Personalised Nutritional Plan and Resistance Exercise Program to Improve Health Parameters in Celiac Women
Abstract
1. Introduction
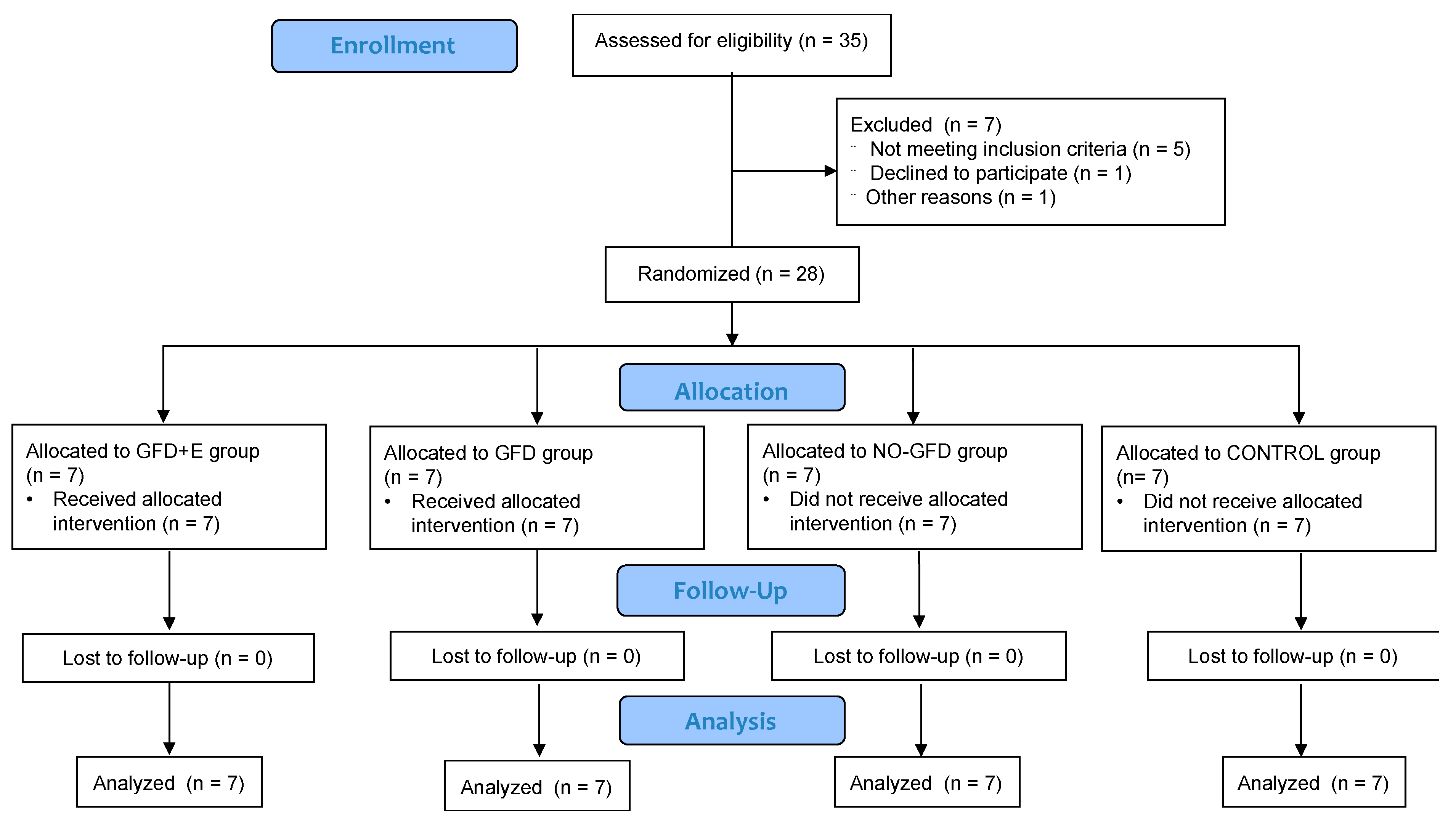
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.2.1. Intervention
2.2.2. Measurement Tools
The Menopause Rating Scale (MRS)
Profile of Mood States (POMS-29)
Bone Quality
Blood Sample
2.3. Statistical Analyses
3. Results
3.1. Baseline Characteristics
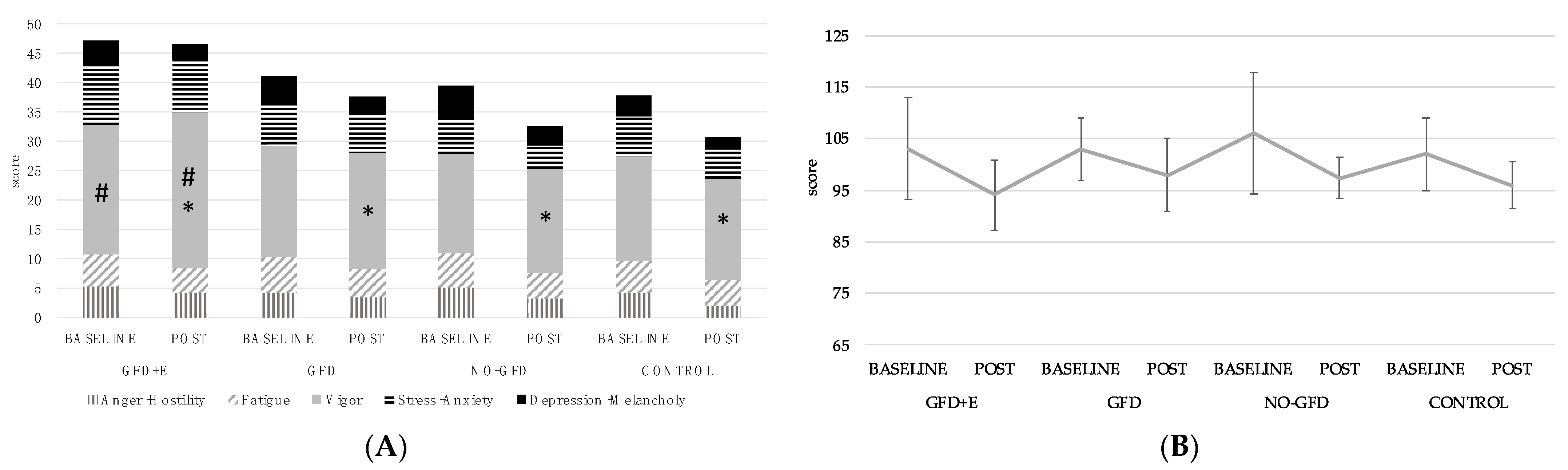
3.2. Menopause Rating Scale (MRS)
3.3. Profile of Mood States (POMS)
3.4. Bone Quality
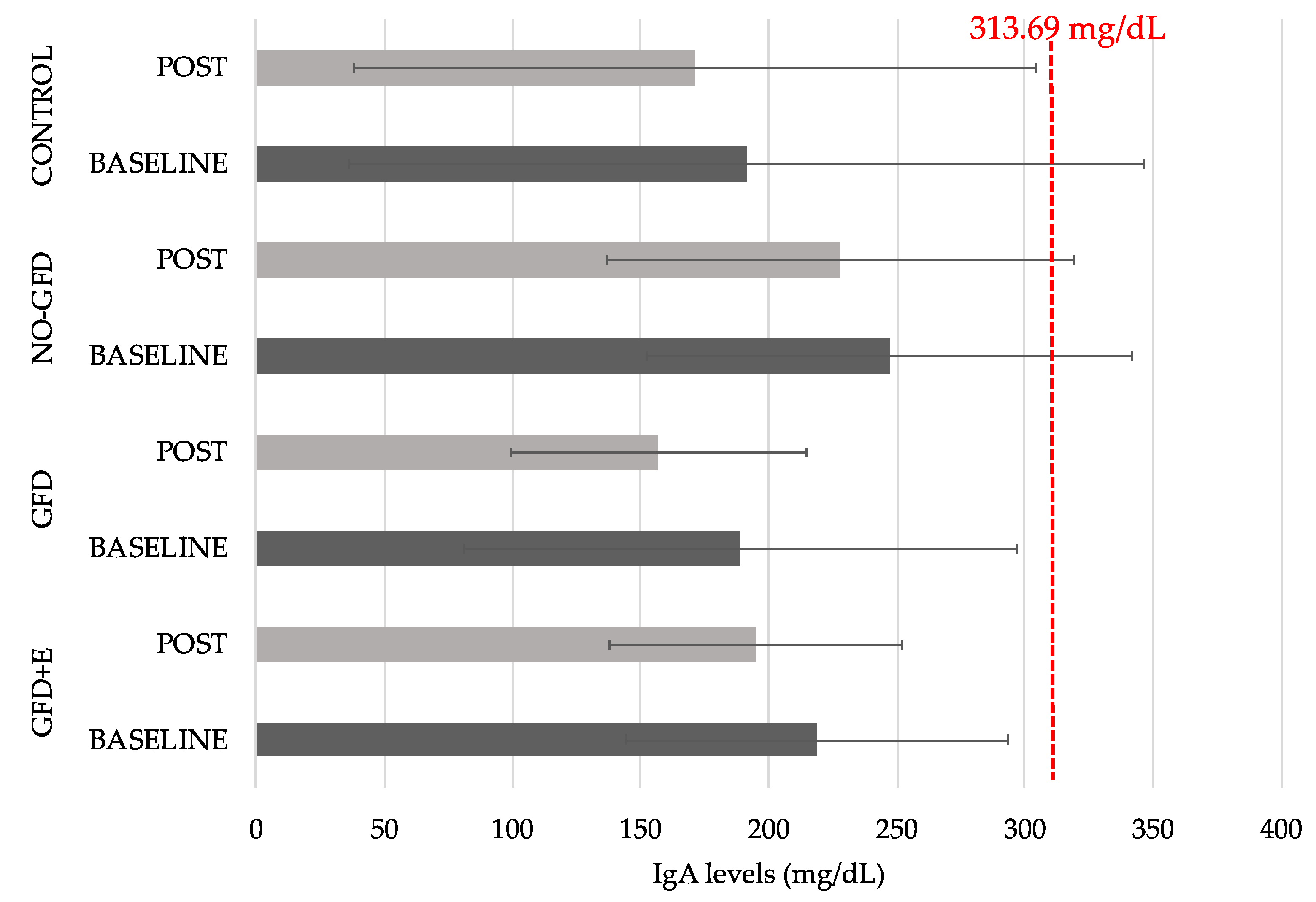
3.5. Immunoglobulin A (IgA)
3.6. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernandez-Lahoz, C.; Mauri-Capdevila, G.; Vega-Villar, J.; Rodrigo, L. Neurological disorders associated with gluten sensitivity. Rev. Neurol. 2011, 53, 287–300. [Google Scholar] [PubMed]
- Pinto-Sanchez, M.I.; Silvester, J.A.; Lebwohl, B.; Leffler, D.A.; Anderson, R.P.; Therrien, A.; Kelly, C.P.; Verdu, E.F. Society for the Study of Celiac Disease position statement on gaps and opportunities in coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 875–884. [Google Scholar]
- Hindryckx, P.; Levesque, B.G.; Holvoet, T.; Durand, S.; Tang, C.M.; Parker, C.; Khanna, R.; Shackelton, L.M.; D’Haens, G.; Sandborn, W.J.; et al. Disease activity indices in coeliac disease: Systematic review and recommendations for clinical trials. Gut 2018, 67, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Oxentenko, A.S.; Grisolano, S.W.; Murray, J.A.; Burgart, L.J.; Dierkhising, R.A.; Alexander, J.A. The insensitivity of endoscopic markers in celiac disease. Am. J. Gastroenterol. 2002, 97, 933–938. [Google Scholar] [CrossRef]
- Moscoso, J.F.; Quera, P.R. Enfermedad celíaca. Revisión. Rev. Médica Chile. 2016, 144, 211–221. [Google Scholar] [CrossRef]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef]
- Cardo, A.; Churruca, I.; Lasa, A.; Navarro, V.; Vázquez-Polo, M.; Perez-Junkera, G.; Larretxi, I. Nutritional imbalances in adult celiac patients following a gluten-free diet. Nutrients 2021, 13, 2877. [Google Scholar] [CrossRef]
- Foschia, M.; Horstmann, S.; Arendt, E.K.; Zannini, E. Nutritional therapy—Facing the gap between coeliac disease and gluten-free food. Int. J. Food Microbiol. 2016, 239, 113–124. [Google Scholar] [CrossRef]
- Satherley, R.M.; Howard, R.; Higgs, S. The prevalence and predictors of disordered eating in women with coeliac disease. Appetite 2016, 107, 260–267. [Google Scholar] [CrossRef]
- Hota, D.; Bhalla, K.; Nanda, S.; Gupta, A.; Mehra, S. Beneficial effects of gluten free diet on IgA tissue transglutaminase levels and various growth parameters in celiac disease patients. J. Fam. Med. Prim. Care 2019, 8, 823. [Google Scholar]
- Rahman, S.; Zainudin, S.; Mun, V. Assessment of menopausal symptoms using modified Menopause Rating Scale (MRS) among middle age women in Kuching, Sarawak, Malaysia. Asia Pac. Fam. Med. 2010, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Masjoudi, M.; Amjadi, M.A.; Leili, E.K.N. Severity and Frequency of Menopausal Symptoms in Middle Aged Women, Rasht, Iran. J. Clin. Diagn. Res. 2017, 11, QC17–QC21. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska-Galas, M.; Dąbrowska, J.; Ptaszkowski, K.; Plinta, R. High Physical Activity Level May Reduce Menopausal Symptoms. Medicina 2019, 55, 466. [Google Scholar] [CrossRef]
- Daley, A.; MacArthur, C.; McManus, R.; Stokes-Lampard, H.; Wilson, S.; Roalfe, A.; Mutrie, N. Factors associated with the use of complementary medicine and non-pharmacological interventions in symptomatic menopausal women. Climacteric 2006, 9, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Holahan, C.K.; Holahan, C.J.; Chen, Y.T.; Li, X. Leisure-time physical activity and affective experience in middle-aged and older women. J. Women Aging 2020, 32, 672–683. [Google Scholar] [CrossRef]
- Moreiras, O.; Carbajal, L.; Cabrera, C.; Cuadrado, C. Ingestas diarias recomendadas de energía y nutrientes para la población española. Ed. Pirámide 2016, 18, 1–5. [Google Scholar]
- Roza, A.M.; Shizgal, H.M. The Harris Benedict equation reevaluated: Resting energy requirements and the body cell mass. Am. J. Clin. Nutr. 1984, 40, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mahajan, N. Menopausal symptoms and its effect on quality of life in urban versus rural women: A cross-sectional study. J. Midlife. Health 2015, 6, 16. [Google Scholar] [CrossRef]
- Ahsan, M.; Mallick, A.; Singh, R.; Prasad, R. Assessment of menopausal symptoms during perimenopause and postmenopause in tertiary care hospital. J. Basic Clin. Reprod. Sci. 2015, 4, 14. [Google Scholar] [CrossRef]
- McNair, D.; Lorr, M.; Droppleman, L. Revised Manual for the Profile of Mood States; Educational and Industrial Testing Services: San Diego, CA, USA, 1992. [Google Scholar]
- Wyrwich, K.W.; Yu, H. Validation of POMS questionnaire in postmenopausal women. Qual. Life Res. 2011, 20, 1111–1121. [Google Scholar] [CrossRef]
- Lara, B.; Salinero, J.J.; Gutiérrez, J.; Areces, F.; Abián-Vicén, J.; Ruiz-Vicente, D.; Gallo-Salazar, C.; Jiménez, F.; Del Coso, J. Influence of endurance running on calcaneal bone stiffness in male and female runners. Eur. J. Appl. Physiol. 2016, 116, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, F.; Martínez, M.; Torres, J.; Rodríguez, S.; Meneses, N. Valores de referencia de inmunoglobulina A sérica total de utilidad para el diagnóstico y seguimiento de la enfermedad celiaca en venezolanos. Gen 2021, 75, 50–54. [Google Scholar]
- Malamut, G.; Cording, S.; Cerf-Bensussan, N. Recent advances in celiac disease and refractory celiac disease. F1000Research 2019, 8, 969. [Google Scholar] [CrossRef]
- Clappison, E.; Hadjivassiliou, M.; Zis, P. Psychiatric Manifestations of Coeliac Disease, a Systematic Review and Meta-Analysis. Nutrients 2020, 12, 142. [Google Scholar] [CrossRef]
- Smith, D.F.; Gerdes, L.U. Meta-analysis on anxiety and depression in adult celiac disease. Acta Psychiatr. Scand. 2012, 125, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Driscoll, D.; Smith, L.M.; Ramaswamy, S. The Role of Inflammation in Late-Life Post-Traumatic Stress Disorder. Mil. Med. 2017, 182, e1815–e1818. [Google Scholar] [CrossRef]
- Parisi, P. The relationship between mucosal damage in celiac disease and the risk of neurological and psychiatric conditions is much more complex than previously thought. Eur. J. Neurol. 2018, 25, 797–798. [Google Scholar] [CrossRef] [PubMed]
- Mirijello, A.; d’Angelo, C.; De Cosmo, S.; Gasbarrini, A.; Addolorato, G. Management of celiac disease in daily clinical practice: Do not forget depression! Eur. J. Intern. Med. 2019, 62, 17. [Google Scholar] [CrossRef] [PubMed]
- Zingone, F.; Swift, G.L.; Card, T.R.; Sanders, D.S.; Ludvigsson, J.F.; Bai, J.C. Psychological morbidity of celiac disease: A review of the literature. United Eur. Gastroenterol. J. 2015, 3, 136–145. [Google Scholar] [CrossRef]
- Li, R.X.; Ma, M.; Xiao, X.R.; Xu, Y.; Chen, X.Y.; Li, B. Perimenopausal syndrome and mood disorders in perimenopause: Prevalence, severity, relationships, and risk factors. Medicine 2016, 95, e4466. [Google Scholar] [CrossRef]
- Kulkarni, J.; Gavrilidis, E.; Hudaib, A.R.; Bleeker, C.; Worsley, R.; Gurvich, C. Development and validation of a new rating scale for perimenopausal depression—The Meno-D. Transl. Psychiatr. 2018, 8, 123. [Google Scholar] [CrossRef]
- Greenblum, C.A.; Rowe, M.A.; Neff, D.F.; Greenblum, J.S. Midlife women: Symptoms associated with menopausal transition and early postmenopause and quality of life. Menopause 2013, 20, 22–27. [Google Scholar] [CrossRef]
- Yılmaz, S.; Arslan, I.; Yengil Taci, D. The effect of physical activity and depressive mood on menopausal symptoms in postmenopausal women. Int. J. Clin. Pract. 2021, 75, e14247. [Google Scholar] [CrossRef]
- Javadivala, Z.; Allahverdipour, H.; Jafarabadi, M.A.; Emami, A. An Interventional strategy of physical activity promotion for reduction of menopause symptoms. Health Promot. Perspect. 2020, 10, 383. [Google Scholar] [CrossRef]
- Nelson, D.B.; Sammel, M.D.; Freeman, E.W.; Lin, H.; Gracia, C.R.; Schmitz, K.H. Effect of physical activity on menopausal symptoms among urban women. Med. Sci. Sports Exerc. 2008, 40, 50–58. [Google Scholar] [CrossRef]
- Da Silva, R.B.; Costa-Paiva, L.; Pinto-Neto, A.M.; Braga, A.D.A.; Morais, S.S. Association between habitual physical activity and parameters of physical fitness in postmenopausal women. Climacteric 2005, 8, 360–370. [Google Scholar] [CrossRef]
- Dugan, S.A.; Bromberger, J.T.; Segawa, E.; Avery, E.; Sternfeld, B. Association between physical activity and depressive symptoms: Midlife women in SWAN. Med. Sci. Sports Exerc. 2015, 47, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Moradpour, F.; Koushkie Jahromi, M.; Fooladchang, M.; Rezaei, R.; Sayar Khorasani, M.R. Association between physical activity, cardiorespiratory fitness, and body composition with menopausal symptoms in early postmenopausal women. Menopause 2020, 27, 230–237. [Google Scholar] [CrossRef]
- Sharifi, N.; Jalili, L.; Khazaeian, S.; Nia, A.N. The Relationship between Physical Activity and General Health among Menopausal Women in Ahvaz, Iran. Electron. Physician 2017, 9, 3639. [Google Scholar] [CrossRef] [PubMed]
- Nazarpour, S.; Simbar, M.; Tehrani, F.R.; Majd, H.A. The relationship between menopausal symptoms and sexual function. Women Health 2018, 58, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Delaney, M.F. Strategies for the prevention and treatment of osteoporosis during early postmenopause. Am. J. Obstet. Gynecol. 2006, 194, S12–S23. [Google Scholar] [CrossRef]
- Porcelli, B.; Verdino, V.; Bossini, L.; Terzuoli, L.; Fagiolini, A. Celiac and non-celiac gluten sensitivity: A review on the association with schizophrenia and mood disorders. Auto-Immun. Highlights 2014, 5, 55. [Google Scholar] [CrossRef]
- Grace-Farfaglia, P. Bones of Contention: Bone Mineral Density Recovery in Celiac Disease—A Systematic Review. Nutrients 2015, 7, 3347. [Google Scholar] [CrossRef]
- Gómez-Cabello, A.; Ara, I.; González-Agüero, A.; Casajús, J.A.; Vicente-Rodríguez, G. Effects of training on bone mass in older adults: A systematic review. Sports Med. 2012, 42, 301–325. [Google Scholar] [CrossRef]
- Polidoulis, I.; Beyene, J.; Cheung, A.M. The effect of exercise on pQCT parameters of bone structure and strength in postmenopausal women—A systematic review and meta-analysis of randomized controlled trials. Osteoporos. Int. 2012, 23, 39–51. [Google Scholar] [CrossRef]
- Anupama, D.S.; Norohna, J.A.; Acharya, K.K.; Ravishankar; George, A. Effect of exercise on bone mineral density and quality of life among postmenopausal women with osteoporosis without fracture: A systematic review. Int. J. Orthop. Trauma Nurs. 2020, 39, 100796. [Google Scholar] [CrossRef]
- Sergi, C.; Villanacci, V.; Carroccio, A. Non-celiac wheat sensitivity: Rationality and irrationality of a gluten-free diet in individuals affected with non-celiac disease: A review. BMC Gastroenterol. 2021, 21, 5. [Google Scholar] [CrossRef]
- Toselli, S.; Badicu, G.; Bragonzoni, L.; Spiga, F.; Mazzuca, P.; Campa, F. Comparison of the effect of different resistance training frequencies on phase angle and handgrip strength in obese women: A randomized controlled trial. Int. J. Environ. Res. Public Health 2020, 17, 1163. [Google Scholar] [CrossRef]
- Mariné, M.; Farre, C.; Alsina, M.; Vilar, P.; Cortijo, M.; Salas, A.; Fernández-Bañares, F.; Rosinach, M.; Santaolalla, R.; Loras, C.; et al. The prevalence of coeliac disease is significantly higher in children compared with adults. Aliment. Pharmacol. Ther. 2011, 33, 477–486. [Google Scholar] [CrossRef]


| GFD+E | GFD | NO-GFD | CONTROL | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BASELINE | POST | BASELINE | POST | BASELINE | POST | BASELINE | POST | |||||||||||||||||
| Stiffness Index (A.U) | 106 | ± | 17.6 | 106 | ± | 17.4 | 94.3 | ± | 15.3 | 95.9 | ± | 14.7 | 88.3 | ± | 12.6 | 89.1 | ± | 12.4 | 87.4 | ± | 6.58 | 89.0 | ± | 4.51 |
| BUA (dB/MHz) | 123 | ± | 12.9 | 124 | ± | 13.1 | 118 | ± | 14.2 | 119 | ± | 14.3 | 115 | ± | 17.2 | 116 | ± | 15.2 | 103 | ± | 30.6 | 105 | ± | 30.1 |
| SOS (m/s) | 1588 | ± | 50.0 | 1588 | ± | 49.9 | 1557 | ± | 28.8 | 1562 | ± | 29.4 | 1541 | ± | 18.9 | 1543 | ± | 22.6 | 1331 | ± | 570 | 1334 | ± | 571 |
| Age (years) | Height (cm) | BMI (kg/m2) | MRS S-V | MRS PSCHY | MRS UG | MRS TOTAL | POMS TOTAL | A-H | Fatigue | Vigour | S-A | D-M | SI (A.U) | BUA (dB/MHz) | SOS (m/s) | IgA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | — | ||||||||||||||||
| Height (cm) | −0.647 ** | — | |||||||||||||||
| BMI (kg/m2) | 0.145 | −0.118 | — | ||||||||||||||
| MRS S-V | 0.681 ** | −0.323 | 0.057 | — | |||||||||||||
| MRS PSCHY | 0.186 | −0.066 | −0.113 | 0.383 * | — | ||||||||||||
| MRS UG | 0.787 ** | −0.391 * | 0.235 | 0.674 ** | 0.152 | — | |||||||||||
| MRS TOTAL | 0.782 ** | −0.393 | 0.149 | 0.899 ** | 0.539 * | 0.851 ** | — | ||||||||||
| POMS TOTAL | 0.185 | −0.240 | 0.260 | 0.264 | 0.053 | 0.216 | 0.277 | — | |||||||||
| A-H | −0.339 | 0.192 | −0.060 | −0.283 | −0.310 | −0.274 | −0.359 | 0.051 | — | ||||||||
| Fatigue | 0.028 | −0.022 | 0.328 | 0.104 | 0.197 | 0.081 | 0.174 | 0.614 ** | 0.086 | — | |||||||
| Vigour | −0.550 * | 0.508 * | −0.162 | −0.470 * | −0.147 | −0.285 | −0.447 * | −0.377 | 0.586 * | −0.034 | — | ||||||
| S-A | −0.406 * | 0.300 | 0.044 | −0.162 | −0.022 | −0.039 | −0.136 | 0.371 | 0.105 | 0.151 | 0.458 * | — | |||||
| D-M | 0.132 | −0.073 | −0.021 | 0.011 | −0.075 | 0.160 | 0.070 | 0.622 ** | 0.379 * | 0.320 | 0.148 | 0.294 | — | ||||
| SI (A.U) | −0.410 * | 0.497 * | 0.059 | −0.069 | −0.035 | −0.187 | −0.132 | 0.114 | 0.306 | 0.249 | 0.335 | 0.328 | 0.064 | — | |||
| BUA (dB/MHz) | −0.192 | 0.309 | −0.029 | 0.013 | −0.319 | −0.176 | −0.177 | 0.119 | 0.276 | 0.035 | 0.114 | 0.023 | 0.289 | 0.561 * | — | ||
| SOS (m/s) | −0.150 | 0.018 | −0.363 | 0.029 | −0.274 | −0.203 | −0.187 | 0.009 | 0.221 | −0.136 | 0.044 | −0.129 | 0.287 | 0.173 | 0.739 ** | — | |
| IgA | 0.115 | 0.245 | 0.088 | 0.173 | 0.161 | 0.216 | 0.252 | 0.186 | 0.110 | 0.196 | 0.064 | 0.175 | 0.092 | 0.205 | −0.276 | −0.574 ** | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Rodríguez, A.; Loaiza-Martínez, D.A.; Sánchez-Sánchez, J.; Rubio-Arias, J.Á.; Alacid, F.; Prats-Moya, S.; Martínez-Olcina, M.; Yáñez-Sepúlveda, R.; Marcos-Pardo, P.J. Personalised Nutritional Plan and Resistance Exercise Program to Improve Health Parameters in Celiac Women. Foods 2022, 11, 3238. https://doi.org/10.3390/foods11203238
Martínez-Rodríguez A, Loaiza-Martínez DA, Sánchez-Sánchez J, Rubio-Arias JÁ, Alacid F, Prats-Moya S, Martínez-Olcina M, Yáñez-Sepúlveda R, Marcos-Pardo PJ. Personalised Nutritional Plan and Resistance Exercise Program to Improve Health Parameters in Celiac Women. Foods. 2022; 11(20):3238. https://doi.org/10.3390/foods11203238
Chicago/Turabian StyleMartínez-Rodríguez, Alejandro, Daniela Alejandra Loaiza-Martínez, Javier Sánchez-Sánchez, Jacobo Á. Rubio-Arias, Fernando Alacid, Soledad Prats-Moya, María Martínez-Olcina, Rodrigo Yáñez-Sepúlveda, and Pablo J. Marcos-Pardo. 2022. "Personalised Nutritional Plan and Resistance Exercise Program to Improve Health Parameters in Celiac Women" Foods 11, no. 20: 3238. https://doi.org/10.3390/foods11203238
APA StyleMartínez-Rodríguez, A., Loaiza-Martínez, D. A., Sánchez-Sánchez, J., Rubio-Arias, J. Á., Alacid, F., Prats-Moya, S., Martínez-Olcina, M., Yáñez-Sepúlveda, R., & Marcos-Pardo, P. J. (2022). Personalised Nutritional Plan and Resistance Exercise Program to Improve Health Parameters in Celiac Women. Foods, 11(20), 3238. https://doi.org/10.3390/foods11203238











