Improvement of the Lyophilization Survival Rate of Lactobacillus casei via Regulation of Its Surface Substances
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
2.2. Surface Substance Stripping Method
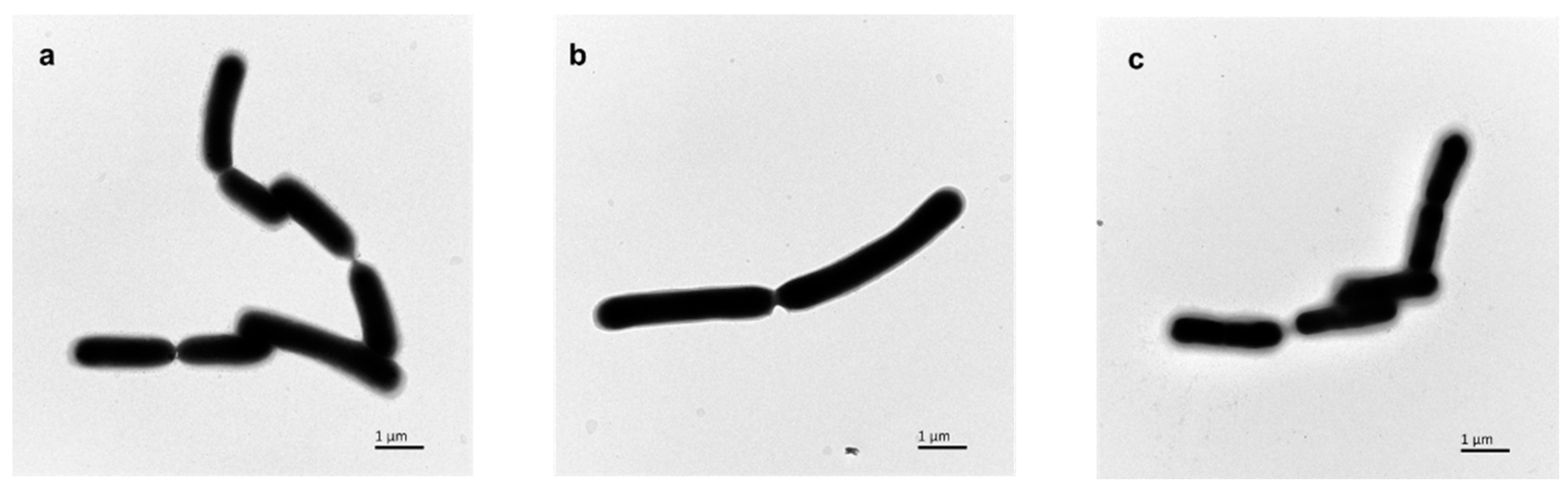
2.3. Capsule Observation and Determination
2.4. Freeze-Dried Bacterial Cell Preparation with Physiological Saline Solution
2.5. Lyophilized Bacterial Cell Pellets with Different Cryoprotectants
2.6. Determination of the Content of the Surface Substances of L. casei
2.7. Cultivation with Different Carbon Sources and Lyophilization
2.8. Cultivation with EDTA and Lyophilization
2.9. Determination and Lyophilization of Surface Substances of Bacteria under Different Fermentation Conditions
2.9.1. Surface Substance Content and Lyophilization Survival Rate of L. casei at a Constant pH with Different Carbon Sources
2.9.2. Surface Substance Content and Lyophilization Survival Rate of L. casei at a Constant pH with Different Nitrogen Sources
2.9.3. Surface Substance Content and Lyophilization Survival rate of L. casei at a Constant pH with Different Carbon–Nitrogen Consumption Ratios
2.9.4. Surface Substance Content and Lyophilization Survival Rate of L. casei at Different pH
2.10. Viable Counting Method
2.11. Statistical Analysis
3. Results
3.1. Effects of Surface Substances and Lyophilized Protectants on the Survival Rate of Lyophilization
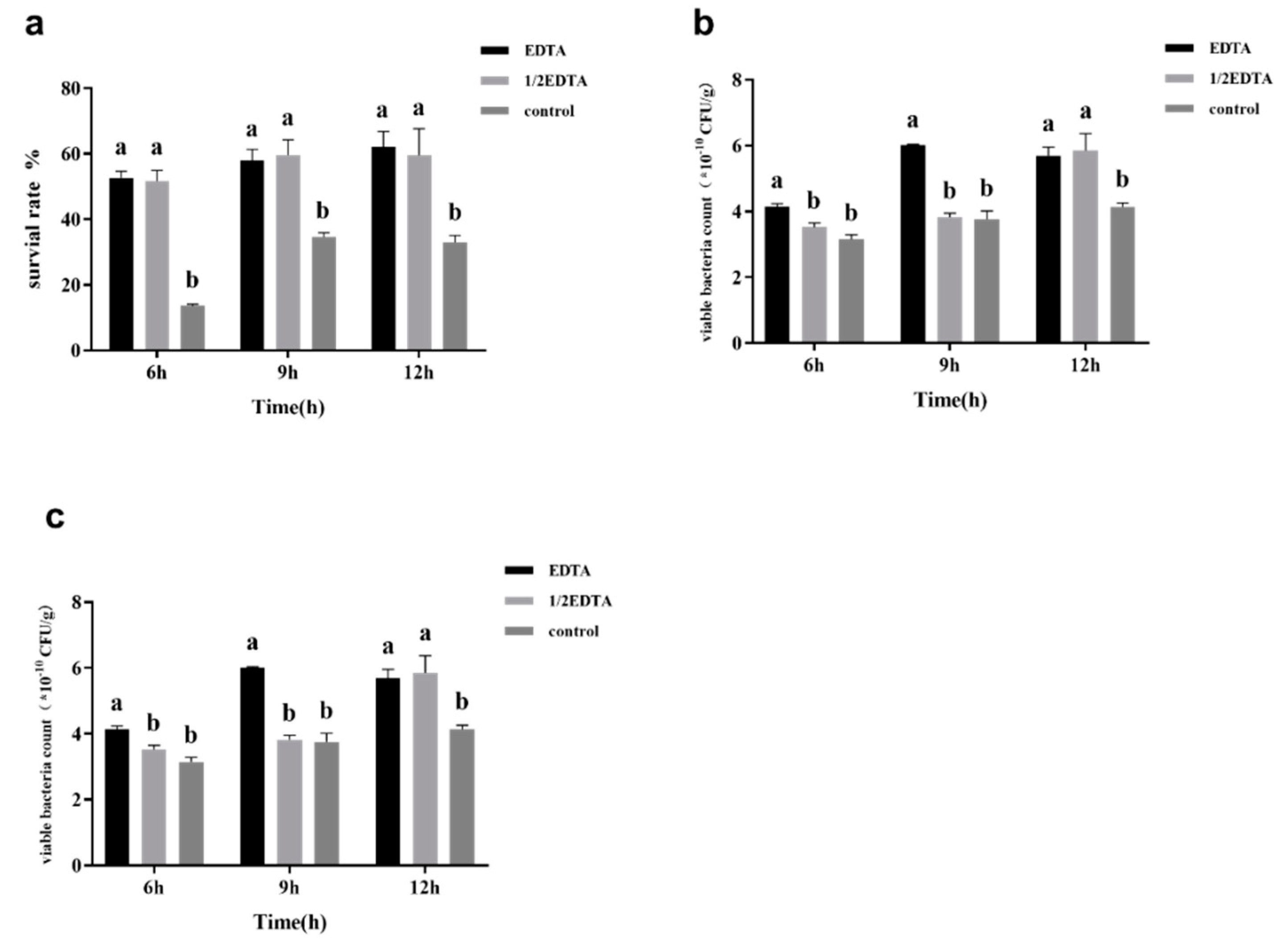
3.2. Effects of Adding EDTA on Surface Substance Yield and Survival Rate after Freeze-Drying
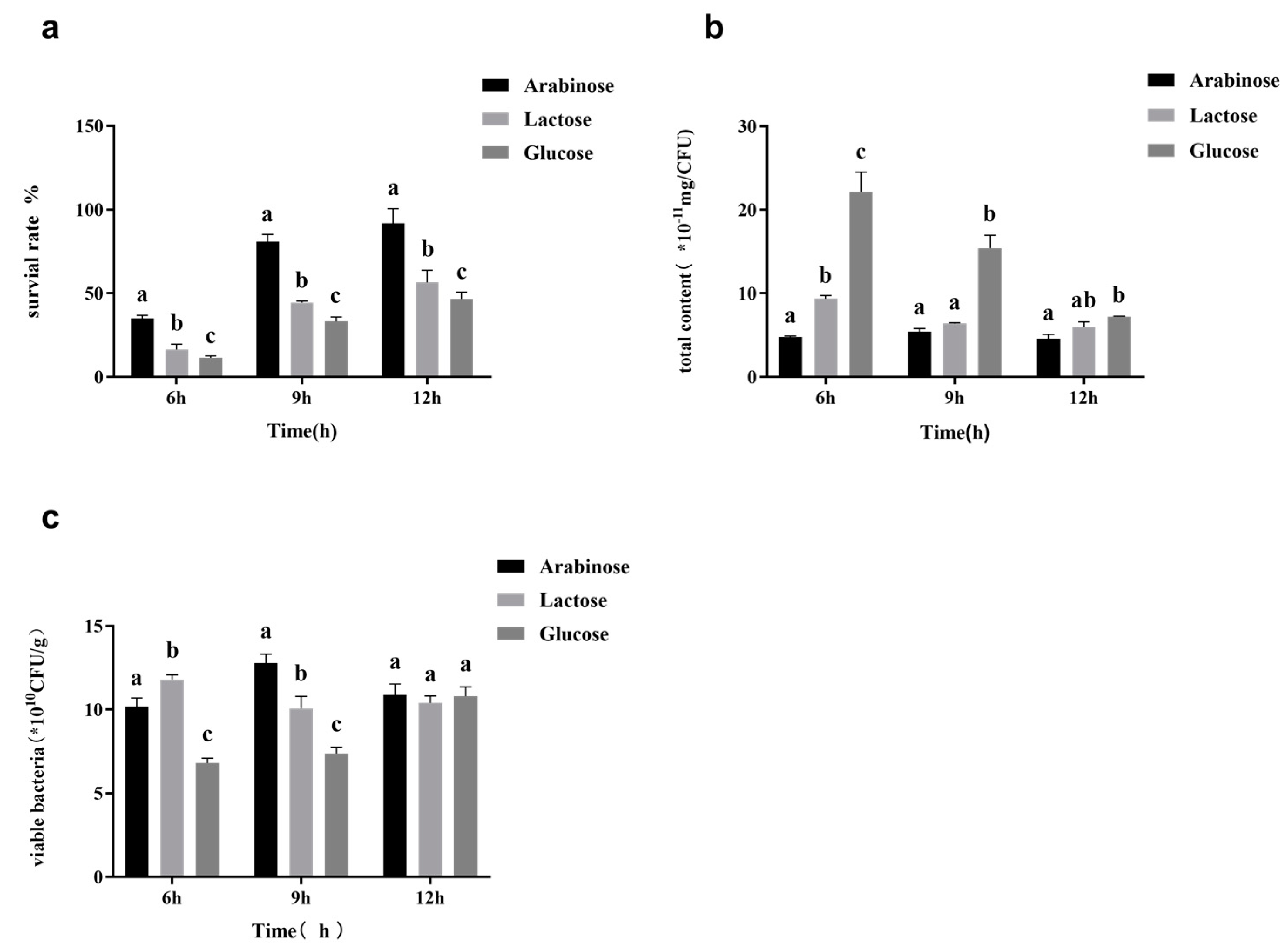
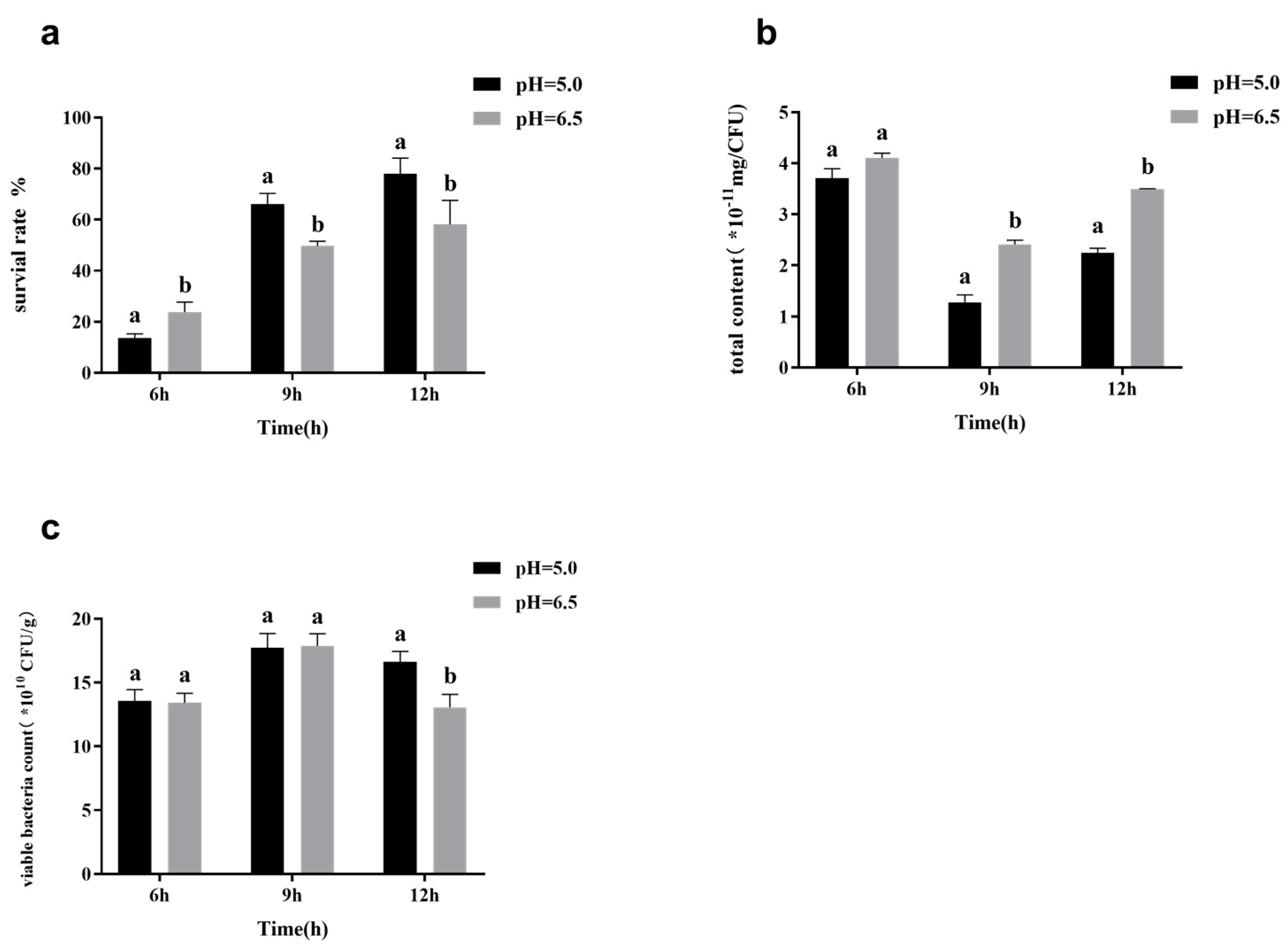
3.3. Effects of Fermentation Conditions on Surface Substance Yield and Survival Rate during Freeze-Drying
3.4. Mechanism of Influence of Surface Substances on Lyophilization Protectants of Stachyose
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Caggianiello, G.; Kleerebezem, M.; Spano, G. Exopolysaccharides produced by lactic acid bacteria: From health-promoting benefits to stress tolerance mechanisms. Appl. Microbiol. Biotechnol. 2016, 100, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Factories 2020, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Zeidan, A.A.; Poulsen, V.K.; Janzen, T.; Buldo, P.; Derkx, P.M.F.; Oregaard, G.; Neves, A.R. Polysaccharide production by lactic acid bacteria: From genes to industrial applications. Fems Microbiol. Rev. 2017, 41, S168–S200. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Fels, L.; Wefers, D.; Behr, J.; Jakob, F.; Vogel, R.F. Lactobacillus hordei dextrans induce Saccharomyces cerevisiae aggregation and network formation on hydrophilic surfaces. Int. J. Biol. Macromol. 2018, 115, 236–242. [Google Scholar] [CrossRef]
- Hynonen, U.; Palva, A. Lactobacillus surface layer proteins: Structure, function and applications. Appl. Microbiol. Biotechnol. 2013, 97, 5225–5243. [Google Scholar] [CrossRef]
- Bradshaw, W.J.; Roberts, A.K.; Shone, C.C.; Acharya, K.R. The structure of the S-layer of Clostridium difficile. J. Cell Commun. Signal. 2018, 12, 319–331. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Wu, Y.-J.; Hu, C.-Y. Monosaccharide composition influence and immunomodulatory effects of probiotic exopolysaccharides. Int. J. Biol. Macromol. 2019, 133, 575–582. [Google Scholar] [CrossRef]
- Saadat, Y.R.; Khosroushahi, A.Y.; Gargari, B.P. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef]
- Sorensen, H.M.; Rochfort, K.D.; Maye, S.; MacLeod, G.; Brabazon, D.; Loscher, C.; Freeland, B. Exopolysaccharides of Lactic Acid Bacteria: Production, Purification and Health Benefits towards Functional Food. Nutrients 2022, 14, 2938. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, P.T.; Nguyen, T.T.V.; Nguyen, T.T.U.; Nguyen, T.B.N.; Bui, N.B.; Hoang, Q.K.; Nguyen, H.T. Correlation Between the Amount of Extracellular Polymeric Substances and the Survival Rate to Freeze-Drying of Probiotics. Curr. Microbiol. 2022, 79, 165. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulou, M.; Bardeau, T.; Ramonet, P.-Y.; Miot-Certier, C.; Claisse, O.; Doco, T.; Petrel, M.; Lucas, P.; Dols-Lafargue, M. Exopolysaccharides produced by Oenococcus oeni: From genomic and phenotypic analysis to technological valorization. Food Microbiol. 2016, 53, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Effendi, D.B.; Sakamoto, T.; Ohtani, S.; Awai, K.; Kanesaki, Y. Possible involvement of extracellular polymeric substrates of Antarctic cyanobacterium Nostoc sp. strain SO-36 in adaptation to harsh environments. J. Plant Res. 2022, 14, 1–14. [Google Scholar] [CrossRef]
- Finore, I.; Di Donato, P.; Mastascusa, V.; Nicolaus, B.; Poli, A. Fermentation Technologies for the Optimization of Marine Microbial Exopolysaccharide Production. Mar. Drugs 2014, 12, 3005–3024. [Google Scholar] [CrossRef]
- Hernandez, A.; Larsson, C.U.; Sawicki, R.; van Niel, E.W.J.; Roos, S.; Hakansson, S. Impact of the fermentation parameters pH and temperature on stress resilience of Lactobacillus reuteri DSM 17938. Amb. Express 2019, 9, 66. [Google Scholar] [CrossRef]
- Liu, X.T.; Hou, C.L.; Zhang, J.; Zeng, X.F.; Qiao, S.Y. Fermentation conditions influence the fatty acid composition of the membranes of Lactobacillus reuteri I5007 and its survival following freeze-drying. Lett. Appl. Microbiol. 2014, 59, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Troxler, L.J.; Werren, J.P.; Schaffner, T.O.; Mostacci, N.; Vermathen, P.; Vermathen, M.; Wuthrich, D.; Simillion, C.; Brugger, S.D.; Bruggmann, R.; et al. Carbon source regulates polysaccharide capsule biosynthesis in Streptococcus pneumoniae. J. Biol. Chem. 2019, 294, 17224–17238. [Google Scholar] [CrossRef]
- Khanal, S.N.; Lucey, J.A. Effect of fermentation temperature on the properties of exopolysaccharides and the acid gelation behavior for milk fermented by Streptococcus thermophilus strains DGCC7785 and St-143. J. Dairy Sci. 2018, 101, 3799–3811. [Google Scholar] [CrossRef]
- Fraunhofer, M.E.; Jakob, F.; Vogel, R.F. Influence of Different Sugars and Initial pH on beta-Glucan Formation by Lactobacillus brevis TMW 1.2112. Curr. Microbiol. 2018, 75, 794–802. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Michelon, M.; Burkert, C.A.V. Biotechnological potential of soybean molasses for the production of extracellular polymers by diazotrophic bacteria. Biocatal. Agric. Biotechnol. 2020, 25, 101609. [Google Scholar] [CrossRef]
- Khadem, H.; Tirtouil, A.M.; Drabo, M.S.; Boubakeur, B. Ultrasound conditioning of Streptococcus thermophilus CNRZ 447: Growth, biofilm formation, exopolysaccharide production, and cell membrane permeability. BioTechnologia. J. Biotechnol. Comput. Biol. Bionanotechnol. 2020, 101, 159–165. [Google Scholar] [CrossRef]
- Robertson, E.J.; Wolf, J.M.; Casadevall, A. EDTA inhibits biofilm formation, extracellular vesicular secretion, and shedding of the capsular polysaccharide glucuronoxylomannan by Cryptococcus neoformans. J. Appl. Environ. Microbiol. 2012, 78, 7977–7984. [Google Scholar] [CrossRef] [PubMed]
- Racioppo, A.; Corbo, M.R.; Piccoli, C.; Sinigaglia, M.; Speranza, B.; Bevilacqua, A. Ultrasound attenuation of lactobacilli and bifidobacteria: Effect on some technological and probiotic properties. Int. J. Food Microbiol. 2017, 243, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Upadhyay, L.S.B. Microbial exopolysaccharides: Synthesis pathways, types and their commercial applications. Int. J. Biol. Macromol. 2020, 157, 577–583. [Google Scholar] [CrossRef]
- Saito, K.; Tomita, S.; Nakamura, T. Aggregation of Lactobacillus brevis associated with decrease in pH by glucose fermentation. Biosci. Biotechnol. Biochem. 2019, 83, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Tonnis, W.F.; Mensink, M.A.; de Jager, A.; Maarschalk, K.V.; Frijlink, H.W.; Hinrichs, W.L.J. Size and Molecular Flexibility of Sugars Determine the Storage Stability of Freeze-Dried Proteins. Mol. Pharm. 2015, 12, 684–694. [Google Scholar] [CrossRef]
- Soltanizadeh, N.; Mirmoghtadaie, L.; Nejati, F.; Najafabadi, L.I.; Heshmati, M.K.; Jafari, M. Solid-State Protein-Carbohydrate Interactions and Their Application in the Food Industry. Compr. Rev. Food Sci. Food Saf. 2014, 13, 860–870. [Google Scholar] [CrossRef]
- Ayyash, M.; Shehabi, A.A.; Mahmoud, N.N.; Al-Bakri, A.G. Antibiofilm properties of triclosan with EDTA or cranberry as Foley Catheter lock solutions. J. Appl. Microbiol. 2019, 127, 1876–1888. [Google Scholar] [CrossRef]
- Archacka, M.; Bialas, W.; Dembczynski, R.; Olejnik, A.; Sip, A.; Szymanowska, D.; Celinska, E.; Jankowski, T.; Olejnik, A.; Rogodzinska, M. Method of preservation and type of protective agent strongly influence probiotic properties of Lactococcus lactis: A complete process of probiotic preparation manufacture and use. Food Chem. 2019, 274, 733–742. [Google Scholar] [CrossRef]
- Romano, N.; Schebor, C.; Mobili, P.; Gomez-Zavaglia, A. Role of mono- and oligosaccharides from FOS as stabilizing agents during freeze-drying and storage of Lactobacillus delbrueckii subsp bulgaricus. Food Res. Int. 2016, 90, 251–258. [Google Scholar] [CrossRef]
- Mensink, M.A.; Van Bockstal, P.-J.; Pieters, S.; De Meyer, L.; Frijlink, H.W.; Maarschalk, K.v.d.V.; Hinrichs, W.L.J.; De Beer, T. In-line near infrared spectroscopy during freeze-drying as a tool to measure efficiency of hydrogen bond formation between protein and sugar, predictive of protein storage stability. Int. J. Pharm. 2015, 496, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Mensink, M.A.; Frijlink, H.W.; Maarschalk, K.v.d.V.; Hinrichs, W.L.J. How sugars protect proteins in the solid state and during drying (review): Mechanisms of stabilization in relation to stress conditions. Eur. J. Pharm. Biopharm. 2017, 114, 288–295. [Google Scholar] [CrossRef] [PubMed]




| Absorption Peak (cm−1) | Intensity |
|---|---|
| 598.15 | 0.865 |
| 861.16 | 3.205 |
| 1053.09 | 0.036 |
| 1412.48 | 0.709 |
| 1654.86 | 0.141 |
| 2931.11 | 0.487 |
| 3399.74 | 0.036 |
| Absorption Peak (cm−1) | Intensity |
|---|---|
| 562.55 | 0.746 |
| 860.27 | 1.603 |
| 927.38 | 9.613 |
| 1052.95 | 0.083 |
| 1245.17 | 0.745 |
| 1413.11 | 0.707 |
| 1654.71 | 0.198 |
| 2931.39 | 0.542 |
| 3351.32 | 0.533 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, S.; Pan, Z.; Wu, S.; Mao, B.; Tang, X.; Zhang, Q.; Zhang, H.; Zhao, J. Improvement of the Lyophilization Survival Rate of Lactobacillus casei via Regulation of Its Surface Substances. Foods 2022, 11, 3468. https://doi.org/10.3390/foods11213468
Cui S, Pan Z, Wu S, Mao B, Tang X, Zhang Q, Zhang H, Zhao J. Improvement of the Lyophilization Survival Rate of Lactobacillus casei via Regulation of Its Surface Substances. Foods. 2022; 11(21):3468. https://doi.org/10.3390/foods11213468
Chicago/Turabian StyleCui, Shumao, Ziyi Pan, Sheng Wu, Bingyong Mao, Xin Tang, Qiuxiang Zhang, Hao Zhang, and Jianxin Zhao. 2022. "Improvement of the Lyophilization Survival Rate of Lactobacillus casei via Regulation of Its Surface Substances" Foods 11, no. 21: 3468. https://doi.org/10.3390/foods11213468
APA StyleCui, S., Pan, Z., Wu, S., Mao, B., Tang, X., Zhang, Q., Zhang, H., & Zhao, J. (2022). Improvement of the Lyophilization Survival Rate of Lactobacillus casei via Regulation of Its Surface Substances. Foods, 11(21), 3468. https://doi.org/10.3390/foods11213468






