Effect of a Multifunctional Biosurfactant Extract Obtained from Corn Steep Liquor on Orange and Apple Juices
Abstract
1. Introduction
2. Materials and Methods
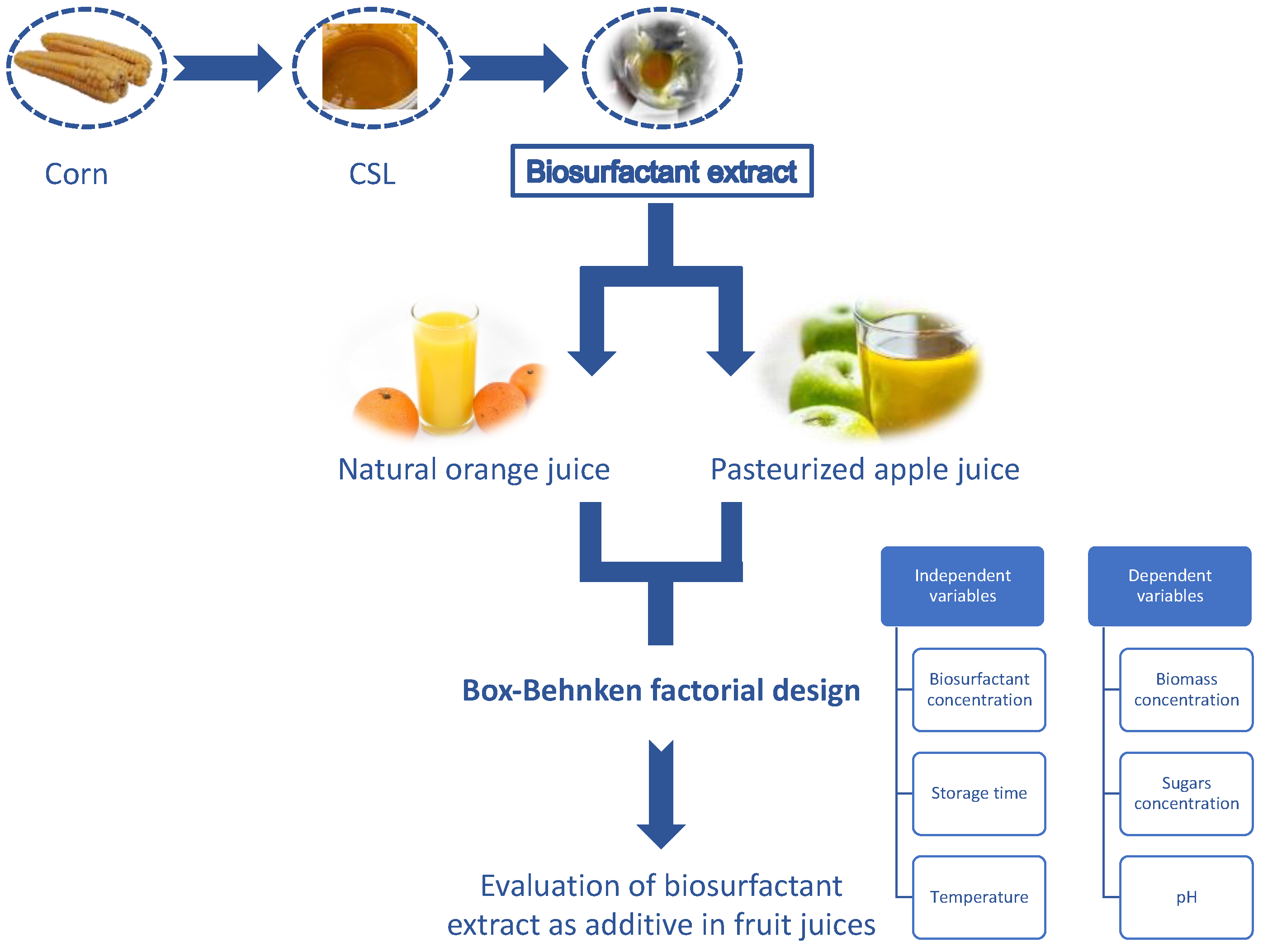
2.1. Obtention of a Multifunctional Biosurfactant Extract from Corn Steep Liquor
2.2. Factorial Design to Evaluate the Effect of a Biosurfactant Extract in Orange and Apple Juices
2.3. pH Determination
2.4. Determination of Biomass
2.5. Determination of Total Soluble Sugars
2.6. Statistical Treatment of Data
3. Results and Discussion
3.1. Factorial Design to Evaluate the Stability of Fruit Juices
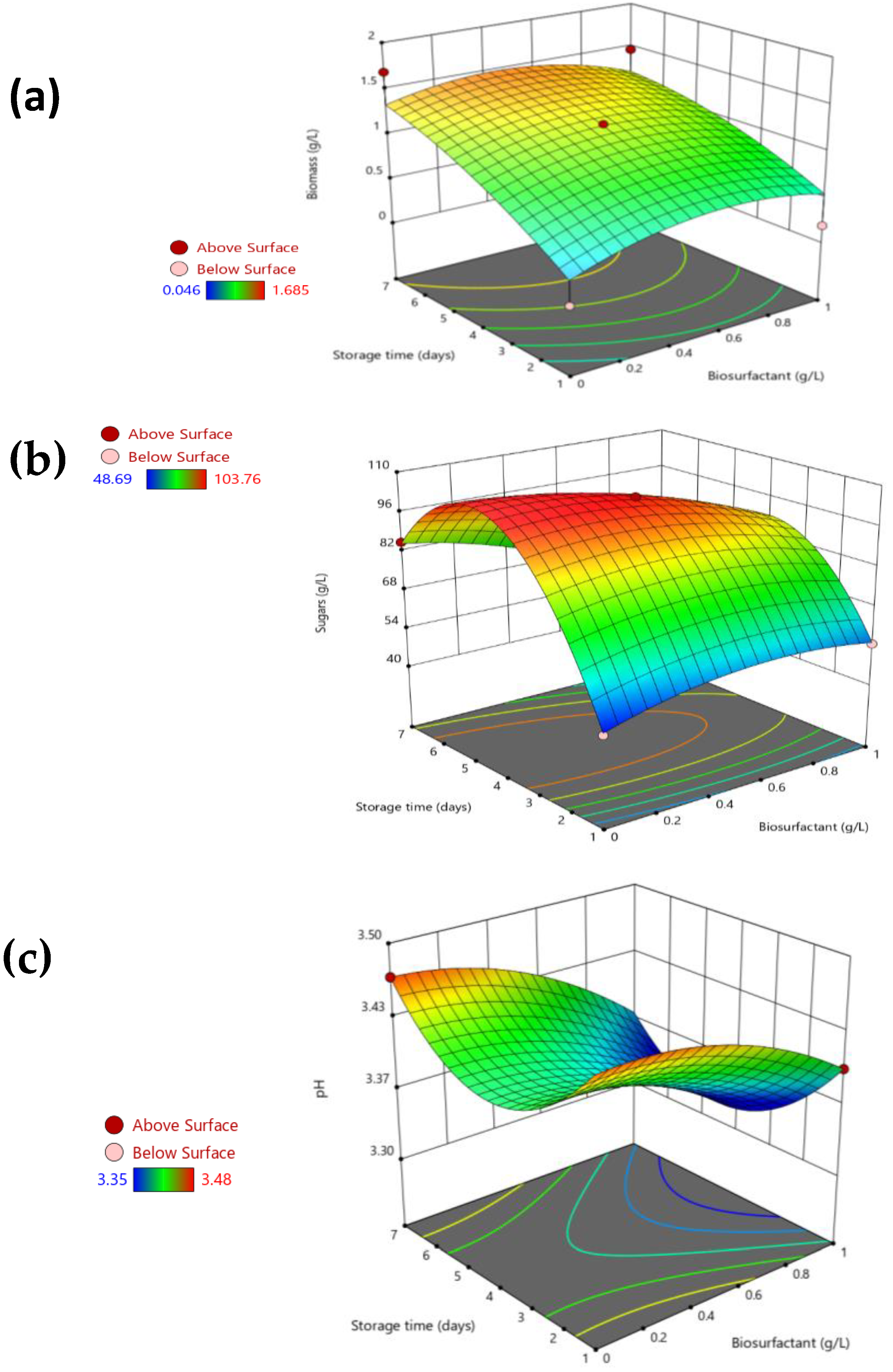
3.2. Effect of a Biosurfactant Extract in Pasteurized Apple Juice
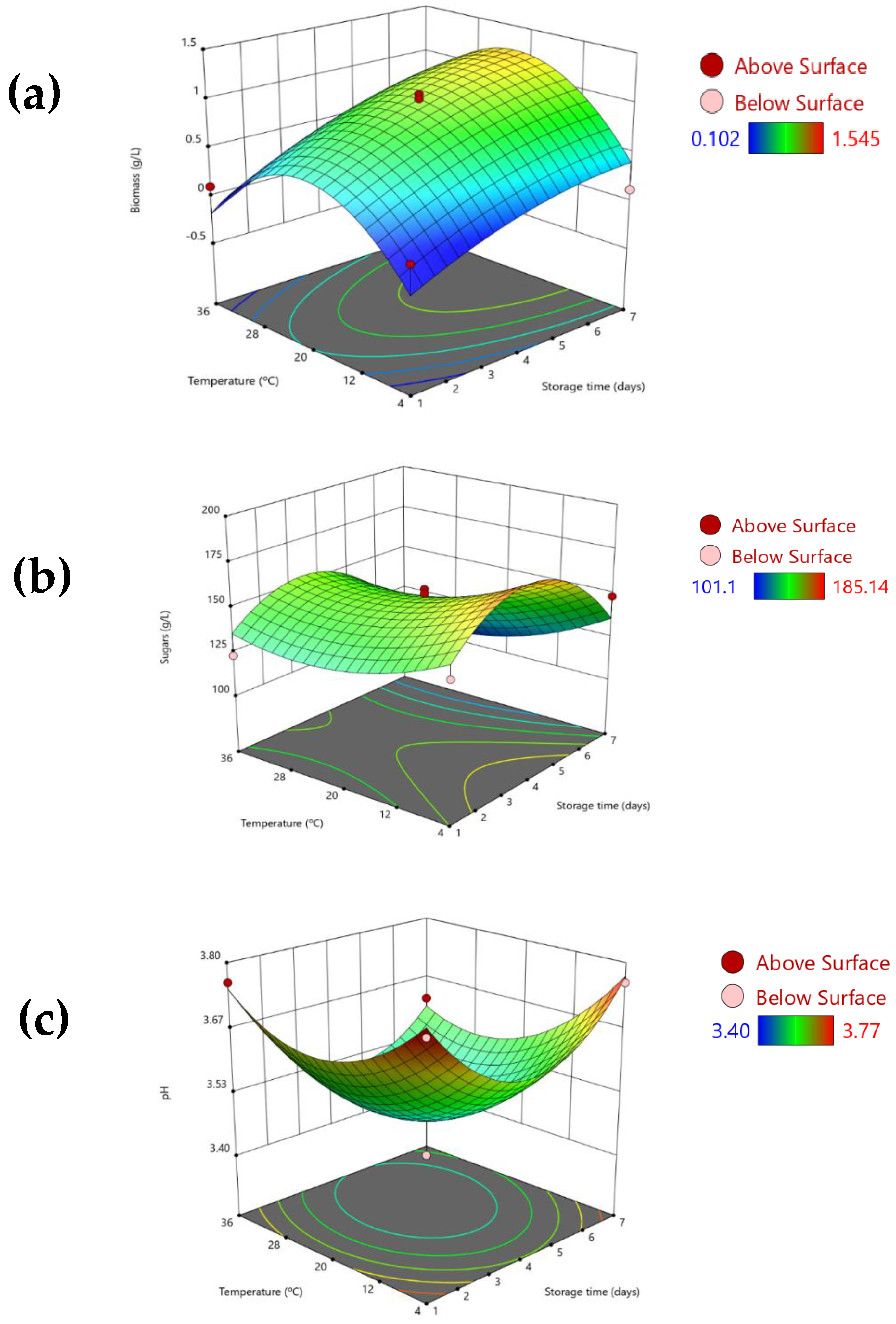
3.3. Effect of the Biosurfactant Extract in Natural Orange Juice
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarubbo, L.A.; Maria da Gloria, C.S.; Durval, I.J.B.; Bezerra, K.G.O.; Ribeiro, B.G.; Silva, I.A.; Twigg, M.S.; Banat, I.M. Biosurfactants: Production, Properties, Applications, Trends, and General Perspectives. Biochem. Eng. J. 2022, 181, 108377. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Rocha, V.; Teixeira, J.A.; Rodrigues, L.R. Antimicrobial and Antiadhesive Properties of a Biosurfactant Isolated from Lactobacillus Paracasei Ssp. Paracasei A20. Lett. Appl. Microbiol. 2010, 50, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Vecino, X.; Rodríguez-López, L.; Ferreira, D.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Bioactivity of Glycolipopeptide Cell-Bound Biosurfactants against Skin Pathogens. Int. J. Biol. Macromol. 2018, 109, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Durval, I.J.B.; Ribeiro, B.G.; Aguiar, J.S.; Sarubbo, L.A.; Rufino, R.D.; Converti, A. Application of a Biosurfactant Produced by Bacillus Cereus Ucp 1615 from Waste Frying Oil as an Emulsifier in a Cookie Formulation. Fermentation 2021, 7, 189. [Google Scholar] [CrossRef]
- Ghazala, I.; Charfeddine, S.; Charfeddine, M.; Gargouri, R.; Semia, B.; Chaabouni, E. Antimicrobial and Antioxidant Activities of Bacillus Mojavensis I4 Lipopeptides and Their Potential Application against the Potato Dry Rot Causative Fusarium Solani. Arch. Microbiol. 2022, 204, 1–12. [Google Scholar] [CrossRef]
- Mouafo, H.T.; Sokamte, A.T.; Mbawala, A.; Ndjouenkeu, R.; Devappa, S. Biosurfactants from Lactic Acid Bacteria: A Critical Review on Production, Extraction, Structural Characterization and Food Application. Food Biosci. 2022, 46, 101598. [Google Scholar] [CrossRef]
- Hoffmann, M.; Mück, D.; Grossmann, L.; Greiner, L.; Klausmann, P.; Henkel, M.; Lilge, L.; Weiss, J.; Hausmann, R. Surfactin from Bacillus Subtilis Displays Promising Characteristics as O/W-Emulsifier for Food Formulations. Colloids Surf. B Biointerfaces 2021, 203, 111749. [Google Scholar] [CrossRef]
- Ribeiro, B.G.; Campos Guerra, J.M.; Sarubbo, L.A. Production of a Biosurfactant from S. Cerevisiae and Its Application in Salad Dressing. Biocatal. Agric. Biotechnol. 2022, 42, 102358. [Google Scholar] [CrossRef]
- López-Prieto, A.; Rodríguez-López, L.; Rincón-Fontán, M.; Moldes, A.B.; Cruz, J.M. Effect of Biosurfactant Extract Obtained from the Corn-Milling Industry on Probiotic Bacteria in Drinkable Yogurt. J. Sci. Food Agric. 2019, 99, 824–830. [Google Scholar] [CrossRef]
- Ravindran, A.; Kiran, G.S.; Selvin, J. Revealing the Effect of Lipopeptide on Improving the Probiotics Characteristics: Flavor and Texture Enhancer in the Formulated Yogurt. Food Chem. 2022, 375, 131718. [Google Scholar] [CrossRef]
- Martínez-Arcos, A.; López-Prieto, A.; Rodríguez-López, L.; Pérez-Cid, B.; Vecino, X.; Moldes, A.B.; Manuel, C.J. Evaluation of Morphological Changes in Grapes Coated with a Biosurfactant Extract Obtained from Corn Steep Liquor. Appl. Sci. 2021, 11, 5904. [Google Scholar] [CrossRef]
- Scalzini, G.; López-Prieto, A.; Paissoni, M.A.; Englezos, V.; Giacosa, S.; Rolle, L.; Gerbi, V.; Segade, S.R.; Cid, B.P.; Moldes, A.B.; et al. Can a Corn-Derived Biosurfactant Improve Colour Traits of Wine? First Insight on Its Application during Winegrape Skin Maceration versus Oenological Tannins. Foods 2020, 9, 1747. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, L.; Vecino, X.; Barbosa-Pereira, L.; Moldes, A.B.; Cruz, J.M. A Multifunctional Extract from Corn Steep Liquor: Antioxidant and Surfactant Activities. Food Funct. 2016, 7, 3724–3732. [Google Scholar] [CrossRef]
- Box, G.E.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters: Design, Innovation and Discovery, 2nd ed.; Wiley and Sons: Hoboken, NJ, USA, 2005; ISBN 978-0-471-71813-0. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bustos, G.; Arcosa, U.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Recycled Lactobacillus pentosus biomass can regenerate biosurfactants after various fermentative and extractive cycles. Biochem. Eng. J. 2018, 132, 191–195. [Google Scholar] [CrossRef]
- López-Prieto, A.; Vecino, X.; Rodríguez-López, L.; Moldes, A.B.; Cruz, J.M. A Multifunctional Biosurfactant Extract Obtained from Corn Steep Water as Bactericide for Agrifood Industry. Foods 2019, 8, 410. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Oliveira, G.; Deering, A.J.; San Martin-Gonzalez, M.F.; Campanella, O.H. Microwave Pasteurization of Apple Juice: Modeling the Inactivation of Escherichia coli O157:H7 and Salmonella Typhimurium at 80–90 °C. Food Microbiol. 2020, 87, 103382. [Google Scholar] [CrossRef]
- Kim, S.S.; Park, J.; Park, H.; Hong, H.; Kang, D.H. Combined Ohmic Heating and Krypton-Chlorine Excilamp Treatment for the Inactivation of Listeria Monocytogenes, Salmonella Typhimurium, and Escherichia Coli O157:H7 in Apple Juice. J. Food Saf. 2020, 40, e12706. [Google Scholar] [CrossRef]
- López-Prieto, A.; Vecino, X.; Rodríguez-López, L.; Moldes, A.B.; Cruz, J.M. Fungistatic and Fungicidal Capacity of a Biosurfactant Extract Obtained from Corn Steep Water. Foods 2021, 10, 1318. [Google Scholar] [CrossRef]
- Sandi, D.; Paes Chaves, J.B.; Gomes De Sousa, A.C.; Maia Parreiras, J.F.; Coelho Da Silva, M.T.; Lessa Constant, P.B. Hunter Color Dimensions, Sugar Content and Volatile Compounds in Pasteurized Yellow Passion Fruit Juice (Passiflora Edulis Var. Flavicarpa) during Storage. Braz. Arch. Biol. Technol. 2004, 47, 233–245. [Google Scholar] [CrossRef]
- Rehman, M.A.; Khan, M.R.; Sharif, M.K.; Ahmad, S.; Shah, F.-U.-H. Study on the Storage Stability of Fruit Juice Concentrates. Pakistan J. Food Sci. 2014, 24, 101–107. [Google Scholar]
- Wang, H.; Yuan, J.; Chen, L.; Ban, Z.; Zheng, Y.; Jiang, Y.; Jiang, Y.; Li, X. Effects of Fruit Storage Temperature and Time on Cloud Stability of Not from Concentrated Apple Juice. Foods 2022, 11, 2568. [Google Scholar] [CrossRef]
- Cevallos-Cedeño, R.E.; Velásquez-Murillo, L.D. Engineering Thesis of Comparison of Temperature-Pasteurization Retention Time and Its Effect on Vitamin C Concentration in Orange Juice; Escuela Superior Politécnica Agropecuaria de Manabí: Calceta, Ecuador, 2007; pp. 50–52. [Google Scholar]
- Dhenge, R.; Langialonga, P.; Alinovi, M.; Lolli, V.; Aldini, A.; Rinaldi, M. Evaluation of Quality Parameters of Orange Juice Stabilized by Two Thermal Treatments (Helical Heat Exchanger and Ohmic Heating) and Non-Thermal (High-Pressure Processing). Food Control 2022, 141, 109150. [Google Scholar] [CrossRef]
- Pham, H.T.T.; Kityo, P.; Buvé, C.; Hendrickx, M.E.; Van Loey, A.M. Influence of Ph and Composition on Nonenzymatic Browning of Shelf-Stable Orange Juice during Storage. J. Agric. Food Chem. 2020, 68, 5402–5411. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, S.; Grauwet, T.; Santiago, J.S.; Tomic, J.; Vervoort, L.; Hendrickx, M.; Van Loey, A. Quality Changes of Pasteurised Orange Juice during Storage: A Kinetic Study of Specific Parameters and Their Relation to Colour Instability Scheling. Food Chem. 2015, 187, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Q.; Zhang, W.; Zhang, L.; Zhou, L.; Cai, S.; Hu, X.; Yi, J. Evaluation of Quality Changes of Differently Formulated Cloudy Mixed Juices during Refrigerated Storage after High Pressure Processing. Curr. Res. Food Sci. 2021, 4, 627–635. [Google Scholar] [CrossRef] [PubMed]
- FDA/Center for Food and Drug Administration. Approximate PH of Foods and Food Products; FDA/Center for Food and Drug Administration: Silver Spring, MD, USA, 2008. [Google Scholar]
| Independent Variables | |||
| Units | Range | ||
| Biosurfactant concentration | g/L | 0–1 | |
| Storage time | days | 1–7 | |
| Temperature | °C | 4–36 | |
| Coded Independent Variables | |||
| Nomenclature | Equation | Range | |
| Biosurfactant concentration | x1 | (x1 − 0.5)/0.5 | (−1, 1) |
| Storage time | x2 | (x2 − 4)/3 | (−1, 1) |
| Temperature | x3 | (x3 − 20)/16 | (−1, 1) |
| Dependent Variables | |||
| Nomenclature | Units | ||
| Biomass concentration | y1 | g/L | |
| Sugars concentration | y2 | g/L | |
| pH | y3 | --- | |
| Non Coded Independent Variables | Coded Independent Variables | |||||
|---|---|---|---|---|---|---|
| Experiment | x1 | x2 | x3 | x1 | x2 | x3 |
| 1 | 0.5 | 1 | 4 | 0 | −1 | −1 |
| 2 | 0.5 | 7 | 4 | 0 | 1 | −1 |
| 3 | 0.5 | 1 | 36 | 0 | −1 | 1 |
| 4 | 0.5 | 7 | 36 | 0 | 1 | 1 |
| 5 | 0 | 1 | 20 | −1 | −1 | 0 |
| 6 | 0 | 7 | 20 | −1 | 1 | 0 |
| 7 | 1 | 1 | 20 | 1 | −1 | 0 |
| 8 | 1 | 7 | 20 | 1 | 1 | 0 |
| 9 | 0 | 4 | 4 | −1 | 0 | −1 |
| 10 | 0 | 4 | 36 | −1 | 0 | 1 |
| 11 | 1 | 4 | 4 | 1 | 0 | −1 |
| 12 | 1 | 4 | 36 | 1 | 0 | 1 |
| 13 | 0.5 | 4 | 20 | 0 | 0 | 0 |
| 14 | 0.5 | 4 | 20 | 0 | 0 | 0 |
| 15 | 0.5 | 4 | 20 | 0 | 0 | 0 |
| y1 | y2 | y3 | y1 | y2 | y3 | |
|---|---|---|---|---|---|---|
| Expt | Apple Juice | Orange Juice | ||||
| 1 | 0.107 ± 0.005 | 57.1 ± 0.2 | 3.47 ± 0.03 | 0.107 ± 0.004 | 144 ± 6 | 3.77 ± 0.01 |
| 2 | 0.151 ± 0.001 | 56.6 ± 1.0 | 3.48 ± 0.01 | 0.120 ± 0.001 | 148 ± 5 | 3.76 ± 0.01 |
| 3 | 0.191 ± 0.009 | 52.7 ± 0.5 | 3.48 ± 0.01 | 0.102 ± 0.002 | 123 ± 6 | 3.76 ± 0.01 |
| 4 | 0.566 ± 0.001 | 78.8 ± 3.7 | 3.47 ± 0.03 | 0.351 ± 0.011 | 115 ± 5 | 3.62 ± 0.02 |
| 5 | 0.075 ± 0.003 | 48.7 ± 0.2 | 3.48 ± 0.01 | 0.110 ± 0.003 | 143 ± 2 | 3.75 ± 0.01 |
| 6 | 1.68 ± 0.01 | 85.4 ± 1.1 | 3.47 ± 0.02 | 1.54 ± 0.02 | 101 ± 4 | 3.64 ± 0.01 |
| 7 | 0.188 ± 0.009 | 54.9 ± 0.1 | 3.40 ± 0.02 | 0.128 ± 0.003 | 146 ± 6 | 3.67 ± 0.01 |
| 8 | 1.46 ± 0.05 | 49.0 ± 0.8 | 3.36 ± 0.04 | 1.28 ± 0.01 | 102 ± 1 | 3.61 ± 0.01 |
| 9 | 0.046 ± 0.001 | 101 ± 1 | 3.43 ± 0.03 | 0.108 ± 0.001 | 185 ± 6 | 3.70 ± 0.01 |
| 10 | 0.130 ± 0.006 | 99.9 ± 0.1 | 3.41 ± 0.02 | 0.241 ± 0.009 | 135 ± 4 | 3.53 ± 0.01 |
| 11 | 0.165 ± 0.007 | 92.4 ± 1.0 | 3.35 ± 0.01 | 0.122 ± 0.005 | 165 ± 3 | 3.67 ± 0.01 |
| 12 | 0.28 ± 0.002 | 77.5 ± 3.6 | 3.35 ± 0.01 | 0.275 ± 0.005 | 180 ± 1 | 3.57 ± 0.02 |
| 13 | 1.18 ± 0.01 | 101 ± 3 | 3.38 ± 0.01 | 1.07 ± 0.01 | 145 ± 1 | 3.40 ± 0.01 |
| 14 | 1.21 ± 0.02 | 104 ± 4 | 3.39 ± 0.01 | 1.03 ± 0.01 | 157 ± 0 | 3.51 ± 0.02 |
| 15 | 1.18 ± 0.01 | 104 ± 2 | 3.38 ± 0.01 | 0.967 ± 0.005 | 154 ± 0 | 3.52 ± 0.02 |
| Apple Juice | ||||||
|---|---|---|---|---|---|---|
| Coeff. | y1 | p-Values | y2 | p-Values | y3 | p-Values |
| β0 | 1.19 | 0.1309 | 102.8 | 0.0013 * | 3.38 | 0.0010 * |
| β1 | 0.0201 | 0.8917 | −7.69 | 0.0111 * | −0.0413 | 0.0002 * |
| β2 | 0.413 | 0.0323 * | 7.07 | 0.0154 * | −0.0063 | 0.2064 |
| β3 | 0.0875 | 0.5607 | 0.196 | 0.9241 | −0.0025 | 0.5867 |
| β1β2 | −0.0840 | 0.6900 | −10.67 | 0.0120 * | −0.0075 | 0.2729 |
| β1β3 | 0.0082 | 0.9685 | −3.39 | 0.2764 | 0.0050 | 0.4490 |
| β2β3 | 0.0827 | 0.6943 | 6.66 | 0.0615 | −0.0050 | 0.4490 |
| β12 | −0.2179 | 0.3402 | −5.93 | 0.0951 | −0.0229 | 0.0153 * |
| β22 | −0.1199 | 0.5871 | −37.41 | < 0.0001 * | 0.0671 | 0.0001 * |
| β32 | −0.8167 | 0.0109 * | −4.13 | 0.2116 | 0.0246 | 0.0117 * |
| Orange Juice | ||||||
| β0 | 1.02 | 0.171 | 151.9 | 0.0626 | 3.48 | 0.0229 * |
| β1 | −0.0252 | 0.855 | 3.53 | 0.511 | −0.0125 | 0.511 |
| β2 | 0.356 | 0.0424 * | −11.19 | 0.0750 | −0.0400 | 0.0732 |
| β3 | 0.0640 | 0.647 | −11.29 | 0.0731 | −0.0525 | 0.0312 * |
| β1β2 | −0.0715 | 0.716 | −0.517 | 0.944 | 0.0125 | 0.638 |
| β1β3 | 0.00500 | 0.979 | 16.33 | 0.0685 | 0.0175 | 0.515 |
| β2β3 | 0.0590 | 0.764 | −3.11 | 0.678 | −0.0325 | 0.251 |
| β12 | −0.120 | 0.561 | 2.20 | 0.777 | 0.0400 | 0.181 |
| β22 | −0.137 | 0.511 | −31.21 | 0.0081 * | 0.150 | 0.0022 * |
| β32 | −0.715 | 0.0140 * | 11.93 | 0.165 | 0.100 | 0.0119 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Cid, B.; Rodríguez-López, L.; Moldes, A.B.; Cruz, J.M.; Vecino, X. Effect of a Multifunctional Biosurfactant Extract Obtained from Corn Steep Liquor on Orange and Apple Juices. Foods 2022, 11, 3506. https://doi.org/10.3390/foods11213506
Pérez-Cid B, Rodríguez-López L, Moldes AB, Cruz JM, Vecino X. Effect of a Multifunctional Biosurfactant Extract Obtained from Corn Steep Liquor on Orange and Apple Juices. Foods. 2022; 11(21):3506. https://doi.org/10.3390/foods11213506
Chicago/Turabian StylePérez-Cid, Benita, Lorena Rodríguez-López, Ana Belén Moldes, José Manuel Cruz, and Xanel Vecino. 2022. "Effect of a Multifunctional Biosurfactant Extract Obtained from Corn Steep Liquor on Orange and Apple Juices" Foods 11, no. 21: 3506. https://doi.org/10.3390/foods11213506
APA StylePérez-Cid, B., Rodríguez-López, L., Moldes, A. B., Cruz, J. M., & Vecino, X. (2022). Effect of a Multifunctional Biosurfactant Extract Obtained from Corn Steep Liquor on Orange and Apple Juices. Foods, 11(21), 3506. https://doi.org/10.3390/foods11213506






