3.1. Autochthonous Microbial Populations of Legume-Based Water Extracts
Viable cell counts of autochthonous microbial populations of legume-based water extracts, before and after 24 h of fermentation at 37 °C, are shown in
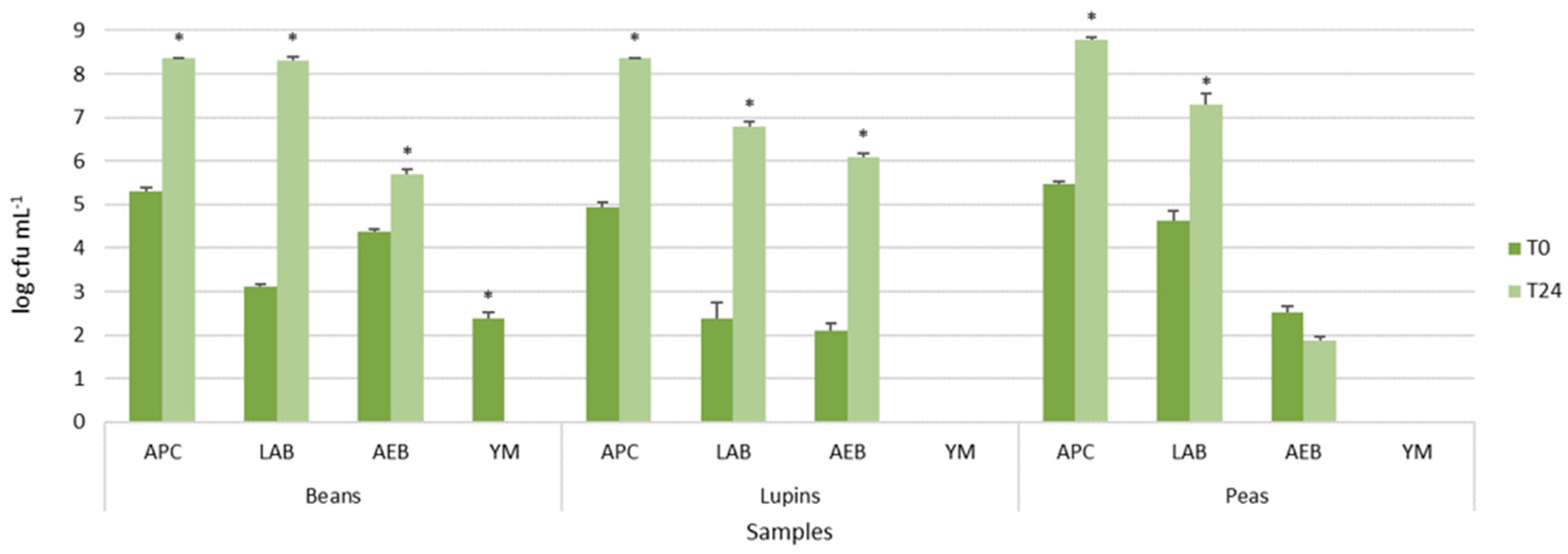
Figure 1. The legume-based water extracts showed the presence of different types of autochthonous microbial populations, mainly belonging to presumptive lactic acid rods and cocci. In particular, LAB counts, presumptively represented by lactic acid rods, ranged from 2.39 ± 0.36 log cfu mL
−1 to 4.62 ± 0.22 log cfu mL
−1, with lupin and peas having the lowest and highest density, respectively (
Figure 1). Aerobic endospore-forming bacteria were found before incubation only in peas and beans. However, after the incubation, they were detected in all samples, reaching 5.85 ± 0.01 log cfu mL
−1 in beans, 6.10 ± 0.09 log cfu mL
−1 in lupin, and approximately 1.88 ± 0.09 cfu mL
−1 in pea water extracts (
Figure 1). Yeast and molds were found only in the bean extracts, but, after incubation, they were not further detected (data not shown).
The comparison of the means of each population before and after incubation within the same extract resulted in statistically significant values for all microbial populations, as shown by the asterisks in
Figure 1, excluding aerobic endospore-forming bacteria of peas.
These results confirmed that the legume grains were characterized by autochthonous and potentially useful lactic acid bacteria, able to grow during incubation, and spoilage bacteria, such as the endospore-forming ones.
As far as the lactic acid microbial population is concerned, the viable loads here reported are in agreement with those found in several fruit and vegetables [
35,
36,
37,
38], belonging to species such as
Lactiplantibacillus plantarum,
Lactiplantibacillus pentosus,
Lm. fermentum,
Latilactobacillus curvatus,
Levilactobacillus brevis,
Leuconostoc mesenteroides,
Weissella spp., and
Enterococcus spp.
As found for lactic acid bacteria, endospore-forming bacterial loads also increased during incubation. This result, in agreement with the occurrence of
Bacillus cereus, B. nitratireducens, B. pumilus, B. safensis, and
B. australimaris in different types of fermented food [
39,
40], forced us to heat-treat extracts before fermentation.
3.2. Fermentation Assays of Legume-Based Water Extracts
In order to select the LAB strains for the production of fermented legume-based beverages, legume-based water extracts, after sterilization (110 °C for 10 min), were first inoculated with each strain at 5–6 log cfu mL
−1 and incubated for 48 h at 37 °C. As shown in
Table 2 and
Table 3,
L. acidophilus ATCC 4356,
Lm. fermentum DSM 20052, and
Lc. paracasei DSM 20312 showed the highest increase in average viable cell counts and a marked decrease in pH values within 48 h of fermentation in comparison to the beginning of incubation.
All LAB strains grew in legume extracts, but only
L. acidophilus ATCC 4356,
Lm. fermentum DSM 20052, and
Lc. paracasei DSM 20312 showed Δlog cfu mL
−1 higher than one in their viable loads in the three legume extracts after 48 h at 37 °C (
Table 2).
The fermentation assays employing these strains were repeated, lowering the initial inoculum level to 4–5 log cfu mL
−1 and evaluating pH changes and cell viability at 24 h and 48 h (
Table 4 and
Table 5).
Two-way ANOVA analysis showed that legume-based beverage, starter strain, and their interaction significantly (p ≤ 0.05) affected cell density and pH values at each sampling time. The effect of the strain on cell density values was not significant at 48 h. All strains confirmed their ability to grow in all legume-based water extracts already after 24 h, without excessive acidification, except in the case of L. acidophilus ATCC 4356 in the pea extract. After 48 h of incubation, the pea extract showed the lowest viable cell increase. The same legume-based beverage showed the highest reduction in pH values for all three inoculated strains after 48 h of incubation. Generally, the pH decreased differently during fermentation, depending on the legume extract and the strain used.
The extension in incubation time from 24 to 48 h increased the average viable cell count only by 0.53 Δlog cfu mL
−1 (as calculated from values reported in
Table 4), with a drop in the pH values from 5.57 ± 0.49 to 4.76 ± 0.19 (
Table 5). Results confirmed the ability of these strains to grow and reduce the pH in fermented legumes, as reported in soy for
L. acidophilus ATCC 4356 [
41], and for these three strains in lentil grains [
17].
Thus, the fermentation step of the legume-based beverage for these strains was set up following incubation for 24 h at 37 °C, avoiding the increase in the concentration of acids from sugar fermentation, which could be unpleasant for the consumer.
As far as the bean water extract is concerned, the release of off-odors at the end of fermentation, probably due to volatile sulfur compounds, prevented its use for the production of a bean-based fermented beverage. As a consequence, bean water extracts were not further characterized.
3.3. Evaluation of the Shelf-Life during Cold Storage
Based on previous results, the fermented legume-based beverages were prepared from lupin and pea water extracts singly fermented for 24 h at 37 °C with L. acidophilus ATCC 4356, Lm. fermentum DSM 20052, and Lc. paracasei DSM 20312.
The microbial viability during 28 days of cold storage of these strains is reported in
Table 6. During 28 days of cold storage, the cell density of all strains reached values close to 8 log cfu mL
−1, remaining stable or, in some cases, increasing by one order of magnitude in comparison to the beginning of incubation.
Here, we underline the ability of
L. acidophilus ATCC 4356,
L. fermentum DSM 20052, and
L. paracasei DSM 20312 to survive and grow in legume-based beverages during cold storage, at viable loads higher than 7 log cfu mL
−1, respecting the minimum value recommended for the daily intake of lactic acid bacteria throughout these products [
42].
In particular, L. acidophilus ATCC 4356 and Lc. paracasei DSM 20312 showed a significant increase in cell density in both pea and lupin beverages throughout storage, whereas Lm. fermentum DSM 20052 showed an increase in viable load followed by a decrease in the late stages of cold storage in both beverages.
Results of this work are in agreement with those of Liao et al. [
43], who reported lactic acid bacteria loads higher than 8 log cfu mL
−1 in adzuki bean beverages fermented with
Lactococcus lactis or
Lacticaseibacillus rhamnosus GG, and stored for 28 days at 4 °C.
Even though the preparation of legume-based water extracts can be carried out following different protocols, we can conclude that lactic acid bacteria are able to ferment legume extracts and survive in these beverages during cold storage, as also demonstrated for the strains
Lacticaseibacillus rhamnosus GR-1 and
Streptococcus thermophilus found in cowpea beverages after 28 days [
44].
The two-way ANOVA of both lupin and pea grain beverages showed that the cell density was significantly affected by the strain and incubation period, as well as their interaction (p ≤ 0.05). The interaction between the strain and incubation period factors significantly influenced the microbial cell densities of Lactobacillus strains.
The high viable cell loads of the three LAB strains determined the decrease in pH values for both the fermented legume-based beverages throughout cold storage. In particular, the pH decreased from 4.81 to 4.00 in lupin-based beverages fermented with
L. acidophilus and 4.08 to 3.30 in pea-based beverages fermented with
Lc. paracasei. Despite the high viable concentration found for all strains in both fermented beverages during cold storage, no post-acidification phenomenon was found. This condition is considered useful to preserve both the microbial viability of probiotic strains in foods and the sensory characteristics of the fermented product [
45,
46,
47,
48]. During cold storage, the viable cell load increased by one log, on average. The growth of these strains during cold storage, probably resulting from the availability of different types of nutrients [
49], was already reported for other lactic acid bacteria strains in different legume-based products [
49,
50].
The lactobacilli strains selected in this work, even though isolated from different matrices, were found to be able to grow in the legume-based water extracts and to survive for one month of cold storage. These results are in agreement with the ability of autochthonous and allochthonous lactic acid bacteria as starters of vegetable matrices [
35,
51,
52] and their survival during cold storage.
3.3.1. Proteins and Free Peptides/Amino Acids throughout Cold Storage
Table 7 shows the concentration of total protein in fermented legume-based beverages throughout 28 days of cold storage.
The amount of total free peptides and amino acids remained almost the same during cold storage, as shown in
Table 8. Thus, it could be assumed that the water-soluble proteins extracted during the soaking and blending process are a preferential source of organic nitrogen and are metabolized by these strains for their survival. A slight decrease in total protein content during cold storage was found only in beverages inoculated with
L. acidophilus ATCC 4356 and
L. paracasei DSM 20312 (
Table 7). A limited degree of proteolysis, depending on the strain and fermentation duration, was also reported by Arteaga et al. [
53] in lacto-fermented pea protein isolate. On the contrary, Schlegel et al. [
54] found that
Limosilactobacillus reuteri and
Lentilactobacillus parabuchneri hydrolyzed medium- and low-molecular-weight polypeptides from lupin protein isolate. Based on the extraction and fermentation process here displayed, we speculate that the proteolysis degree of fermented legume grains is the result of the process of protein extraction, the protein profile of the matrix, the LAB strain, and the fermentation conditions.
As already found for total protein content, the concentrations of free peptides and amino acids were significantly affected by the strain but not by the storage period.
Fermented plant-based foods are often designed as dairy alternatives. However, often, these foods are claimed to contain lower concentrations of nutrients, mainly proteins and peptides, than their milk-based counterparts. It is noteworthy that the concentrations of free peptides and amino acids here reported, and measured by the OPA method, were always higher that those reported by Bhattacharya et al. [
55] for 10 commercial dairy products, including milk, ranging from 60 to 130 mg L
−1, as measured by ion-exchange chromatography.
3.3.2. Free Amino Acids
The concentrations of amino acids released after the fermentation, as well as those still detectable at the end of cold storage, were quantified by HPLC.
Table 9 summarizes the concentrations of different groups of amino acids, which are individually detailed in
Tables S1 and S2. As already observed for the total concentrations of free peptides and amino acids, the total amount of free amino acids decreased in all fermented legume extracts. The highest reduction in the concentration of total free amino acids was observed in legume grain water extracts fermented with
Lm. fermentum DSM 20052 (
Table 9). As far as the concentration of each amino acid group is concerned, no relevant changes were observed in essential amino acids and ɤ-aminobutyric acid. However, pea water extract fermented with
Lm. fermentum DSM 20052 showed a great reduction in essential amino acids, branched-chain amino acids, and other amino acids (
Table 9).
As reported in
Tables S1 and S2, cysteine and tyrosine were largely consumed by all strains, and aspartic acid was reduced preferentially by the
Lm. fermentum DSM 20052, whereas ɤ-aminobutyric acid remained almost stable in all fermented extracts. In some cases, the reduction in the total content of free amino acids after fermentation was accompanied by an increase in the concentration of certain amino acids. We can speculate that the increased concentration of some amino acids could be related to the reduction in the total protein concentration recorded during cold storage, as reported above (
Table 7).
Free amino acids in lupin-based beverages decreased on average by 42% after fermentation. Similar results were also reported for yogurt by Germani et al. [
56], who observed a reduction after fermentation of more than 30% in the total amount of free amino acids.
In the case of pea-based beverages, a sharp reduction in the free amino acid concentration was found only after fermentation with
Lm. fermentum DSM 20052. It is interesting to note that the free amino acid content of fermented pea beverages is in line with that of cow milk (ca. 450 µmol/L) [
57]. It is possible to conclude that, as reported for the total concentration of free peptides and amino acids (
Table 8), the concentration of total free amino acids decreased after fermentation but remained almost stable during cold storage (
Tables S1 and S2).
At the end of the refrigerated period, the total free amino acid content ranged from 223.19 mg L
−1 to 915.37 mg L
−1 in lupin-based beverages fermented with
Lm. fermentum DSM 20052 and pea-based ones fermented with
L. acidophilus ATCC 4356, respectively (
Tables S1 and S2). The consumption of fermented legume extracts characterized by a high cell density of probiotic strains and high free amino acid content could improve the concentration of post-prandial blood amino acids. Indeed, Jägeret et al. [
58] found that pea extract fermented with the probiotic strains
Lc. paracasei DSM 20312 and
L. acidophilus ATCC 4356 increased amino acid absorption after pea protein ingestion.
In addition, it is interesting to note that a glass (150 mL) of fermented lupin-based beverage contains approximately the same amount of ɤ-aminobutyric acid potentially able to lower systolic blood pressure as demonstrated in humans consuming 50 g per day of GABA-enriched cheese [
59].
3.3.3. Sensory Properties
The sensory characteristics of the legume-based beverages were evaluated every seven days during the cold storage period using a simplified check-all-that-apply (CATA) method. Due to the innovative characteristics of these lab-scale fermented beverages and the absence of any similar sensory experience in the untrained panelists, the sensory acceptance of these beverage was considered the main result to be achieved. Thus, questionnaires were compiled describing only a single, the main, trait for each macro-descriptor. The simplified CATA method here applied considered only the acceptability (good/not good) of each descriptor, resulting in a binary response score.
These scores were then organized in a contingency table, combined, and normalized. In some cases, the same descriptor (e.g., acid taste) was considered acceptable (score 1) or not according to the personal preferences of panelists. Here, we considered that scores higher than 0.7 are representative of a sufficient level of acceptability.
Since there was no information about the sensory descriptors of fermented legume-based beverages in the literature, the descriptors of the CATA method, belonging to macro-descriptors “Appearance”, “Odor”, and “Taste”, were freely defined by each panelist and then compared to each other. Due to the water-like consistency of these beverages, the “Texture” macro-descriptor, necessary to describe yogurt-like fermented beverages, was not included.
The sensory characteristics of the legume-based beverages were largely and specifically affected by the lactic acid fermentation. In comparison with the unfermented control, lactic acid fermentation of the lupin water extract moderately affected the three sensory macro-descriptors, whereas fermentation of the pea water extract was positively affected by the inoculation of L. acidophilus ATCC 4356 and Lc. paracasei DSM 20312.
Among the three lactobacilli assayed, Lm. fermentum DSM 20052 was the strain that produced limited or no improvements in acceptability scores for all legume-based beverages. None of the three lactobacilli were able to produce a significant improvement in lupin acceptability scores. In this case, the taste was the worst macro-descriptor, since it was characterized by an unpleasant, bitter, and persistent taste in the innermost area of the tongue.
This unacceptable sensory characteristic was reduced thanks to lactic acid fermentation, increasing the taste acceptability from 0 to 0.6. In particular, a milk flavor in samples fermented with
L. acidophilus ATCC 4356, and vegetable notes in samples fermented with
Lm. fermentum DSM 20052 and
Lc. paracasei DSM 20312, partially masked the bitterness (
Table S3). Reduced bitterness was recently found in lupin protein isolates fermented with
Latilactobacillus sakei subsp.
carnosus [
54].
As far as the appearance of legume-based beverages is concerned, lactic acid fermentation did not negatively affect the appearance of legume samples, with lupin samples characterized by a transparent straw yellow color and with a negligible amount of sediment, and pea samples characterized by a greenish-yellow color (
Tables S3 and S4).
All fermented lupin-based beverages were characterized by a cooked ham odor (
Table S3). Similarly, Schlegel et al. [
54] found notes of cooked products (cooked potato, roasty, and oatmeal) in lupin fermented with lactic acid bacteria. The best result for the macro-descriptor “Odor” was assigned to pea extracts fermented with
Lc. paracasei DSM20132, which, as a result of fermentation, produced pleasant notes of “green peas”, and “fruity”, “floral”, and “fresh-cut grass” notes, resulting in a score of 0.9 (
Table S4). These results agree with El Youssef et al. [
60], which found “green flavor/vegetal” and “leguminous plant” as the main descriptors of pea protein fermented with lactic acid bacteria. The sensory characteristics of the legume-based beverages showed moderate changes under cold storage, leading to only an increase in odor and taste of acidity (data not shown).
Our results partially agree with other works in which both extracts of lupin and pea grains were fermented, even though differences in sensory notes described cannot be correctly compared due to differences in fermenting strains, extraction processes, and fermentation steps [
61,
62]. Even though the method applied is less informative than others based on hedonic scales, it was able to define the level of acceptability of each beverage and underline which sensory trait needs to be improved in order to increase the average level of acceptability.