Effects of Five Different Lactic Acid Bacteria on Bioactive Components and Volatile Compounds of Oat
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oat Substrate
2.3. Fermentation of Oat Substrates with LAB Strains
2.4. Determination of Viable Counts and pH Value
2.5. Determination of β-Glucan Content
2.6. Extraction of Phenolic Compounds
2.7. Determination of Phenolic Content
2.8. Determination of Flavonoid Content
2.9. Determination of Volatile Components
2.10. Statistical Analysis
3. Results and Discussion
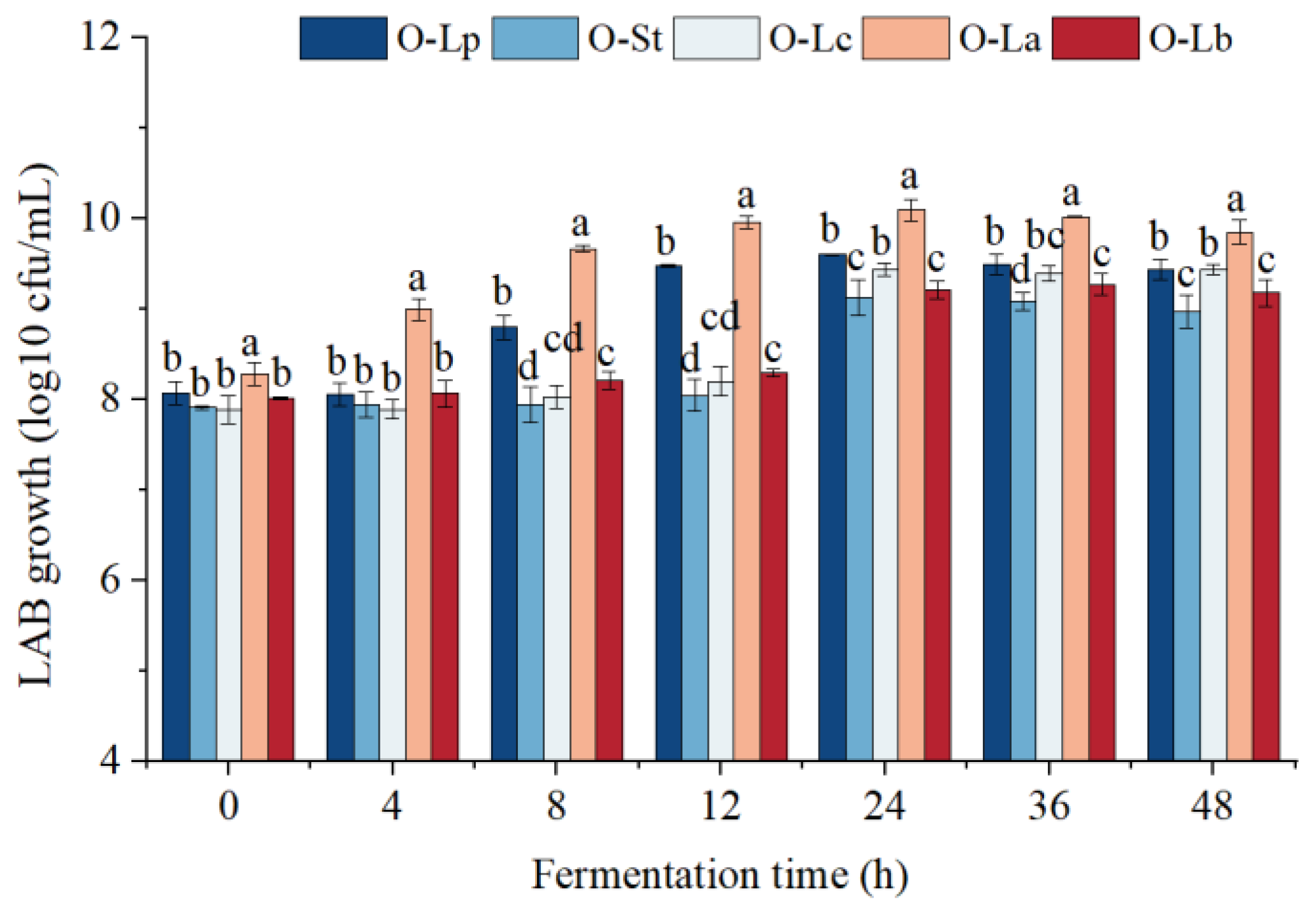
3.1. LAB Growth Curve
3.2. Effect of Fermentation on pH
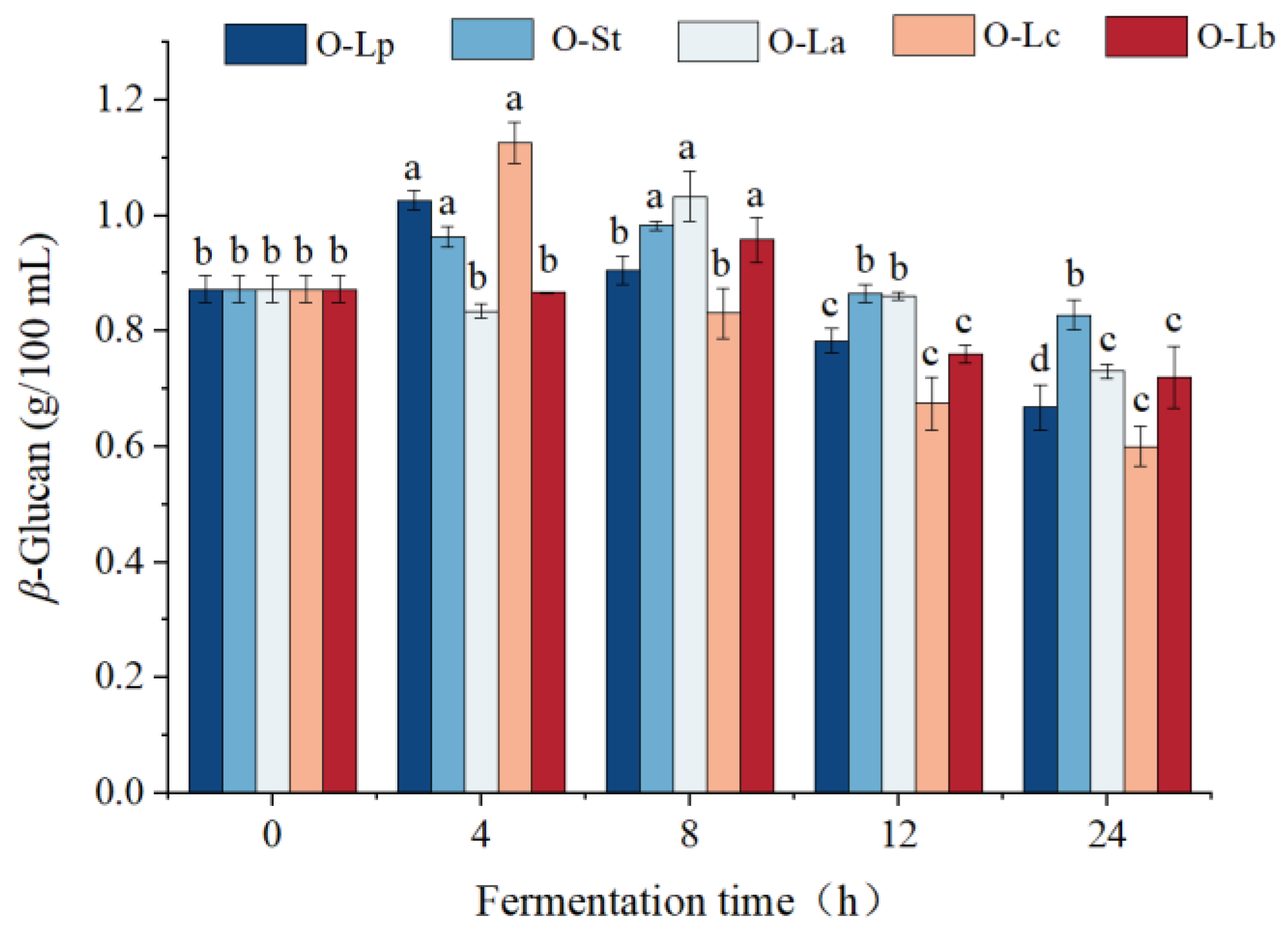
3.3. Effect of Fermentation on β-Glucan Content
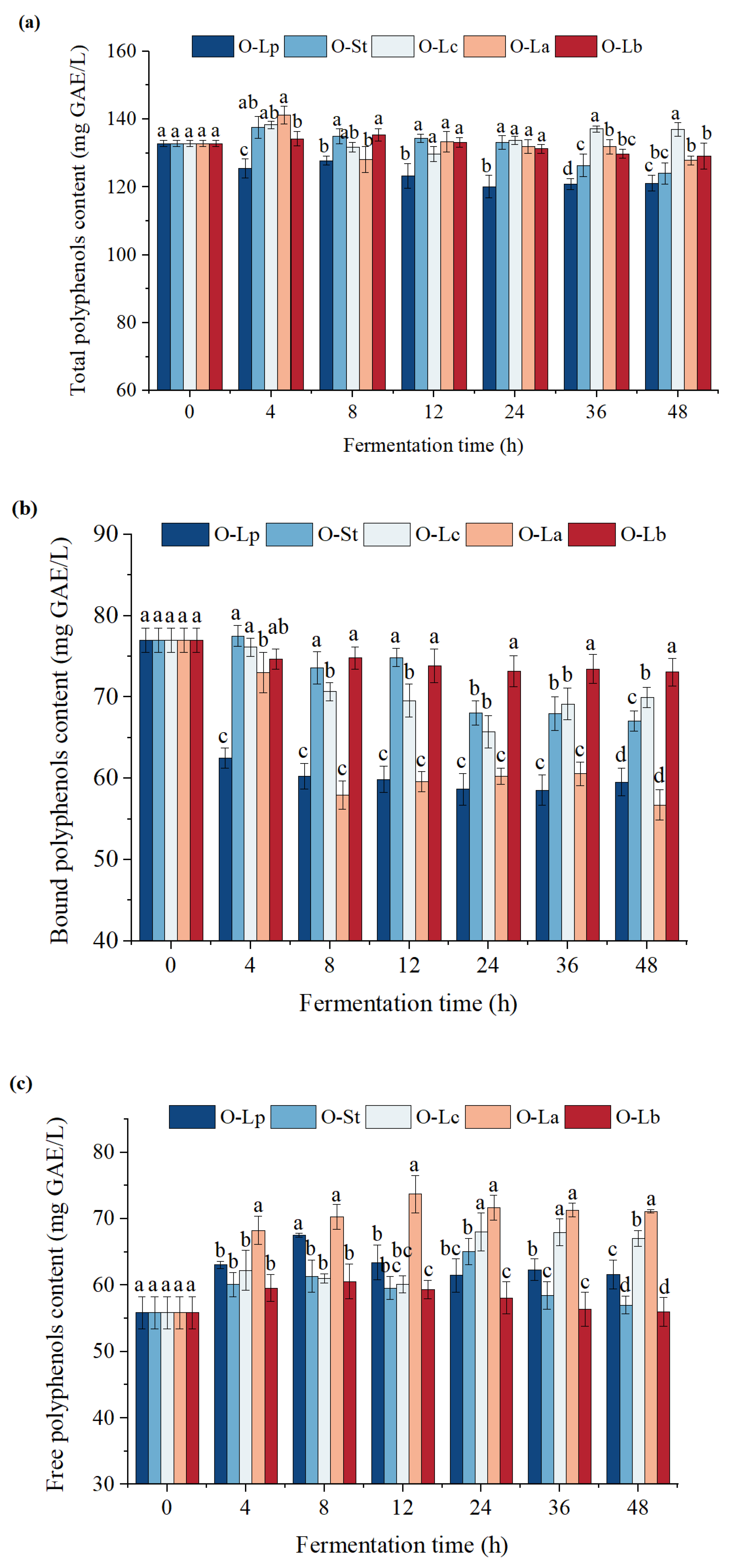
3.4. Effect of Fermentation on Phenolic Content
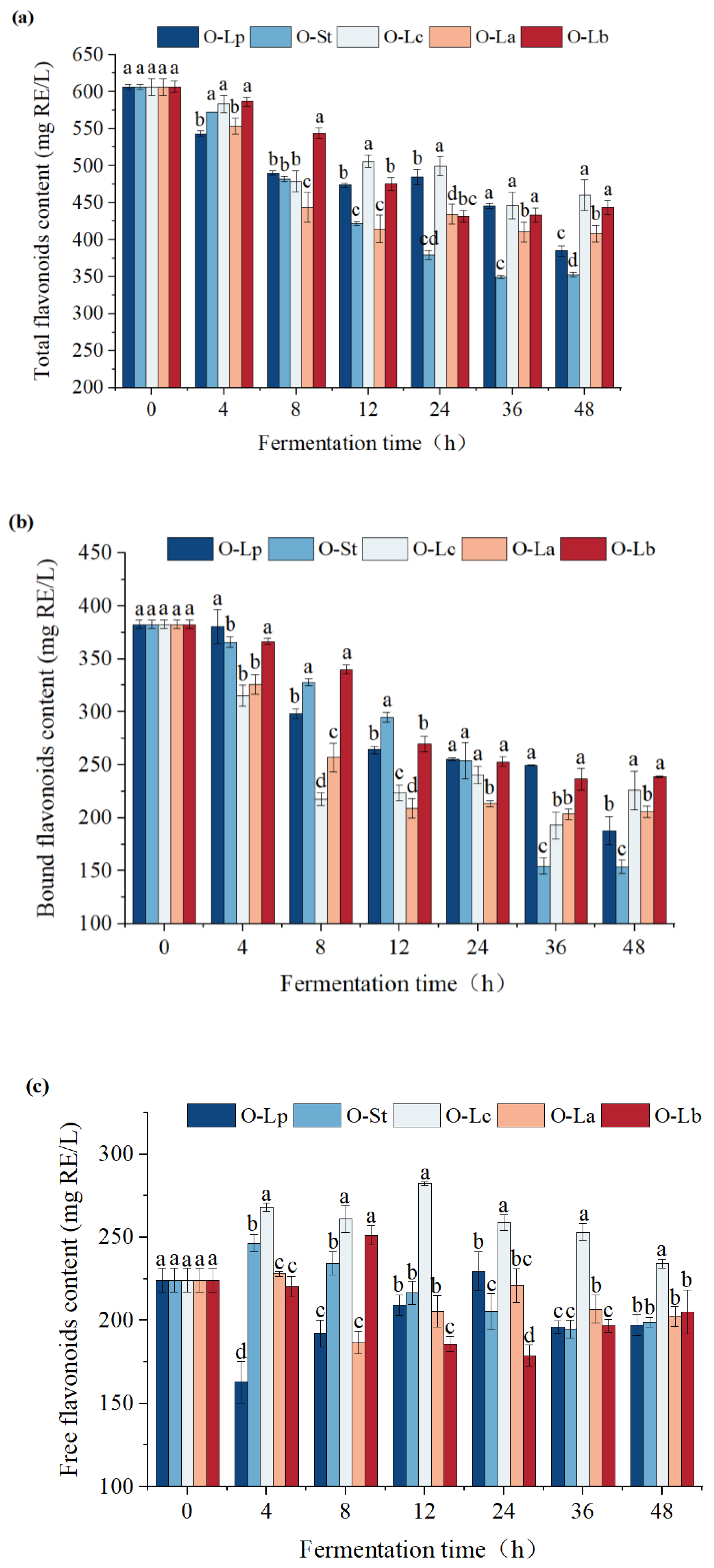
3.5. Effect of Fermentation on Flavonoid Content
3.6. Effect of Fermentation on Volatile Components
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bai, X.; Zhang, M.L.; Zhang, Y.Y.; Zhang, J.; Wang, C.; Zhang, Y.K. Effect of steam, microwave, and hot-air drying on antioxidant capacity and in vitro digestion properties of polyphenols in oat bran. J. Food Process. Preserv. 2021, 45, 12. [Google Scholar] [CrossRef]
- Paudel, D.; Dhungana, B.; Caffe, M.; Krishnan, P. A Review of Health-Beneficial Properties of Oats. Foods 2021, 10, 2591. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.L.; Gilbert, R.G.; Gidley, M.J.; Fox, G.P. The contribution of beta-glucan and starch fine structure to texture of oat-fortified wheat noodles. Food Chem. 2020, 324, 8. [Google Scholar] [CrossRef] [PubMed]
- Salmeron, I. Fermented cereal beverages: From probiotic, prebiotic and synbiotic towards Nanoscience designed healthy drinks. Lett. Appl. Microbiol. 2017, 65, 114–124. [Google Scholar] [CrossRef]
- Nignpense, B.E.; Francis, N.; Blanchard, C.; Santhakumar, A.B. Bioaccessibility and Bioactivity of Cereal Polyphenols: A Review. Foods 2021, 10, 1595. [Google Scholar] [CrossRef]
- Hozlar, P.; Gregusova, V.; Nemecek, P.; Slikova, S.; Havrlentova, M. Study of Dynamic Accumulation in beta-D-Glucan in Oat (Avena sativa L.) during Plant Development. Polymers 2022, 14, 2668. [Google Scholar] [CrossRef] [PubMed]
- Karademir, E.; Yalcin, S.K.; Yalcin, E. Effect of fermentation process on nutritional properties and bioactive compounds of cereal and legume based foods. Gida 2018, 43, 163–173. [Google Scholar] [CrossRef]
- Reque, P.M.; Pinilla, C.M.B.; Tinello, F.; Corich, V.; Lante, A.; Giacomini, A.; Brandelli, A. Biochemical and functional properties of wheat middlings bioprocessed by lactic acid bacteria. J. Food Biochem. 2020, 44, 11. [Google Scholar] [CrossRef]
- Blandino, A.; Al-Aseeri, M.E.; Pandiella, S.S.; Cantero, D.; Webb, C. Cereal-based fermented foods and beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Nematollahi, A.; Sohrabvandi, S.; Mortazavian, A.M.; Jazaeri, S. Viability of probiotic bacteria and some chemical and sensory characteristics in cornelian cherry juice during cold storage. Electron. J. Biotechnol. 2016, 21, 49–53. [Google Scholar] [CrossRef]
- Yepez, A.; Russo, P.; Spano, G.; Khomenko, I.; Biasioli, F.; Capozzi, V.; Aznar, R. In situ riboflavin fortification of different kefir-like cereal-based beverages using selected Andean LAB strains. Food Microbiol. 2019, 77, 61–68. [Google Scholar] [CrossRef]
- Ziarno, M.; Cichonska, P. Lactic Acid Bacteria-Fermentable Cereal- and Pseudocereal-Based Beverages. Microorganisms 2021, 9, 2532. [Google Scholar] [CrossRef] [PubMed]
- Nionelli, L.; Coda, R.; Curiel, J.A.; Poutanen, K.; Marco, G.; Rizzello, C.G. Manufacture and characterization of a yogurt-like beverage made with oat flakes fermented by selected lactic acid bacteria. Int. J. Food Microbiol. 2014, 185, 17–26. [Google Scholar] [CrossRef]
- Bocchi, S.; Rocchetti, G.; Elli, M.; Lucini, L.; Lim, C.Y.; Morelli, L. The combined effect of fermentation of lactic acid bacteria and in vitro digestion on metabolomic and oligosaccharide profile of oat beverage. Food Res. Int. 2021, 142, 12. [Google Scholar] [CrossRef] [PubMed]
- Martensson, O.; Biorklund, M.; Lambo, A.M.; Duenas-Chasco, M.; Irastorza, A.; Holst, O.; Norin, E.; Welling, G.; Oste, R.; Onning, G. Fermented, ropy, oat-based products reduce cholesterol levels and stimulate the bifidobacteria flora in humans. Nutr. Res. 2005, 25, 429–442. [Google Scholar] [CrossRef]
- Zhai, Y.H.; Pan, L.H.; Luo, X.H.; Zhang, Y.W.; Wang, R.; Chen, Z.X. Effect of electron beam irradiation on storage, moisture and eating properties of high-moisture rice during storage. J. Cereal Sci. 2022, 103, 6. [Google Scholar] [CrossRef]
- Zhang, J.G.; Li, M.Y.; Cheng, J.H.; Zhang, X.H.; Li, K.X.; Li, B.; Wang, C.Y.; Liu, X.M. Viscozyme L hydrolysis and Lactobacillus fermentation increase the phenolic compound content and antioxidant properties of aqueous solutions of quinoa pretreated by steaming with alpha-amylase. J. Food Sci. 2021, 86, 1726–1736. [Google Scholar] [CrossRef]
- Rekha, C.R.; Vijayalakshmi, G. Biomolecules and Nutritional Quality of Soymilk Fermented with Probiotic Yeast and Bacteria. Appl. Biochem. Biotechnol. 2008, 151, 452–463. [Google Scholar] [CrossRef]
- Kim, M.Y.; Jang, G.Y.; Lee, Y.; Li, M.; Ji, Y.M.; Yoon, N.; Lee, S.H.; Kim, K.M.; Lee, J.; Jeong, H.S. Free and bound form bioactive compound profiles in germinated black soybean (Glycine max L.). Food Sci. Biotechnol. 2016, 25, 1551–1559. [Google Scholar] [CrossRef]
- Peyer, L.C.; Zannini, E.; Jacob, F.; Arendt, E.K. Growth Study, Metabolite Development, and Organoleptic Profile of a Malt-Based Substrate Fermented by Lactic Acid Bacteria. J. Am. Soc. Brew. Chem. 2015, 73, 303–313. [Google Scholar] [CrossRef]
- Angelov, A.; Gotcheva, V.; Hristozova, T.; Gargova, S. Application of pure and mixed probiotic lactic acid bacteria and yeast cultures for oat fermentation. J. Sci. Food Agric. 2005, 85, 2134–2141. [Google Scholar] [CrossRef]
- Rathore, S.; Salmeron, I.; Pandiella, S.S. Production of potentially probiotic beverages using single and mixed cereal substrates fermented with lactic acid bacteria cultures. Food Microbiol. 2012, 30, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.; Altaf, M.; Naveena, B.J.; Venkateshwar, M.; Kumar, E.V. Amylolytic bacterial lactic acid fermentation—A review. Biotechnol. Adv. 2008, 26, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Charalampopoulos, D.; Pandiella, S.S.; Webb, C. Growth studies of potentially probiotic lactic acid bacteria in cereal-based substrates. J. Appl. Microbiol. 2002, 92, 851–859. [Google Scholar] [CrossRef]
- Cui, Y.H.; Xu, T.T.; Qu, X.J.; Hu, T.; Jiang, X.; Zhao, C.Y. New Insights into Various Production Characteristics of Streptococcus thermophilus Strains. Int. J. Mol. Sci. 2016, 17, 1701. [Google Scholar] [CrossRef]
- Degrain, A.; Manhivi, V.; Remize, F.; Garcia, C.; Sivakumar, D. Effect of Lactic Acid Fermentation on Color, Phenolic Compounds and Antioxidant Activity in African Nightshade. Microorganisms 2020, 8, 1324. [Google Scholar] [CrossRef]
- Mirmohammadi, R.; Zamindar, N.; Razavi, S.H.; Mirmohammadi, M.; Paidari, S. Investigation of the possibility of fermentation of red grape juice and rice flour by Lactobacillus plantarum and Lactobacillus casei. Food Sci. Nutr. 2021, 9, 5370–5378. [Google Scholar] [CrossRef]
- Lu, J.; Shan, L.K.; Xie, Y.T.; Min, F.F.; Gao, J.Y.; Guo, L.C.; Ren, C.Z.; Yuan, J.L.; Gilissen, L.; Chen, H.B. Effect of fermentation on content, molecule weight distribution and viscosity of beta-glucans in oat sourdough. Int. J. Food Sci. Technol. 2019, 54, 62–67. [Google Scholar] [CrossRef]
- Goncerzewicz, A.; Misiewicz, A.; Owczarek, L.; Jasinska, U.; Skapska, S. The Effect of a Newly Developed Oat-Banana Fermented Beverage with a Beta-glucan Additive on ldhL Gene Expression in Streptococcus thermophilus TKM3 KKP 2030p. Curr. Microbiol. 2016, 73, 773–780. [Google Scholar] [CrossRef]
- Sims, I.M.; Ryan, J.L.J.; Kim, S.H. In vitro fermentation of prebiotic oligosaccharides by Bifidobacterium lactis HN019 and Lactobacillus spp. Anaerobe 2014, 25, 11–17. [Google Scholar] [CrossRef]
- Ferri, M.; Serrazanetti, D.I.; Tassoni, A.; Baldissarri, M.; Gianotti, A. Improving the functional and sensorial profile of cereal-based fermented foods by selecting Lactobacillus plantarum strains via a metabolomics approach. Food Res. Int. 2016, 89, 1095–1105. [Google Scholar] [CrossRef]
- Li, T.; Jiang, T.; Liu, N.; Wu, C.; Xu, H.; Lei, H. Biotransformation of phenolic profiles and improvement of antioxidant capacities in jujube juice by select lactic acid bacteria. Food Chem. 2021, 339, 10. [Google Scholar] [CrossRef]
- Adebo, O.A.; Njobeh, P.B.; Adebiyi, J.A.; Kayitesi, E. Co-influence of fermentation time and temperature on physicochemical properties, bioactive components and microstructure of ting (a Southern African food) from whole grain sorghum. Food Biosci. 2018, 25, 118–127. [Google Scholar] [CrossRef]
- Huynh, N.T.; Van Camp, J.; Smagghe, G.; Raes, K. Improved Release and Metabolism of Flavonoids by Steered Fermentation Processes: A Review. Int. J. Mol. Sci. 2014, 15, 19369–19388. [Google Scholar] [CrossRef] [PubMed]
- Georgetti, S.R.; Vicentini, F.; Yokoyama, C.Y.; Borin, M.F.; Spadaro, A.C.C.; Fonseca, M.J.V. Enhanced in vitro and in vivo antioxidant activity and mobilization of free phenolic compounds of soybean flour fermented with different beta-glucosidase-producing fungi. J. Appl. Microbiol. 2009, 106, 459–466. [Google Scholar] [CrossRef]
- Matejcekova, Z.; Mikulajova, A.; Vlkova, E.; Liptakova, D.; Mosovska, S.; Hybenova, E.; Valik, L. Design of Bacterial Cultures in Fermented Functional Maize Product Formulation. Pol. J. Food Nutr. Sci. 2019, 69, 417–426. [Google Scholar] [CrossRef]
- Adebo, O.A.; Oyedeji, A.B.; Adebiyi, J.A.; Chinma, C.E.; Oyeyinka, S.A.; Olatunde, O.O.; Green, E.; Njobeh, P.B.; Kondiah, K. Kinetics of Phenolic Compounds Modification during Maize Flour Fermentation. Molecules 2021, 26, 6702. [Google Scholar] [CrossRef]
- Lee, Y.; Oh, J.; Jeong, Y.S. Lactobacillus plantarum-mediated conversion of flavonoid glycosides into flavonols, quercetin, and kaempferol in Cudrania tricuspidata leaves. Food Sci. Biotechnol. 2015, 24, 1817–1821. [Google Scholar] [CrossRef]
- Xu, C.; Ji, G.E. Bioconversion of Flavones during Fermentation in Milk Containing Scutellaria baicalensis Extract by Lactobacillus brevis. J. Microbiol. Biotechnol. 2013, 23, 1422–1427. [Google Scholar] [CrossRef]
- Lee, S.M.; Oh, J.; Hurh, B.S.; Jeong, G.H.; Shin, Y.K.; Kim, Y.S. Volatile Compounds Produced by Lactobacillus paracasei during Oat Fermentation. J. Food Sci. 2016, 81, C2915–C2922. [Google Scholar] [CrossRef]
- Peyer, L.C.; Zannini, E.; Arendt, E.K. Lactic acid bacteria as sensory biomodulators for fermented cereal-based beverages. Trends Food Sci. Tech. 2016, 54, 17–25. [Google Scholar] [CrossRef]
| Time (h) | O-Lp | O-La | O-St | O-Lb | O-Lc |
|---|---|---|---|---|---|
| 0 | 7.01 ± 0.01 a | 7.01 ± 0.03 a | 7.01 ± 0.03 a | 7.01 ± 0.03 a | 7.01 ± 0.03 a |
| 4 | 6.23 ± 0.02 d | 6.24 ± 0.01 d | 6.76 ± 0.01 a | 6.54 ± 0.01 b | 6.35 ± 0.04 c |
| 8 | 5.09 ± 0.08 d | 5.07 ± 0.03 d | 6.27 ± 0.02 a | 5.95 ± 0.04 b | 5.85 ± 0.02 c |
| 12 | 4.27 ± 0.05 d | 4.12 ± 0.03 e | 5.85 ± 0.06 a | 5.46 ± 0.07 b | 5.36 ± 0.05 c |
| 24 | 3.54 ± 0.05 c | 3.5 ± 0.06 c | 5.17 ± 0.06 a | 4.48 ± 0.01 b | 4.56 ± 0 b |
| 36 | 3.3 ± 0.06 d | 3.23 ± 0.01 e | 4.48 ± 0.02 a | 3.79 ± 0.01 b | 3.55 ± 0.02 c |
| 48 | 3.15 ± 0.01 d | 3.14 ± 0.03 d | 4.25 ± 0.01 a | 3.76 ± 0.04 b | 3.22 ± 0.03 c |
| Volatile Compounds | RT (s) | Control (%) | O-Lp (%) | O-Lc (%) | O-La (%) | O-St (%) | O-Lb (%) |
|---|---|---|---|---|---|---|---|
| Alcohols | |||||||
| 2-Butanol | 411.88 | ND | ND | ND | ND | 0.33 ± 0.02 a | ND |
| 1-Pentanol | 603.74 | ND | 0.49 ± 0.03 b | 0.46 ± 0.04 b | 0.59 ± 0.02 a | 0.29 ± 0.02 d | 0.34 ± 0.02 c |
| 1-Hexanol | 696.64 | 0.09 ± 0.01 d | 4.81 ± 0.32 a | 1.96 ± 0.23 b | 5.22 ± 0.41 a | 0.63 ± 0.06 c | 0.10 ± 0.03 d |
| 1-Octen-3-ol | 778.63 | 0.46 ± 0.03 b | 0.39 ± 0.02 c | 0.44 ± 0.03 bc | 0.75 ± 0.03 a | 0.38 ± 0.05 c | 0.43 ± 0.03 bc |
| 1-Heptanol | 782.84 | 0.25 ± 0.02 c | 0.69 ± 0.03 a | 0.35 ± 0.05 b | 0.76 ± 0.08 a | 0.18 ± 0.03 c | 0.19 ± 0.04 c |
| 1-Octanol | 863.64 | 0.46 ± 0.03 d | 0.95 ± 0.04 b | 0.58 ± 0.02 c | 1.08 ± 0.02 a | 0.40 ± 0.04 e | 0.47 ± 0.03 d |
| 2-Octen-1-ol, (Z)- | 908.51 | 0.05 ± 0.01 b | 0.04 ± 0.00 b | 0.03 ± 0.01 b | 0.11 ± 0.01 a | 0.03 ± 0.00 b | 0.03 ± 0.02 b |
| 1-Nonanol | 939.49 | ND | 0.99 ± 0.12 a | 0.32 ± 0.02 b | 0.96 ± 0.17 a | ND | 0.04 ± 0.02 c |
| Benzenemethanol | 1098.00 | 0.02 ± 0.00 b | 0.12 ± 0.05 a | 0.09 ± 0.03 a | 0.12 ± 0.02 a | 0.02 ± 0.01 b | 0.03 ± 0.01 b |
| Benzeneethanol | 1121.35 | 0.02 ± 0.00 b | 0.09 ± 0.01 a | 0.08 ± 0.01 a | 0.09 ± 0.02 a | 0.02 ± 0.00 b | 0.02 ± 0.00 b |
| Aldehydes | |||||||
| Pentanal | 321.59 | 0.35 ± 0.09 a | ND | ND | ND | 0.25 ± 0.05 a | 0.28 ± 0.02 a |
| Hexanal | 431.62 | 4.63 ± 0.34 a | 0.06 ± 0.01 c | ND | ND | 3.34 ± 0.37 b | 3.84 ± 0.30 b |
| Heptanal | 542.25 | 0.60 ± 0.04 b | ND | ND | ND | 0.42 ± 0.02 c | 0.74 ± 0.02 a |
| 2-Hexenal, (E)- | 578.54 | 0.10 ± 0.02 a | 0.04 ± 0.00 b | ND | 0.04 ± 0.01 b | 0.09 ± 0.02 a | 0.11 ± 0.01 a |
| Octanal | 644.40 | 1.81 ± 0.25 a | ND | ND | 0.36 ± 0.04 c | 0.95 ± 0.03 b | 0.88 ± 0.05 b |
| 2-Heptenal, (E)- | 679.51 | 0.41 ± 0.02 a | 0.27 ± 0.04 c | 0.14 ± 0.01 d | 0.35 ± 0.02 b | 0.26 ± 0.05 c | 0.30 ± 0.04 bc |
| Nonanal | 738.364 | 2.32 ± 0.32 a | 0.95 ± 0.04 c | 0.95 ± 0.03 c | 1.10 ± 0.05 c | 1.96 ± 0.12 b | 2.48 ± 0.31 a |
| 2 Octenal | 771.40 | 0.19 ± 0.02 a | 0.14 ± 0.01 c | 0.06 ± 0.02 d | 0.16 ± 0.02 bc | 0.02 ± 0.00 e | 0.17 ± 0.01 ab |
| Benzaldehyde | 852.46 | ND | ND | ND | 0.51 ± 0.03 b | 0.56 ± 0.04 a | ND |
| 2-Nonenal, (E)- | 856.75 | 0.55 ± 0.03 a | 0.44 ± 0.13 b | 0.05 ± 0.04 c | 0.40 ± 0.01 b | 0.47 ± 0.03 ab | 0.56 ± 0.01 a |
| Acids | |||||||
| Acetic acid | 788.20 | ND | 2.31 ± 0.34 a | 1.62 ± 0.12 b | 2.54 ± 0.24 a | 0.39 ± 0.09 c | 0.56 ± 0.11 c |
| Hexanoic acid | 1073.15 | 0.41 ± 0.02 d | 0.73 ± 0.05 c | 1.37 ± 0.12 a | 0.88 ± 0.03 b | 0.50 ± 0.01 d | 0.70 ± 0.02 c |
| Pentanoic acid | 1073.79 | ND | 0.25 ± 0.01 b | 0.08 ± 0.01 c | 0.88 ± 0.07 a | ND | 0.05 ± 0.02 c |
| Heptanoic acid | 1141.51 | 0.31 ± 0.02 a | 0.28 ± 0.03 ab | 0.24 ± 0.01 bc | 0.23 ± 0.03 c | 0.21 ± 0.02 c | 0.23 ± 0.02 c |
| Octanoic acid | 1206.61 | 0.13 ± 0.02 e | 0.65 ± 0.03 a | 0.44 ± 0.03 c | 0.53 ± 0.02 b | 0.28 ± 0.02 d | 0.41 ± 0.01 c |
| Nonanoic acid | 1268.57 | ND | 0.12 ± 0.01 a | 0.11 ± 0.01 a | 0.11 ± 0.00 a | 0.06 ± 0.00 b | ND |
| Decanoic acid | 1327.65 | ND | 0.01 ± 0.00 b | ND | ND | ND | 0.07 ± 0.01 a |
| Ketones | |||||||
| 2,4-Pentanedione | 148.80 | ND | ND | ND | ND | 0.24 ± 0.04 a | ND |
| 2-Propanone | 188.36 | ND | 0.07 ± 0.00 d | 0.09 ± 0.01 c | 0.09 ± 0.01 c | 0.17 ± 0.00 b | 0.19 ± 0.01 a |
| 2- butanone | 245.99 | ND | ND | 0.03 ± 0.00 a | ND | ND | ND |
| 2-Pentadecanone | 260.50 | ND | ND | 0.05 ± 0.00 a | ND | ND | ND |
| 2,3-Butanedione | 322.60 | ND | ND | 0.39 ± 0.02 a | 0.11 ± 0.01 b | ND | ND |
| 2,3-Pentanedione | 409.87 | ND | ND | ND | ND | 0.10 ± 0.01 a | ND |
| 2-Heptanone | 539.31 | 0.58 ± 0.04 b | 0.54 ± 0.07 b | 0.61 ± 0.03 ab | 0.69 ± 0.07 a | 0.54 ± 0.01 b | 0.59 ± 0.03 b |
| 3-Octanone | 610.63 | ND | 0.04 ± 0.01 ab | 0.03 ± 0.00 b | 0.05 ± 0.01 a | 0.03 ± 0.00 b | ND |
| 2-Octanone | 640.36 | ND | ND | 0.15 ± 0.01 a | 0.16 ± 0.02 a | ND | 0.14 ± 0.01 a |
| Methoxy-1-phenyl-2-propanone | 641.12 | ND | ND | 0.71 ± 0.02 a | 0.16 ± 0.03 b | ND | ND |
| 2-Butanone, 3-hydroxy- | 644.82 | ND | ND | 0.56 ± 0.03 a | 0.36 ± 0.02 b | ND | ND |
| 1-Octen-3-one | 656.24 | 0.09 ± 0.00 c | 0.13 ± 0.01 b | 0.07 ± 0.00 d | 0.19 ± 0.01 a | 0.06 ± 0.00 d | ND |
| 2-Propanone, 1-hydroxy- | 659.94 | 0.01 ± 0.00 b | 0.02 ± 0.01 ab | 0.02 ± 0.00 ab | 0.03 ± 0.01 a | ND | 0.01 ± 0.00 b |
| 3,5-octadiene-2-one | 843.73 | 0.09 ± 0.02 a | 0.10 ± 0.03 a | 0.09 ± 0.02 a | 0.10 ± 0.01 a | 0.01 ± 0.01 b | ND |
| 6-Undecanone | 845.96 | 0.02 ± 0.01 a | ND | 0.02 ± 0.00 a | ND | ND | ND |
| Esters | |||||||
| Formic acid, pentyl ester | 473.96 | 0.07 ± 0.02 a | ND | 0.05 ± 0.01 a | ND | ND | 0.06 ± 0.01 a |
| Heptanoic acid, methyl ester | 639.94 | 1.08 ± 0.04 a | 0.32 ± 0.09 b | ND | ND | 0.32 ± 0.05 b | ND |
| 1-Cyclopropylpentyl acetate | 688.58 | ND | 0.23 ± 0.03 a | 0.19 ± 0.03 a | 0.19 ± 0.02 a | 0.19 ± 0.01 a | 0.22 ± 0.05 a |
| 2-etynyl-3-ethyl-2-buten-4-olide | 738.56 | ND | ND | ND | ND | 1.97 ± 0.32 b | 2.48 ± 0.51 a |
| Hexanoic acid, pentyl ester | 831.13 | ND | 0.02 ± 0.00 a | 0.01 ± 0.00 a | 0.02 ± 0.01 a | 0.01 ± 0.00 a | 0.02 ± 0.01 a |
| 2-methylpropyl heptanoate | 845.64 | ND | 0.03 ± 0.01 a | ND | 0.03 ± 0.00 a | 0.02 ± 0.00 a | 0.02 ± 0.01 a |
| Octadecanoic acid, methyl ester | 1296.47 | 0.03 ± 0.01 ab | 0.05 ± 0.02 a | 0.04 ± 0.00 ab | 0.03 ± 0.00 ab | 0.02 ± 0.00 b | 0.04 ± 0.01 ab |
| Furan derivatives | |||||||
| Furan, 2,3-dihydro- | 124.44 | ND | 0.88 ± 0.06 a | 0.81 ± 0.09 a | ND | ND | ND |
| Furan, 2-methyl- | 222.88 | ND | ND | ND | 0.08 ± 0.02 a | ND | ND |
| Furan, 2-ethyl- | 295.38 | ND | 0.08 ± 0.01 a | 0.02 ± 0.00 b | 0.09 ± 0.01 a | 0.07 ± 0.00 a | 0.08 ± 0.02 a |
| 2-Propylfuran | 377.78 | ND | 0.07 ± 0.02 a | ND | 0.08 ± 0.01 a | 0.06 ± 0.01 a | 0.07 ± 0.00 a |
| 2-Butylfuran | 483.20 | 0.10 ± 0.00 a | ND | 0.07 ± 0.01 b | 0.11 ± 0.01 a | 0.07 ± 0.00 b | 0.11 ± 0.02 a |
| 2-tert-Butoxytetra-hydrofuran | 515.08 | 0.11 ± 0.02 b | ND | 0.19 ± 0.05 a | ND | ND | ND |
| Furan, 2-pentyl- | 585.34 | ND | 2.20 ± 0.09 b | 2.74 ± 0.13 a | 2.27 ± 0.31 b | 2.08 ± 0.08 b | 2.66 ± 0.22 a |
| Furan, 2-(1-pentenyl)-, (E)- | 739.45 | ND | ND | 0.04 ± 0.01 b | 0.08 ± 0.02 b | ND | 2.48 ± 0.27 a |
| 2-Heptyl furan | 769.09 | 0.01 ± 0.01 a | 0.01 ± 0.00 a | 0.000 ± 0.00 a | 0.01 ± 0.01 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| Furan, 2-(methoxymethyl) | 981.74 | 0.14 ± 0.04 a | 0.11 ± 0.03 ab | 0.05 ± 0.01 c | 0.08 ± 0.01 bc | 0.13 ± 0.02 ab | 0.14 ± 0.03 a |
| 2-Phenylfuran | 1087.83 | 0.06 ± 0.02 a | 0.07 ± 0.01 a | 0.05 ± 0.02 a | 0.06 ± 0.01 a | 0.06 ± 0.01 a | 0.06 ± 0.02 a |
| Hydrocarbons | |||||||
| Pentane | 127.57 | ND | 0.48 ± 0.03 a | 0.45 ± 0.03 a | 0.43 ± 0.02 a | 0.49 ± 0.05 a | 0.31 ± 0.04 b |
| Heptane | 176.43 | 0.07 ± 0.02 b | 0.41 ± 0.12 a | ND | ND | 0.06 ± 0.02 b | 0.09 ± 0.01 b |
| Undecane | 338.38 | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.09 ± 0.02 a | 0.01 ± 0.00 b | 0.01 ± 0.00 b |
| Dodecane | 543.61 | ND | 0.25 ± 0.04 a | ND | 0.16 ± 0.03 b | ND | ND |
| Pentadecane | 546.45 | 0.17 ± 0.02 ab | ND | 0.14 ± 0.01 b | ND | 0.19 ± 0.03 a | 0.16 ± 0.02 ab |
| Tetradecane | 643.72 | ND | 0.36 ± 0.03 a | 0.01 ± 0.01 b | 0.36 ± 0.02 a | 0.04 ± 0.00 b | ND |
| Nonadecane | 893.62 | 0.05 ± 0.01 a | 0.02 ± 0.01 c | 0.05 ± 0.00 a | 0.05 ± 0.01 a | 0.03 ± 0.00 bc | 0.04 ± 0.01 ab |
| Terpenes | |||||||
| Limonene | 548.57 | 0.01 ± 0.00 c | 0.03 ± 0.01 b | 0.05 ± 0.02 ab | 0.03 ± 0.01 b | 0.06 ± 0.01 a | 0.05 ± 0.00 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Zhang, H.; Wang, T.; Wang, R.; Luo, X. Effects of Five Different Lactic Acid Bacteria on Bioactive Components and Volatile Compounds of Oat. Foods 2022, 11, 3230. https://doi.org/10.3390/foods11203230
He Z, Zhang H, Wang T, Wang R, Luo X. Effects of Five Different Lactic Acid Bacteria on Bioactive Components and Volatile Compounds of Oat. Foods. 2022; 11(20):3230. https://doi.org/10.3390/foods11203230
Chicago/Turabian StyleHe, Zhishu, Hao Zhang, Tao Wang, Ren Wang, and Xiaohu Luo. 2022. "Effects of Five Different Lactic Acid Bacteria on Bioactive Components and Volatile Compounds of Oat" Foods 11, no. 20: 3230. https://doi.org/10.3390/foods11203230
APA StyleHe, Z., Zhang, H., Wang, T., Wang, R., & Luo, X. (2022). Effects of Five Different Lactic Acid Bacteria on Bioactive Components and Volatile Compounds of Oat. Foods, 11(20), 3230. https://doi.org/10.3390/foods11203230






