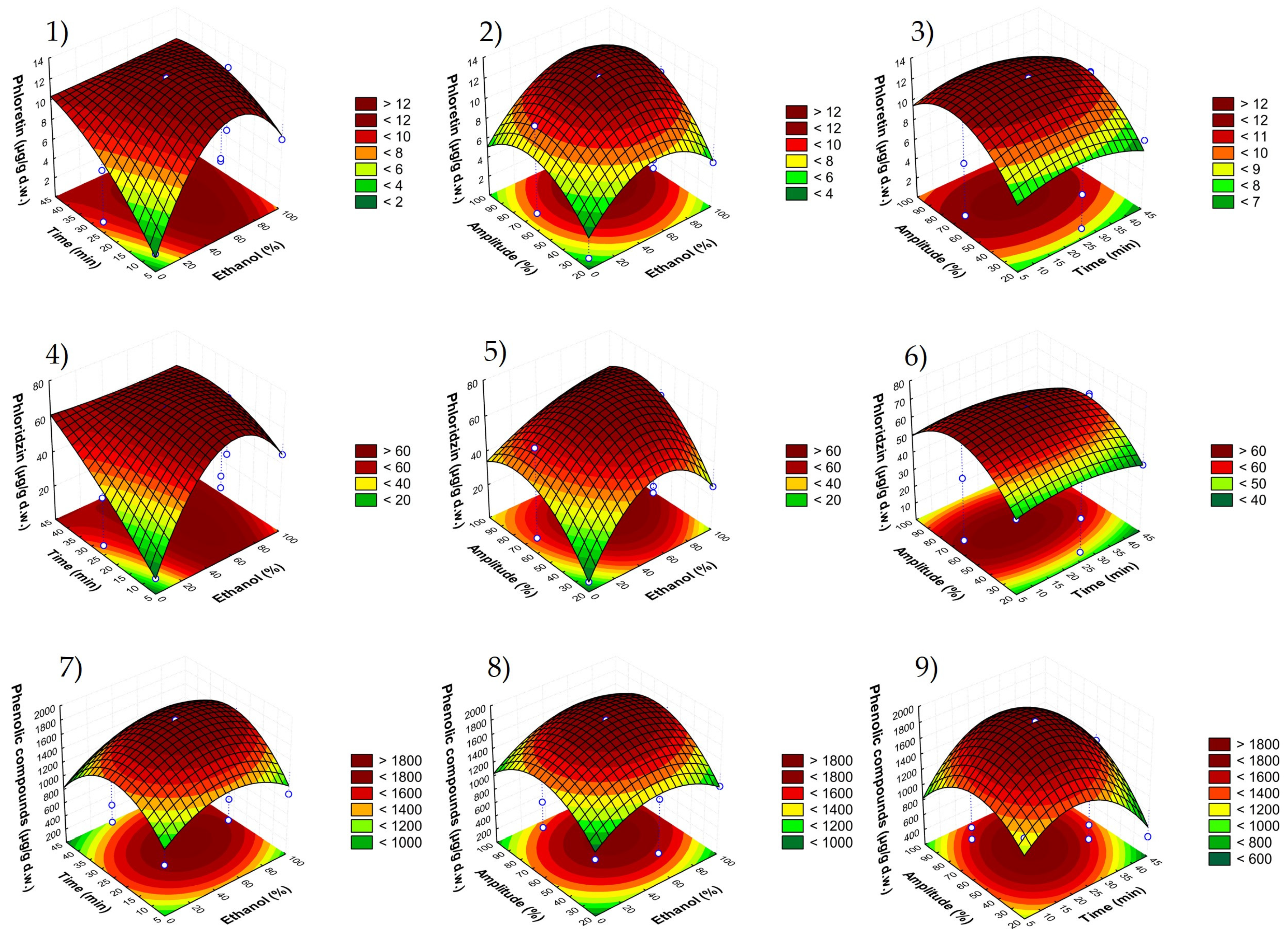
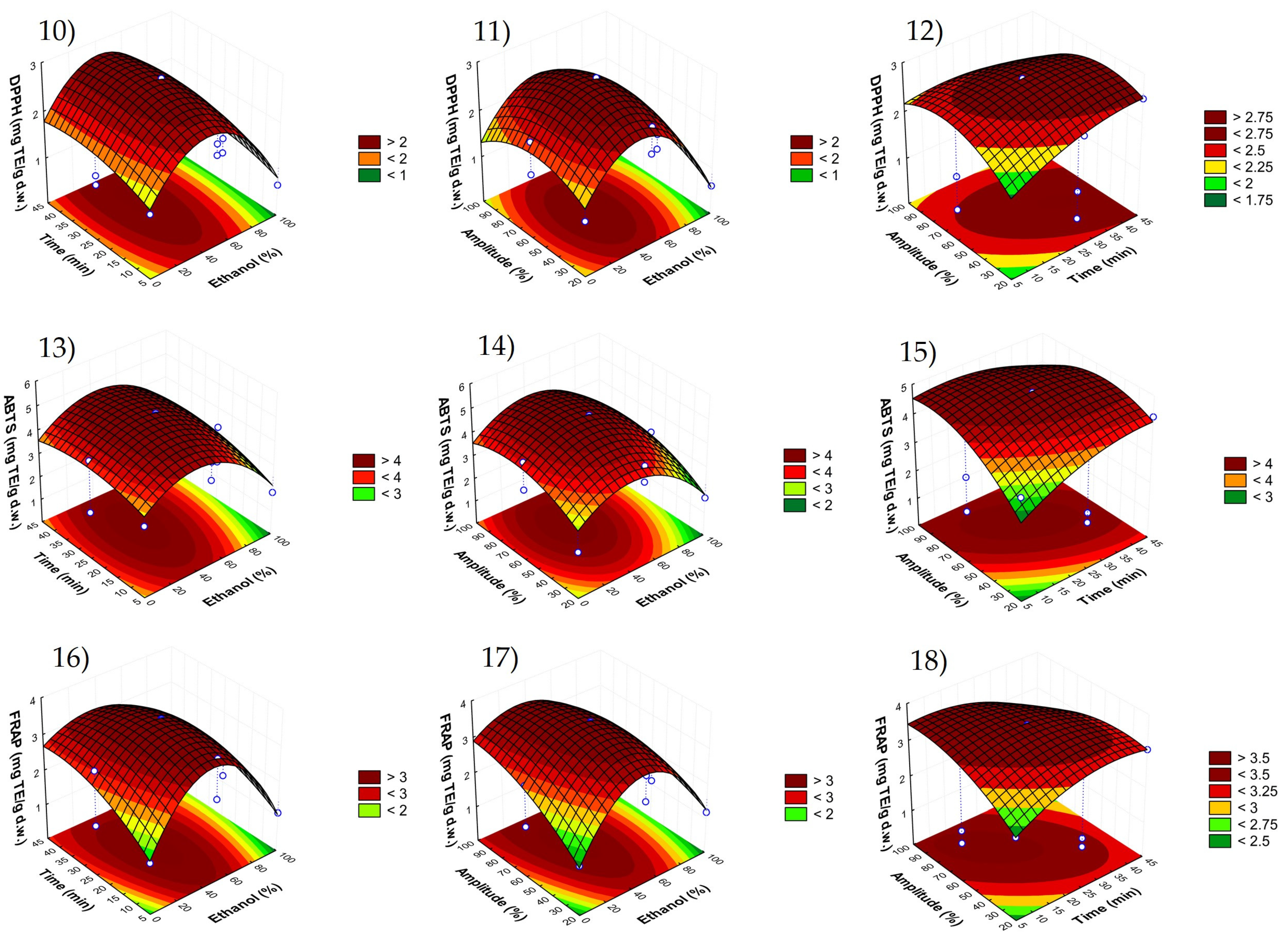
3.2. Fitting the Model
3.3. Comparison of Amygdalin and Phenolic Content in Different Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szabo, K.; Dulf, F.V.; Teleky, B.-E.; Eleni, P.; Boukouvalas, C.; Krokida, M.; Kapsalis, N.; Rusu, A.V.; Socol, C.T.; Vodnar, D.C. Evaluation of the Bioactive Compounds Found in Tomato Seed Oil and Tomato Peels Influenced by Industrial Heat Treatments. Foods 2021, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Scarano, P.; Tartaglia, M.; Zuzolo, D.; Prigioniero, A.; Guarino, C.; Sciarrillo, R. Recovery and Valorization of Bioactive and Functional Compounds from the Discarded of Opuntia ficus-indica (L.) Mill. Fruit Peel. Agronomy 2022, 12, 388. [Google Scholar] [CrossRef]
- Guzzetti, L.; Galimberti, A.; Bruni, I.; Magoni, C.; Ferri, M.; Tassoni, A.; Sangiovanni, E.; Dell’Agli, M.; Labra, M. Bioprospecting on invasive plant species to prevent seed dispersal. Sci. Rep. 2017, 7, 13799. [Google Scholar] [CrossRef] [PubMed]
- Rudovica, V.; Rotter, A.; Gaudêncio, S.P.; Novoveská, L.; Akgül, F.; Akslen-Hoel, L.K.; Alexandrino, D.A.M.; Anne, O.; Arbidans, L.; Atanassova, M.; et al. Valorization of Marine Waste: Use of Industrial By-Products and Beach Wrack towards the Production of High Added-Value Products. Front. Mar. Sci. 2021, 8, 723333. [Google Scholar] [CrossRef]
- Statista. Available online: https://www.statista.com/ (accessed on 4 May 2022).
- Shalini, R.; Gupta, D.K. Utilization of pomace from apple processing industries: A review. J. Food Sci. Technol. 2010, 47, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple pomace as a functional and healthy ingredient in food products: A review. Processes 2020, 8, 319. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Guido, A.; Liberatore, C.M.; Ganino, T.; Lazzi, C.; Chiancone, B. From byproduct to resource: Fermented apple pomace as beer flavoring. Foods 2019, 8, 309. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Arraibi, A.A.; Ferreira, I.C.F.R. Bioactive and functional compounds in apple pomace from juice and cider manufacturing: Potential use in dermal formulations. Trends Food Sci. Technol. 2019, 90, 76–87. [Google Scholar] [CrossRef]
- Gowman, A.C.; Picard, M.C.; Rodriguez-Uribe, A.; Misra, M.; Khalil, H.; Thimmanagari, M.; Mohanty, A.K. Physicochemical analysis of Apple and Grape Pomaces. BioResources 2019, 14, 3210–3230. [Google Scholar] [CrossRef]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple pomace as food fortification ingredient: A systematic review and meta-analysis. J. Food Sci. 2020, 85, 2977–2985. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Riaño, B.; Hijosa-Valsero, M.; González-García, I.; Paniagua-García, A.I.; Hernández, D.; Garita-Cambronero, J.; Díez-Antolínez, R.; García-González, M.C. Valorization of apple pomaces for biofuel production: A biorefinery approach. Biomass Bioenergy 2020, 142, 105785. [Google Scholar] [CrossRef]
- Rana, S.; Kumar, S.; Rana, A.; Padwad, Y.; Bhushan, S. Biological activity of phenolics enriched extracts from industrial apple pomace. Ind. Crops Prod. 2021, 160, 113158. [Google Scholar] [CrossRef]
- Heras-Ramírez, M.E.; Quintero-Ramos, A.; Camacho-Dávila, A.A.; Barnard, J.; Talamás-Abbud, R.; Torres-Muñoz, J.V.; Salas-Muñoz, E. Effect of Blanching and Drying Temperature on Polyphenolic Compound Stability and Antioxidant Capacity of Apple Pomace. Food Bioprocess Technol. 2012, 5, 2201–2210. [Google Scholar] [CrossRef]
- Yan, H.; Kerr, W.L. Total phenolics content, anthocyanins, and dietary fiber content of apple pomace powders produced by vacuum-belt drying. J. Sci. Food Agric. 2013, 93, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Perussello, C.A.; Zhang, Z.; Marzocchella, A.; Tiwari, B.K. Valorization of Apple Pomace by Extraction of Valuable Compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 776–796. [Google Scholar] [CrossRef] [PubMed]
- Çam, M.; Aaby, K. Optimization of extraction of apple pomace phenolics with water by response surface methodology. J. Agric. Food Chem. 2010, 58, 9103–9111. [Google Scholar] [CrossRef]
- Candrawinata, V.I.; Golding, J.B.; Roach, P.D.; Stathopoulos, C.E. Optimisation of the phenolic content and antioxidant activity of apple pomace aqueous extracts. CYTA-J. Food 2015, 13, 293–299. [Google Scholar] [CrossRef]
- Zheng, H.Z.; Hwang, I.W.; Chung, S.K. Enhancing polyphenol extraction from unripe apples by carbohydrate- hydrolyzing enzymes. J. Zhejiang Univ. Sci. B 2009, 10, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Skrypnik, L.; Novikova, A. Response surface modeling and optimization of polyphenols extraction from apple pomace based on nonionic emulsifiers. Agronomy 2020, 10, 92. [Google Scholar] [CrossRef]
- Bai, X.L.; Yue, T.L.; Yuan, Y.H.; Zhang, H.W. Optimization of microwave-assisted extraction of polyphenols from apple pomace using response surface methodology and HPLC analysis. J. Sep. Sci. 2010, 33, 3751–3758. [Google Scholar] [CrossRef] [PubMed]
- Grigoras, C.G.; Destandau, E.; Fougère, L.; Elfakir, C. Evaluation of apple pomace extracts as a source of bioactive compounds. Ind. Crops Prod. 2013, 49, 794–804. [Google Scholar] [CrossRef]
- Rezaei, S.; Rezaei, K.; Haghighi, M.; Labbafi, M. Solvent and solvent to sample ratio as main parameters in the microwave-assisted extraction of polyphenolic compounds from apple pomace. Food Sci. Biotechnol. 2013, 22, 1–6. [Google Scholar] [CrossRef]
- Casazza, A.A.; Pettinato, M.; Perego, P. Polyphenols from apple skins: A study on microwave-assisted extraction optimization and exhausted solid characterization. Sep. Purif. Technol. 2020, 240, 116640. [Google Scholar] [CrossRef]
- Massias, A.; Boisard, S.; Baccaunaud, M.; Leal Calderon, F.; Subra-Paternault, P. Recovery of phenolics from apple peels using CO2 + ethanol extraction: Kinetics and antioxidant activity of extracts. J. Supercrit. Fluids 2015, 98, 172–182. [Google Scholar] [CrossRef]
- Ferrentino, G.; Morozova, K.; Mosibo, O.K.; Ramezani, M.; Scampicchio, M. Biorecovery of antioxidants from apple pomace by supercritical fluid extraction. J. Clean. Prod. 2018, 186, 253–261. [Google Scholar] [CrossRef]
- Frontuto, D.; Carullo, D.; Harrison, S.M.; Brunton, N.P.; Ferrari, G.; Lyng, J.G.; Pataro, G. Optimization of Pulsed Electric Fields-Assisted Extraction of Polyphenols from Potato Peels Using Response Surface Methodology. Food Bioprocess Technol. 2019, 12, 1708–1720. [Google Scholar] [CrossRef]
- Egüés, I.; Hernandez-Ramos, F.; Rivilla, I.; Labidi, J. Optimization of ultrasound assisted extraction of bioactive compounds from apple pomace. Molecules 2021, 26, 3783. [Google Scholar] [CrossRef]
- Fu, C.; Tian, H.; Li, Q.; Cai, T.; Du, W. Ultrasound-assisted extraction of xyloglucan from apple pomace. Ultrason. Sonochem. 2006, 13, 511–516. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Ultrasound-Assisted Extraction of Pectin from Malus domestica ‘Fălticeni’ Apple Pomace. Processes 2019, 7, 488. [Google Scholar] [CrossRef]
- Shahabi Mohammadabadi, S.; Goli, M.; Naji Tabasi, S. Optimization of Bioactive Compound Extraction from Eggplant Peel by Response Surface Methodology: Ultrasound-Assisted Solvent Qualitative and Quantitative Effect. Foods 2022, 11, 3263. [Google Scholar] [CrossRef]
- Razola-Díaz, M.d.C.; Guerra-Hernández, E.J.; Rodríguez-Pérez, C.; Gómez-Caravaca, A.M.; García-Villanova, B.; Verardo, V. Optimization of Ultrasound-Assisted Extraction via Sonotrode of Phenolic Compounds from Orange By-Products. Foods 2021, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Demirok, N.T.; Yıkmış, S. Combined Effect of Ultrasound and Microwave Power in Tangerine Juice Processing: Bioactive Compounds, Amino Acids, Minerals, and Pathogens. Processes 2022, 10, 2100. [Google Scholar] [CrossRef]
- Fonteles, T.; Leite, A.K.; Miguel, T.; Fernandes, F.; Pinheiro, S.; Miguel, E.; Rodrigues, S. Optimization of Sonication Parameters to Produce a Cashew Apple Bagasse Puree Rich in Superoxide Dismutase. Foods 2022, 11, 2694. [Google Scholar] [CrossRef]
- Medina-Jaramillo, C.; Gomez-Delgado, E.; López-Córdoba, A. Improvement of the Ultrasound-Assisted Extraction of Polyphenols from Welsh Onion (Allium fistulosum) Leaves Using Response Surface Methodology. Foods 2022, 11, 2425. [Google Scholar] [CrossRef] [PubMed]
- Aznar-Ramos, M.J.; Razola-Díaz, M.d.C.; Verardo, V.; Gómez-Caravaca, A.M. Comparison between Ultrasonic Bath and Sonotrode Extraction of Phenolic Compounds from Mango Peel By-Products. Horticulturae 2022, 8, 1014. [Google Scholar] [CrossRef]
- Razola-Díaz, M.d.C.; Gómez-Caravaca, A.M.; López de Andrés, J.; Voltes-Martínez, A.; Zamora, A.; Pérez-Molina, G.M.; Castro, D.J.; Marchal, J.A.; Verardo, V. Evaluation of Phenolic Compounds and Pigments Content in Yellow Bell Pepper Wastes. Antioxidants 2022, 11, 557. [Google Scholar] [CrossRef]
- Verni, M.; Pontonio, E.; Krona, A.; Jacob, S.; Pinto, D.; Rinaldi, F.; Verardo, V.; Díaz-de-Cerio, E.; Coda, R.; Rizzello, C.G. Bioprocessing of Brewers’ Spent Grain Enhances Its Antioxidant Activity: Characterization of Phenolic Compounds and Bioactive Peptides. Front. Microbiol. 2020, 11, 1831. [Google Scholar] [CrossRef]
- Ćetković, G.; Čanadanović-Brunet, J.; Djilas, S.; Savatović, S.; Mandić, A.; Tumbas, V. Assessment of polyphenolic content and in vitro antiradical characteristics of apple pomace. Food Chem. 2008, 109, 340–347. [Google Scholar] [CrossRef]
- Li, W.; Yang, R.; Ying, D.; Yu, J.; Sanguansri, L.; Augustin, M.A. Analysis of polyphenols in apple pomace: A comparative study of different extraction and hydrolysis procedures. Ind. Crops Prod. 2020, 147, 112250. [Google Scholar] [CrossRef]
- Diñeiro García, Y.; Valles, B.S.; Picinelli Lobo, A. Phenolic and antioxidant composition of by-products from the cider industry: Apple pomace. Food Chem. 2009, 117, 731–738. [Google Scholar] [CrossRef]
- Pollini, L.; Blasi, F.; Ianni, F.; Grispoldi, L.; Moretti, S.; Di Veroli, A.; Cossignani, L.; Cenci-goga, B.T. Ultrasound-Assisted Extraction and Characterization of Polyphenols from Apple Pomace, Functional Ingredients for Beef Burger Fortification. Molecules 2022, 27, 1933. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Waldbauer, K.; McKinnon, R.; Kopp, B. Apple Pomace as Potential Source of Natural Active Compounds. Planta Med. 2017, 83, 994–1010. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudlou, Y.; Asghari Ghajari, M.; Tavasoli, S. Effects of heat treatment on the phenolic compounds and antioxidant capacity of quince fruit and its tisane’s sensory properties. J. Food Sci. Technol. 2019, 56, 2365–2372. [Google Scholar] [CrossRef]
- Larrauri, J.A.; Rupérez, P.; Saura-Calixto, F. Effect of Drying Temperature on the Stability of Polyphenols and Antioxidant Activity of Red Grape Pomace Peels. J. Agric. Food Chem. 1997, 45, 1390–1393. [Google Scholar] [CrossRef]
- Pollini, L.; Cossignani, L.; Juan, C.; Mañes, J. Extraction of phenolic compounds from fresh apple pomace by different non-conventional techniques. Molecules 2021, 26, 4272. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Zhang, G.; Wood, E.; Rogel Castillo, C.; Mitchell, A.E. Quantification of Amygdalin in Nonbitter, Semibitter, and Bitter Almonds (Prunus dulcis) by UHPLC-(ESI)QqQ MS/MS. J. Agric. Food Chem. 2013, 61, 7754–7759. [Google Scholar] [CrossRef]
- Guć, M.; Rutecka, S.; Schroeder, G. Analysis of Amygdalin in Various Matrices Using Electrospray Ionization and Flowing Atmospheric-Pressure Afterglow Mass Spectrometry. Biomolecules 2020, 10, 1459. [Google Scholar] [CrossRef]
- Yan, T.; Fu, Q.; Wang, J.; Ma, S. UPLC-MS/MS determination of ephedrine, methylephedrine, amygdalin and glycyrrhizic acid in Beagle plasma and its application to a pharmacokinetic study after oral administration of Ma Huang Tang. Drug Test. Anal. 2015, 7, 158–163. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Richel, A. Fractionation of apple by-products as source of new ingredients: Current situation and perspectives. Trends Food Sci. Technol. 2014, 40, 99–114. [Google Scholar] [CrossRef]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.M.; Tou, J.C. A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Amygdalin content of seeds, kernels and food products commercially- available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Mišina, I.; Olšteine, A.; Krasnova, I.; Pugajeva, I.; Lacis, G.; Siger, A.; Michalak, M.; Soliven, A.; Segliņa, D. Phenolic compounds in different fruit parts of crab apple: Dihydrochalcones as promising quality markers of industrial apple pomace by-products. Ind. Crops Prod. 2015, 74, 607–612. [Google Scholar] [CrossRef]
- CDC—Immediately Dangerous to Life or Health Concentrations (IDLH): Cyanides (as CN)—NIOSH Publications and Products. Available online: https://www.cdc.gov/niosh/idlh/cyanides.html (accessed on 4 July 2022).
- European Commission. Directive (EU) 2017/164 Establishing a Fourth List of Indicative Occupational Exposure Limit Values Pursuant to Council Directive 98/24/EC, and Amending Commission Directives 91/322/EEC, 2000/39/EC and 2009/161/EU; European Union. 2017. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX:32017L0164 (accessed on 22 October 2022).
- Dang, T.; Nguyen, C.; Tran, P.N. Physician Beware: Severe Cyanide Toxicity from Amygdalin Tablets Ingestion. Case Rep. Emerg. Med. 2017, 2017, 4289527. [Google Scholar] [CrossRef] [PubMed]
- Persic, M.; Mikulic-Petkovsek, M.; Slatnar, A.; Veberic, R. Chemical composition of apple fruit, juice and pomace and the correlation between phenolic content, enzymatic activity and browning. LWT-Food Sci. Technol. 2017, 82, 23–31. [Google Scholar] [CrossRef]
- Rana, S.; Rana, A.; Gupta, S.; Bhushan, S. Varietal influence on phenolic constituents and nutritive characteristics of pomace obtained from apples grown in western Himalayas. J. Food Sci. Technol. 2021, 58, 166–174. [Google Scholar] [CrossRef] [PubMed]
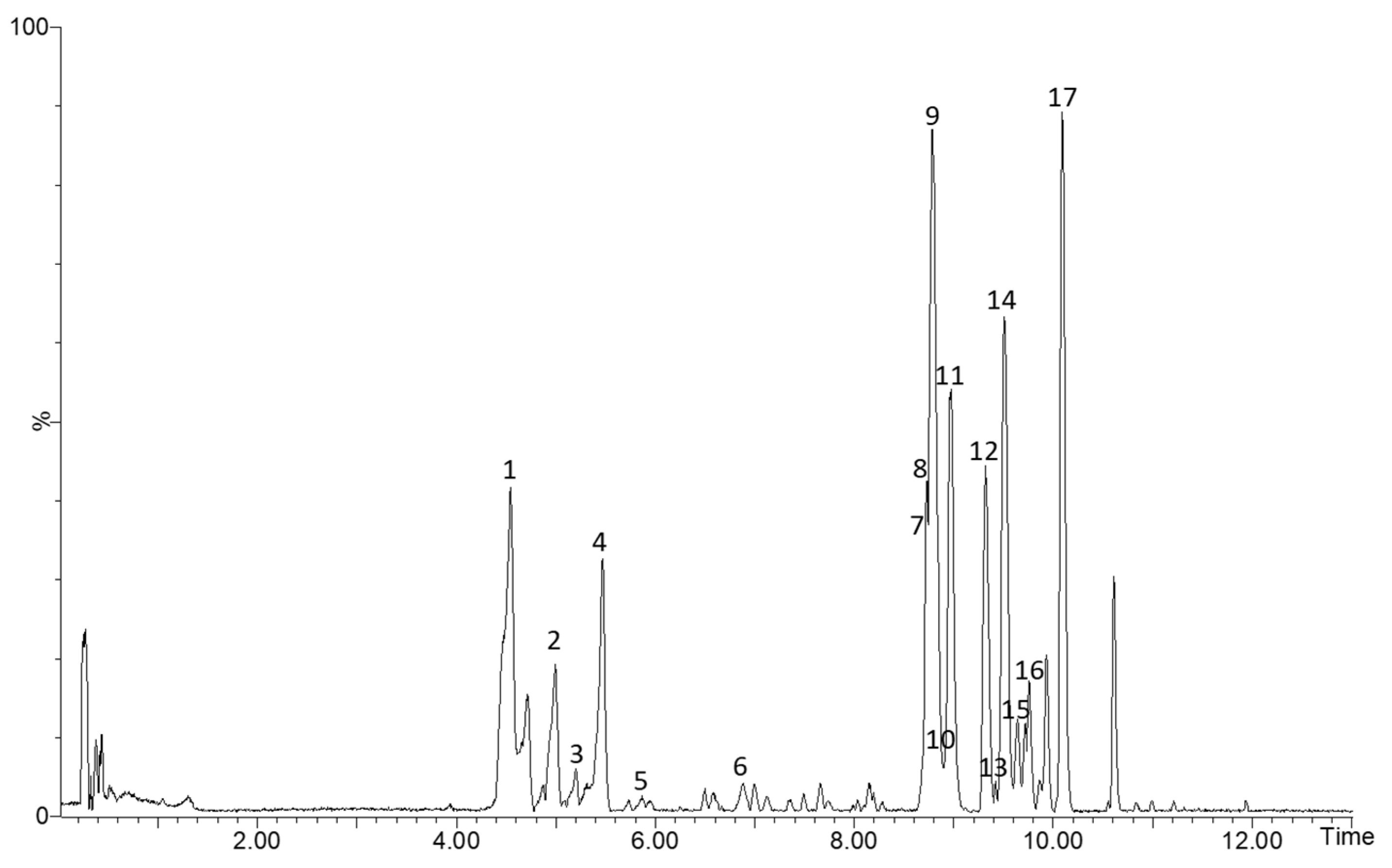
| Peak | Rt (min) | Observed m/z | Calculated m/z | Error (ppm) | Score (%) | Molecular Formula | In Source m/z Fragments | Compound Name |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.56 | 353.0863 | 353.0873 | −2.8 | 98.15 | C16H18O9 | 191.0532; 179.0342; 173.0427; 135.0425 | Caffeoylquinic acid |
| 2 | 4.99 | 289.0703 | 289.0712 | −3.1 | 91.47 | C15H14O6 | 245.0740; 125.0235 | Catechin |
| 3 | 5.20 | 289.0699 | 289.0712 | −4.5 | 90.97 | C15H14O6 | 125.0233 | Epicatechin |
| 4 | 5.31 | 337.0907 | 337.0923 | −4.7 | 93.86 | C16H18O8 | 173.0423; 191.0530; 235.0558 | Coumaroylquinic acid |
| 5 | 5.41 | 577.1357 | 577.1346 | 1.9 | 99.83 | C30H26O12 | 407.007; 289.0711 | Procyanidin dimer |
| 6 | 6.88 | 865.2006 | 865.1980 | 3.0 | 99.71 | C45H38O18 | 407.0749; 289.0721 | Procyanidin trimer |
| 7 | 8.72 | 273.0754 | 273.0763 | −3.3 | 97.23 | C15H14O5 | 167.0319 | Phloretin |
| 8 | 8.73 | 567.1715 | 567.1714 | 0.2 | 97.67 | C26H32O14 | 273.0730; 167.0313 | Phloretin-2′-O-xyloglucoside |
| 9 | 8.78 | 463.0868 | 463.0877 | −1.9 | 93.12 | C21H20O12 | 301.0390; 271.0211; 241.0108 | Quercetin-3-O-galactoside |
| 10 | 8.84 | 609.1438 | 609.1456 | −3.0 | 93.17 | C27H30O16 | 463.0861; 301.0303 | Rutin |
| 11 | 8.97 | 463.0857 | 463.0877 | −4.3 | 99.96 | C21H20O12 | 301.0301; 271.0202; 241.0096 | Quercetin-3-O-glucoside |
| 12 | 9.31 | 435.1276 | 435.1291 | −3.4 | 99.95 | C21H24O10 | 273.0739; 167.0320 | Phloridzin |
| 13 | 9.33 | 477.1010 | 477.1033 | −4.8 | 92.24 | C22H22O12 | 315.0110; 287.0181; 331.0412 | Isorhamnetin-3-O-glucoside |
| 14 | 9.51 | 433.075 | 433.0771 | −4.8 | 99.97 | C20H18O11 | 301.0314; 271.0214; 241.0117 | Quercetin-3-O-arabinopyranoside |
| 15 | 9.64 | 433.0754 | 433.0771 | −3.9 | 99.77 | C20H18O11 | 300.0248; 271.0206; 241.0113 | Quercetin-3-O-arabinofuranoside |
| 16 | 9.72 | 433.0759 | 433.0771 | −2.8 | 90.11 | C20H18O11 | 301.0335; 271.0222; 241.0109 | Quercetin-3-O-xylanoside |
| 17 | 10.09 | 447.0921 | 447.0927 | −1.3 | 97.64 | C21H20O11 | 301.0330; 271.0236; 255.0279 | Quercetin-3-O-rhamnoside |
| Run | Independent Factors | Responses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Phloretin (µg/g d.w.) | Phloridzin (µg/g d.w.) | Sum of Phenolic Compounds (µg/g d.w.) | DPPH (mg TE/g d.w.) | ABTS (mg TE/g d.w.) | FRAP (mg TE/g d.w.) | |
| 1 | 0 (−1) | 5 (−1) | 60 (0) (89 W) | 1.84 ± 0.03 | 9.20 ± 0.03 | 747.28 ± 5.98 | 1.37 ± 0.02 | 3.02 ± 0.33 | 1.46 ± 0.05 |
| 2 | 100 (1) | 5 (−1) | 60 (0) (88 W) | 7.12 ± 0.12 | 44.98 ± 0.18 | 896.37 ± 29.63 | 0.66 ± 0.04 | 1.82 ± 0.00 | 1.10 ± 0.01 |
| 3 | 0 (−1) | 45 (1) | 60 (0) (87) | 10.64 ± 0.09 | 61.10 ± 0.13 | 1116.30 ± 21.78 | 2.06 ± 0.18 | 4.18 ± 0.03 | 2.61 ± 0.08 |
| 4 | 100 (1) | 45 (1) | 60 (0) (85) | 10.52 ± 0.09 | 59.81 ± 0.12 | 1298.88 ± 21.43 | 1.00 ± 0.09 | 3.05 ± 0.00 | 1.81 ± 0.01 |
| 5 | 0 (−1) | 25 (0) | 20 (−1) (38 W) | 1.15 ± 0.03 | 5.87 ± 0.04 | 827.16 ± 6.66 | 1.19 ± 0.03 | 1.99 ± 0.01 | 1.42 ± 0.05 |
| 6 | 100 (1) | 25 (0) | 20 (−1) (29 W) | 4.63 ± 0.08 | 25.67 ± 0.11 | 1009.69 ± 18.90 | 0.61 ± 0.02 | 1.65 ± 0.02 | 1.18 ± 0.01 |
| 7 | 0 (−1) | 25 (0) | 100 (1) (149 W) | 6.40 ± 0.07 | 33.69 ± 0.09 | 1072.79 ± 16.85 | 1.38 ± 0.01 | 4.16 ± 0.38 | 2.95 ± 0.21 |
| 8 | 100 (1) | 25 (0) | 100 (1) (126 W) | 11.02 ± 0.09 | 58.93 ± 0.12 | 1489.76 ± 21.25 | 0.93 ± 0.10 | 3.17 ± 0.34 | 1.58 ± 0.05 |
| 9 | 50 (0) | 5 (−1) | 20 (−1) (36 W) | 7.90 ± 0.09 | 42.94 ± 0.12 | 1240.36 ± 21.14 | 1.93 ± 0.04 | 3.61 ± 0.20 | 2.33 ± 0.03 |
| 10 | 50 (0) | 45 (1) | 20 (−1) (37 W) | 7.06 ± 0.08 | 39.20 ± 0.11 | 462.23 ± 19.03 | 2.49 ± 0.17 | 4.28 ± 0.03 | 3.05 ± 0.12 |
| 11 | 50 (0) | 5 (−1) | 100 (1) (136 W) | 8.17 ± 0.13 | 49.49 ± 0.19 | 940.24 ± 32.30 | 2.17 ± 0.00 | 4.36 ± 0.02 | 3.44 ± 0.14 |
| 12 | 50 (0) | 45 (1) | 100 (1) (140 W) | 7.64 ± 0.12 | 42.74 ± 0.17 | 931.26 ± 29.10 | 1.71 ± 0.03 | 2.95 ± 0.28 | 2.36 ± 0.08 |
| 13 | 50 (0) | 25 (0) | 60 (0) (86 W) | 12.63 ± 0.11 | 67.04 ± 0.15 | 1808.56 ± 26.26 | 2.72 ± 0.00 | 4.72 ± 0.22 | 3.49 ± 0.09 |
| 14 | 50 (0) | 25 (0) | 60 (0) (87 W) | 12.32 ± 0.10 | 67.48 ± 0.14 | 1878.74 ± 23.91 | 2.79 ± 0.00 | 4.92 ± 0.18 | 3.58 ± 0.29 |
| 15 | 50 (0) | 25 (0) | 60 (0) (85 W) | 11.96 ± 0.10 | 67.19 ± 0.14 | 1816.58 ± 23.74 | 2.67 ± 0.04 | 4.85 ± 0.07 | 3.54 ± 0.15 |
| Phloretin (µg/g d.w.) | Phloridzin (µg/g d.w.) | Sum of Phenolic Compounds (µg/g d.w.) | DPPH (mg TE/g d.w.) | ABTS (mg TE/g d.w.) | FRAP (mg TE/g d.w.) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | p Value | Effect | p Value | Effect | p Value | Effect | p Value | Effect | p Value | Effect | p Value | |
| β0 | 7.0078 | 0.0002 ** | 39.4685 | 0.0000 ** | 1002.6926 | 0.0001 ** | 1.4592 | 0.0001 ** | 3.1865 | 0.0001 ** | 2.1070 | 0.0000 ** |
| Linear | ||||||||||||
| β1 | 3.3139 | 0.0050 * | 19.0022 | 0.0001 ** | 232.7921 | 0.0133 * | −0.7016 | 0.0038 ** | −0.9157 | 0.0062 * | −0.6934 | 0.0020 ** |
| β2 | 3.8383 | 0.0042 ** | 20.4948 | 0.0001 ** | 125.9876 | 0.0480 * | 0.3551 | 0.0163 * | 0.6771 | 0.0124 * | 0.5599 | 0.0034 ** |
| β3 | 4.0193 | 0.0038 ** | 22.0404 | 0.0001 ** | 270.0492 | 0.0111 * | 0.0799 | 0.2238 | 1.1331 | 0.0045 ** | 0.7087 | 0.0021 ** |
| Quadratic | ||||||||||||
| β11 | 3.3347 | 0.0027 ** | 18.0086 | 0.0000 ** | 306.7966 | 0.0042 ** | 1.2519 | 0.0007 ** | 1.4344 | 0.0014 ** | 1.4025 | 0.0003 ** |
| β22 | 1.4400 | 0.0142 * | 5.4554 | 0.0005 ** | 513.1239 | 0.0015 ** | 0.2026 | 0.0241 * | 0.3789 | 0.0192 * | 0.3890 | 0.0034 ** |
| β33 | 3.1693 | 0.0030 ** | 18.1889 | 0.0000 ** | 427.9818 | 0.0022 ** | 0.4479 | 0.0051 * | 0.6529 | 0.0066 * | 0.3515 | 0.0041 ** |
| Crossed | ||||||||||||
| β12 | −2.7004 | 0.0149 * | −18.5412 | 0.0001 ** | 16.7476 | 0.7054 | −0.1766 | 0.1032 | 0.0368 | 0.7532 | −0.2264 | 0.0352 * |
| β13 | 0.5730 | 0.2282 | 2.7210 | 0.0069 * | 117.2224 | 0.0927 | 0.0615 | 0.4233 | −0.3261 | 0.0858 * | −0.5643 | 0.0059 * |
| β23 | 0.1538 | 0.6903 | −1.5058 | 0.0219 * | 384.5743 | 0.0098 * | −0.5103 | 0.0143 * | −1.0383 | 0.0096 * | −0.9035 | 0.0023 ** |
| R2 model | 0.9739 | 0.9999 | 0.9706 | 0.9644 | 0.9585 | 0.9883 | ||||||
| p model | 0.0433 * | 0.0430 * | 0.0198 * | 0.0005 ** | 0.0489 * | 0.0114 * | ||||||
| p lack of fit | 0.1937 | 0.3547 | 0.1657 | 0.1201 | 0.1223 | 0.0666 | ||||||
| Parameter | Optimal Conditions | |||||
|---|---|---|---|---|---|---|
| Ethanol (%) | 50 | |||||
| Time (min) | 23 | |||||
| Amplitude (%) | 65 (90 W) | |||||
| Phloretin | Phloridzin | Sum of phenolic compounds | DPPH | ABTS | FRAP | |
| (µg/g d.w.) | (µg/g d.w.) | (µg/g d.w.) | (mg TE/g d.w.) | (mg TE/g d.w.) | (mg TE/g d.w.) | |
| Predicted value | 12.44 ± 2.34 | 67.49 ± 1.59 | 1834.33 ± 269.38 | 2.70 ± 0.43 | 4.85 ± 0.72 | 3.55 ± 0.31 |
| Obtained value | 13.21 ± 0.25 | 69.12 ± 2.30 | 1945.54 ± 24.61 | 2.73 ± 0.02 | 4.82 ± 0.66 | 3.53 ± 0.58 |
| Coefficient of variation (%) | 4.22 | 1.69 | 4.16 | 0.59 | 0.51 | 0.58 |
| Statistical difference | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. |
| Granny Smith | Golden Delicious | Fuji | Gala | |||||
|---|---|---|---|---|---|---|---|---|
| Without Seed | With Seed | Without Seed | With Seed | Without Seed | With Seed | Without Seed | With Seed | |
| Phenolic compounds (µg/g d.w.) | ||||||||
| p-coumaric acid | 25.79 ± 0.21 b | 32.86 ± 0.20 a | 13.92 ± 0.08 e | 13.28 ± 0.19 e | 16.14 ± 0.12 d | 17.25 ± 0.02 c | 17.75 ± 0.09 c | 17.87 ± 0.08 c |
| Caffeoylquinic acid | 75.83 ± 0.32 g | 24.68 ± 0.22 h | 526.65 ± 1.45 a | 367.53 ± 1.76 d | 224.05 ± 0.13 e | 105.08 ± 0.44 f | 377.51 ± 3.10 c | 452.12 ± 1.57 b |
| Catechin | 174.94 ± 0.38 a | 96.37 ± 0.00 f | 126.98 ± 0.70 d | 104.89 ± 0.24 e | 149.88 ± 0.05 b | 79.06 ± 1.13 g | 148.17 ± 0.45 b | 138.31 ± 0.97 c |
| Epicatechin | 30.89 ± 0.2 a | 25.15 ± 0.02 c | 14.57 ± 0.13 e | 13.60 ± 0.15 f | 12.43 ± 0.04 g | 12.45 ± 0.02 g | 19.97 ± 0.06 d | 26.50 ± 0.32 b |
| Procyanidin dimer | 164.82 ± 0.54 a | 95.51 ± 0.23 b | 93.13 ± 0.18 b | 55.80 ± 0.23 e | 61.68 ± 0.86 d | 41.47 ± 0.96 f | 72.31 ± 0.70 b | 71.42 ± 0.92 b |
| Procyanidin trimer | 61.63 ± 0.79 a | 38.50 ± 0.11 b | 24.59 ± 0.13 e | 16.19 ± 0.11 f | 23.83 ± 0.48 e | 16.14 ± 0.46 f | 28.41 ± 0.31 c | 29.24 ± 1.17 d |
| Phloretin-2′-O-xyloglucoside | 70.23 ± 0.48 a | 40.37 ± 1.14 d | 54.97 ± 0.14 c | 36.19 ± 0.34 e | 3.95 ± 0.21 f | < LOQ | 51.56 ± 0.79 c | 60.93 ± 0.83 b |
| Quercetin-3-O-galactoside | 60.54 ± 0.71 e | 137.50 ± 2.11 b | 37.16 ± 1.40 f | 55.30 ± 1.69 e | 106.88 ± 0.17 d | 145.45 ± 2.51 b | 124.97 ± 0.09 c | 209.40 ± 2.77 a |
| Quercetin-3-O-glucoside | <LOQ | 153.65 ± 3.99 a | <LOQ | <LOQ | 39.12 ± 0.05 d | 90.83 ± 2.44 c | 24.88 ± 0.17 e | 107.90 ± 1.29 b |
| Rutin | <LOQ | 131.32 ± 3.29 b | <LOQ | <LOQ | 67.03 ± 0.86 c | 150.67 ± 1.97 a | <LOQ | 38.81 ± 0.77 d |
| Phloretin | 38.89 ± 0.35 h | 64.71 ± 0.16 f | 84.41 ± 0.08 e | 115.63 ± 0.11 a | 97.89 ± 0.00 c | 106.13 ± 0.18 b | 43.12 ± 0.15 g | 95.96 ± 0.07 d |
| Phloridzin | 30.43 ± 0.25 f | 92.95 ± 1.16 e | 139.13 ± 1.46 d | 263.38 ± 3.75 a | 202.92 ± 1.47 b,c | 211.35 ± 0.12 b | 39.15 ± 0.30 f | 198.93 ± 1.02 c |
| Quercetin-3-O-arabinopyranoside | 39.97 ± 0.38 f | 111.15 ± 2.85 c | 11.95 ± 0.11 g | 35.17 ± 1.35 f | 90.53 ± 0.60 d | 141.98 ± 2.28 a | 75.37 ± 1.07 e | 123.29 ± 2.44 b |
| Quercetin-3-O-arabinofuranoside | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 7.14 ± 0.00 | <LOQ | <LOQ |
| Quercetin-3-O-xylanoside | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Isorhamnetin-3-O-glucoside | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Quercetin-3-O-rhamnoside | 73.41 ± 0.53 e | 124.32 ± 2.24 c | 50.00 ± 0.15 f | 96.33 ± 1.47 d | 103.66 ± 0.07 d | 175.99 ± 2.38 a | 101.99 ± 0.88 d | 153.79 ± 1.55 b |
| Sum of phenolic compounds | 922.39 ± 6.43 e | 1169.04 ± 17.01 c,d | 1177.46 ± 6.00 c,d | 1177.76 ± 11.39 c,d | 1199.98 ± 5.10 c | 1297.41 ± 14.91 b | 1125.14 ± 8.15 d | 1724.47 ± 15.77 a |
| Amygdalin (µg/g d.w.) | n.d. | 17.48 ± 0.74 c | n.d. | 60.07 ± 0.28 a | n.d. | 48.32 ± 0.26 b | n.d. | 13.14 ± 0.13 d |
| Antioxidant assays (mg TE/g d.w.) | ||||||||
| DPPH | 5.11 ± 0.34 a,b,c | 5.47 ± 0.06 a,b | 3.89 ± 0.03 b,c | 4.65 ± 0.30 c | 5.23 ± 0.46 a,b | 6.10 ± 0.20 a | 5.00 ± 0.49 a,b,c | 6.26 ± 0.38 a |
| ABTS | 11.28 ± 0.29 a,b,c | 12.68 ± 0.25 a | 7.72 ± 0.26 d | 8.54 ± 0.46 d | 9.43 ± 0.93 c,d | 10.76 ± 0.61 a,b,c | 10.57 ± 0.28 b,c | 11.94 ± 0.54 a,b |
| FRAP | 7.74 ± 0.74 a,b | 8.26 ± 0.02 a | 4.00 ± 0.45 c | 4.78 ± 0.00 b,c | 6.17 ± 0.69 a,b,c | 7.22 ± 1.07 a,b | 6.74 ± 0.33 a,b,c | 8.37 ± 0.52 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razola-Díaz, M.d.C.; Aznar-Ramos, M.J.; Guerra-Hernández, E.J.; García-Villanova, B.; Gómez-Caravaca, A.M.; Verardo, V. Establishment of a Sonotrode Ultrasound-Assisted Extraction of Phenolic Compounds from Apple Pomace. Foods 2022, 11, 3809. https://doi.org/10.3390/foods11233809
Razola-Díaz MdC, Aznar-Ramos MJ, Guerra-Hernández EJ, García-Villanova B, Gómez-Caravaca AM, Verardo V. Establishment of a Sonotrode Ultrasound-Assisted Extraction of Phenolic Compounds from Apple Pomace. Foods. 2022; 11(23):3809. https://doi.org/10.3390/foods11233809
Chicago/Turabian StyleRazola-Díaz, María del Carmen, María José Aznar-Ramos, Eduardo Jesús Guerra-Hernández, Belén García-Villanova, Ana María Gómez-Caravaca, and Vito Verardo. 2022. "Establishment of a Sonotrode Ultrasound-Assisted Extraction of Phenolic Compounds from Apple Pomace" Foods 11, no. 23: 3809. https://doi.org/10.3390/foods11233809
APA StyleRazola-Díaz, M. d. C., Aznar-Ramos, M. J., Guerra-Hernández, E. J., García-Villanova, B., Gómez-Caravaca, A. M., & Verardo, V. (2022). Establishment of a Sonotrode Ultrasound-Assisted Extraction of Phenolic Compounds from Apple Pomace. Foods, 11(23), 3809. https://doi.org/10.3390/foods11233809