Biodegradation of PLA-PHBV Blend Films as Affected by the Incorporation of Different Phenolic Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Obtaining Active Blend Films
2.3. Film Characterisation
2.3.1. Moisture Content, Thickness, Elementary Composition and Visual Appearance
2.3.2. Thermogravimetric Analysis
2.4. Compost and Synthetic Solid Residue (SSR)
2.5. Disintegration Test
2.6. Biodegradation Test
2.7. Statistical Analysis
3. Results and Discussion
3.1. Moisture Content, Thickness and Elementary Composition
3.2. Compost Characteristics
3.3. Disintegration Test
3.4. Thermogravimetric Analysis
3.5. Biodegradation Kinetics under Laboratory-Scale Composting Conditions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilfred, O.; Tai, H.; Marriott, R.; Liu, Q.; Tverezovskiy, V.; Curling, S.; Tai, H.; Fan, Z.; Wang, W. Biodegradation of Polylactic Acid and Starch Composites in Compost and Soil. Int. J. Nano Res. 2018, 1, 1–11. [Google Scholar]
- Abdin, M.; El-Beltagy, A.E.; El-sayed, M.E.; Naeem, M.A. Production and Characterization of Sodium Alginate/Gum Arabic Based Films Enriched with Syzygium cumini Seeds Extracts for Food Application. J. Polym. Environ. 2021, 171, 1–12. [Google Scholar] [CrossRef]
- European Bioplastics, Market Update 2020: Bioplastics Continue to Become Mainstream as the Global Bioplastics Market Is Set to Grow by 36 Percent over the Next 5 Years. 2020. Available online: https://www.european-bioplastics.org (accessed on 1 December 2020).
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable polymers and green-based antimicrobial packaging materials: A mini-review. Adv. Ind. Eng. Polym. Res. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Boufarguine, M.; Guinault, A.; Miquelard-Garnier, G.; Sollogoub, C. PLA/PHBV Films with Improved Mechanical and Gas Barrier Properties. Macromol. Mater. Eng. 2013, 298, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Requena, R.; Vargas, M.; Chiralt, A. Obtaining antimicrobial bilayer starch and polyester-blend films with carvacrol. Food Hydrocoll. 2018, 83, 118–133. [Google Scholar] [CrossRef]
- Arrieta, M.P.; García, A.D.; López, D.; Fiori, S.; Peponi, L. Antioxidant Bilayers Based on PHBV and Plasticized Electrospun PLA-PHB Fibers Encapsulating Catechin. Nanomaterials 2019, 9, 346. [Google Scholar] [CrossRef] [Green Version]
- Hernández-García, E.; Vargas, M.; Chiralt, A. Thermoprocessed starch-polyester bilayer films as affected by the addition of gellan or xanthan gum. Food Hydrocoll. 2021, 113, 106509. [Google Scholar] [CrossRef]
- Rivera-Briso, A.L.; Serrano-Aroca, Á. Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate): Enhancement Strategies for Advanced Applications. Polymers 2018, 10, 732. [Google Scholar] [CrossRef] [Green Version]
- Eltabakh, M.; Kassab, H.; Badawy, W.; Abdin, M.; Abdelhady, S. Active Bio-composite Sodium Alginate/Maltodextrin Packaging Films for Food Containing Azolla pinnata Leaves Extract as Natural Antioxidant. J. Polym. Environ. 2021, 1–11. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.F.R.; Froufe, H.J.C.; Abreu, R.M.V.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef]
- Takahashi, H.; Takahashi, T.; Miya, S.; Yokoyama, H.; Kuda, T.; Kimura, B. Growth inhibition effects of ferulic acid and glycine/sodium acetate on Listeria monocytogenes in coleslaw and egg salad. Food Control. 2015, 57, 105–109. [Google Scholar] [CrossRef]
- Meira, N.V.; Holley, R.A.; Bordin, K.; de Macedo, R.E.; Luciano, F.B. Combination of essential oil compounds and phenolic acids against Escherichia coli O157:H7 in vitro and in dry-fermented sausage production. Int. J. Food Microbiol. 2017, 260, 59–64. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Physicochemical and antimicrobial properties of cassava starch films with ferulic or cinnamic acid. LWT 2021, 144, 111242. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Stojkovic, D.; Živković, J.; Sokovic, M.; Glamočlija, J.; Ferreira, I.C.; Janković, T.; Maksimović, Z. Antibacterial activity of Veronica montana L. extract and of protocatechuic acid incorporated in a food system. Food Chem. Toxicol. 2013, 55, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of Bioplastic Packaging Materials: An Overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.; Das, M. Biodegradability of Starch Based Self-Supporting Antimicrobial Film and Its Effect on Soil Quality. J. Polym. Environ. 2018, 26, 4331–4337. [Google Scholar] [CrossRef]
- Tang, S.; Zou, P.; Xiong, H.; Tang, H. Effect of nano-SiO2 on the performance of starch/polyvinyl alcohol blend films. Carbohydr. Polym. 2008, 72, 521–526. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; González-Martínez, C.; Chiralt, A. Biodegradable Antimicrobial Films for Food Packaging: Effect of Antimicrobials on Degradation. Foods 2021, 10, 1256. [Google Scholar] [CrossRef]
- Gonçalves, S.P.C.; Strauss, M.; Martinez, D.S.T. The Positive Fate of Biochar Addition to Soil in the Degradation of PHBV-Silver Nanoparticle Composites. Environ. Sci. Technol. 2018, 52, 13845–13853. [Google Scholar] [CrossRef]
- Muller, J.; González-Martínez, C.; Chiralt, A. Poly(lactic) acid (PLA) and starch bilayer films, containing cinnamaldehyde, obtained by compression moulding. Eur. Polym. J. 2017, 95, 56–70. [Google Scholar] [CrossRef]
- Miyague, L.; Macedo, R.E.; Meca, G.; Holley, R.A.; Luciano, F.B. Combination of phenolic acids and essential oils against Listeria monocytogenes. LWT 2015, 64, 333–336. [Google Scholar] [CrossRef]
- ISO 20200, ISO 20200, Plastics-Determination of the Degree of Disintegration pf Plastic Materials under Simulated Composting in a Laboratory -Scale Test. 2004. Available online: https://www.une.org (accessed on 1 December 2020).
- UNE-EN ISO 14855-1, Asociación Española de Normalización. Determinación de la Biodegradabilidad Aeróbica Final de Materiales Plásticos en Condiciones de Compostaje Controladas. In Método Según el Análisis de Dióxido de Carbono Generado; Parte 1: Método general; Asociación Española de Normalización: Madrid, Spain, 2012.
- Balaguer, M.P.; Villanova, J.; Cesar, G.; Gavara, R.; Hernandez-Munoz, P. Compostable properties of antimicrobial bioplastics based on cinnamaldehyde cross-linked gliadins. Chem. Eng. J. 2015, 262, 447–455. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Lopez, J.; Hernández, A.; Rayón, E. Ternary PLA–PHB–Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Muniyasamy, S.; Ofosu, O.; John, M.J.; Anandjiwala, R.D. Mineralization of Poly(lactic acid) (PLA), Poly(3-hydroxybutyrate-co-valerate) (PHBV) and PLA/PHBV Blend in Compost and Soil Environments. J. Renew. Mater. 2016, 4, 133–145. [Google Scholar] [CrossRef]
- Berthet, M.-A.; Angellier-Coussy, H.; Chea, V.; Guillard, V.; Gastaldi, E.; Gontard, N. Sustainable food packaging: Valorising wheat straw fibres for tuning PHBV-based composites properties. Compos. Part A Appl. Sci. Manuf. 2015, 72, 139–147. [Google Scholar] [CrossRef]
- Requena, R.; Jiménez, A.; Vargas, M.; Chiralt, A. Effect of plasticizers on thermal and physical properties of compression-moulded poly[(3-hydroxybutyrate)-co-(3-hydroxyvalerate)] films. Polym. Test. 2016, 56, 45–53. [Google Scholar] [CrossRef]
- Sharma, S.; Jaiswal, A.K.; Duffy, B.; Jaiswal, S. Ferulic acid incorporated active films based on poly(lactide)/poly(butylene adipate-co-terephthalate) blend for food packaging. Food Packag. Shelf Life 2020, 24, 100491. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Chiralt, A. Use of Phenolic Acids to Obtain Active Films Based on PLA-PHBV. Food Packaging and Shelf Life 2022. under review. [Google Scholar]
- Arrieta, M. Films de PLA y PLA-PHB Plastificados Para su Aplicación en Envases de Alimentos. Caracterización y Análisis de Los Procesos de Degradación. Ph.D. Thesis, Universidad Politécnica de Valencia, Valencia, Spain, 2014; pp. 239–242. Available online: http://hdl.handle.net/10251/39338 (accessed on 1 December 2020).
- Cano, A.I.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Biodegradation behavior of starch-PVA films as affected by the incorporation of different antimicrobials. Polym. Degrad. Stab. 2016, 132, 11–20. [Google Scholar] [CrossRef]
- El-Hadi, A.M.; Schnabel, R.; Straube, E.; Müller, G.; Henning, S. Correlation between degree of crystallinity, morphology, glass temperature, mechanical properties and biodegradation of poly (3-hydroxyalkanoate) PHAs and their blends. Polym. Test. 2002, 21, 665–674. [Google Scholar] [CrossRef]
- Balaguer, M.; Aliaga, C.; Fito, C.; Hortal, M. Compostability assessment of nano-reinforced poly(lactic acid) films. Waste Manag. 2016, 48, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Tampau, A.; González-Martínez, C.; Chiralt, A. Biodegradability and disintegration of multilayer starch films with electrospun PCL fibres encapsulating carvacrol. Polym. Degrad. Stab. 2020, 173, 109100. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Puglia, D.; Dominici, F.; Santulli, C.; Kenny, J.; Torre, L. Investigation of thermo-mechanical, chemical and degradative properties of PLA-limonene films reinforced with cellulose nanocrystals extracted from Phormium tenax leaves. Eur. Polym. J. 2014, 56, 77–91. [Google Scholar] [CrossRef]
- Weng, Y.-X.; Wang, Y.; Wang, X.-L.; Wang, Y.-Z. Biodegradation behavior of PHBV films in a pilot-scale composting condition. Polym. Test. 2010, 29, 579–587. [Google Scholar] [CrossRef]
- Husárová, L.; Pekařová, S.; Stloukal, P.; Kucharzcyk, P.; Verney, V.; Commereuc, S.; Ramone, A.; Koutny, M. Identification of important abiotic and biotic factors in the biodegradation of poly(l-lactic acid). Int. J. Biol. Macromol. 2014, 71, 155–162. [Google Scholar] [CrossRef]
- Ozkoc, G.; Kemaloglu, S. Morphology, Biodegradability, Mechanical, and Thermal Properties of Nanocomposite Films Based on PLA and Plasticized PLA. J. Appl. Polym. Sci. 2009, 114, 2481–2487. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R. Hydrolysis and Biodegradation of Poly (lactic acid). Adv. Polym. Sci. 2018, 279, 119–151. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Herald, P.J.; Davidson, P.M. Antibacterial Activity of Selected Hydroxycinnamic Acids. J. Food Sci. 1983, 48, 1378–1379. [Google Scholar] [CrossRef]
- Ramos-Nino, M.; Clifford, M.; Adams, M. Quantitative structure activity relationship for the effect of benzoic acids, cinnamic acids and benzaldehydes onListeria monocytogenes. J. Appl. Bacteriol. 1996, 80, 303–310. [Google Scholar] [CrossRef]
- Phan, T.-N.; Nguyen, P.T.M.; Abranches, J.; Marquis, R.E. Fluoride and organic weak acids as respiration inhibitors for oral streptococci in acidified environments. Oral Microbiol. Immunol. 2002, 17, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.; Couto, J.A.; Figueiredo, A.; Tóth, I.; Rangel, A.; Hogg, T. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Int. J. Food Microbiol. 2009, 135, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Maldonado, A.; Schieber, A.; Gänzle, M. Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
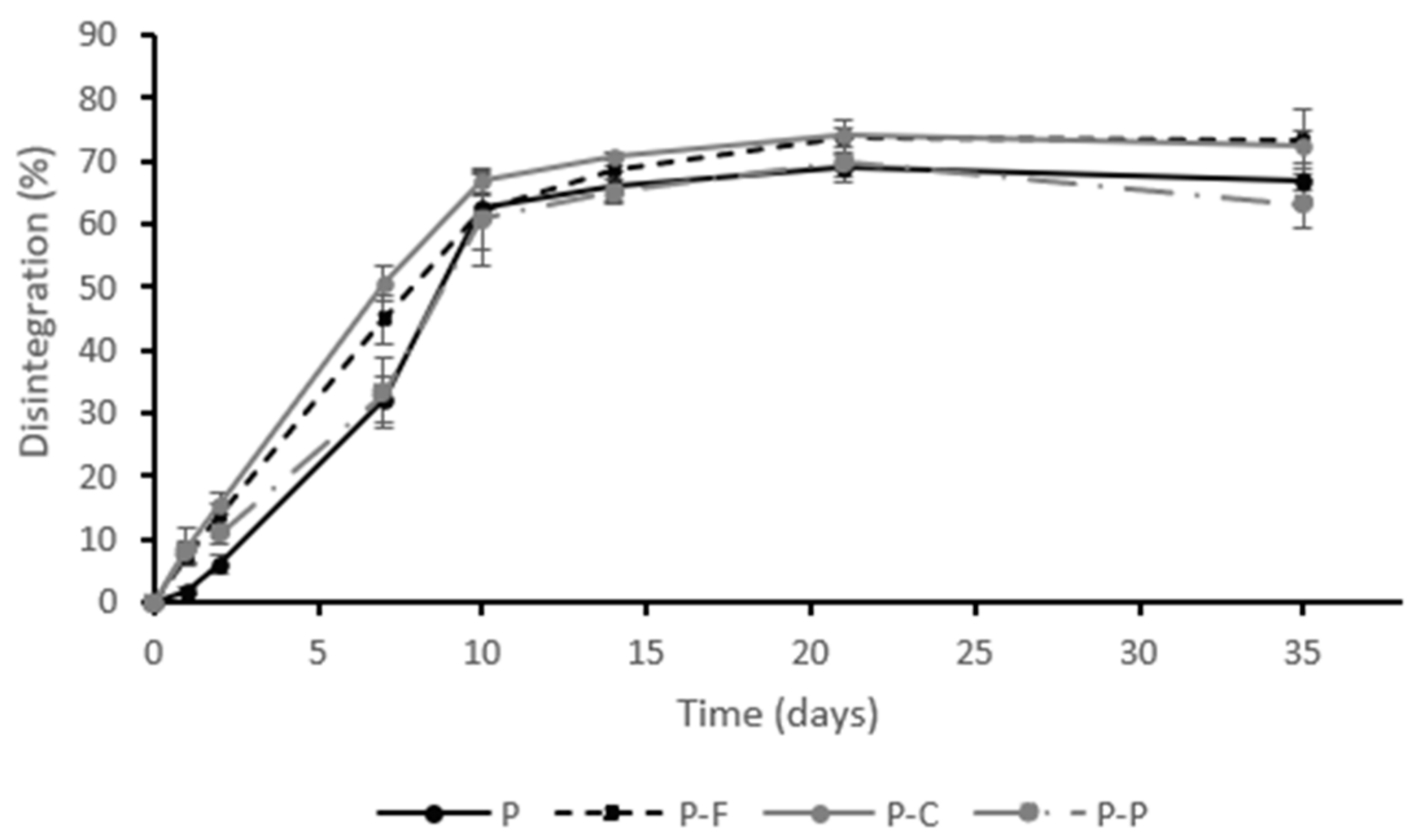
 ) and PLA-PHBV films P (
) and PLA-PHBV films P (  ), PLA-PHBV films containing ferulic acid P-F (
), PLA-PHBV films containing ferulic acid P-F (  ), p-coumaric acid P-C (+) and protocatechuic acid P-P (
), p-coumaric acid P-C (+) and protocatechuic acid P-P (  ) throughout the composting time. Experimental data (symbols) and Hill’s fitted model (lines).
) throughout the composting time. Experimental data (symbols) and Hill’s fitted model (lines).
 ) and PLA-PHBV films P (
) and PLA-PHBV films P (  ), PLA-PHBV films containing ferulic acid P-F (
), PLA-PHBV films containing ferulic acid P-F (  ), p-coumaric acid P-C (+) and protocatechuic acid P-P (
), p-coumaric acid P-C (+) and protocatechuic acid P-P (  ) throughout the composting time. Experimental data (symbols) and Hill’s fitted model (lines).
) throughout the composting time. Experimental data (symbols) and Hill’s fitted model (lines).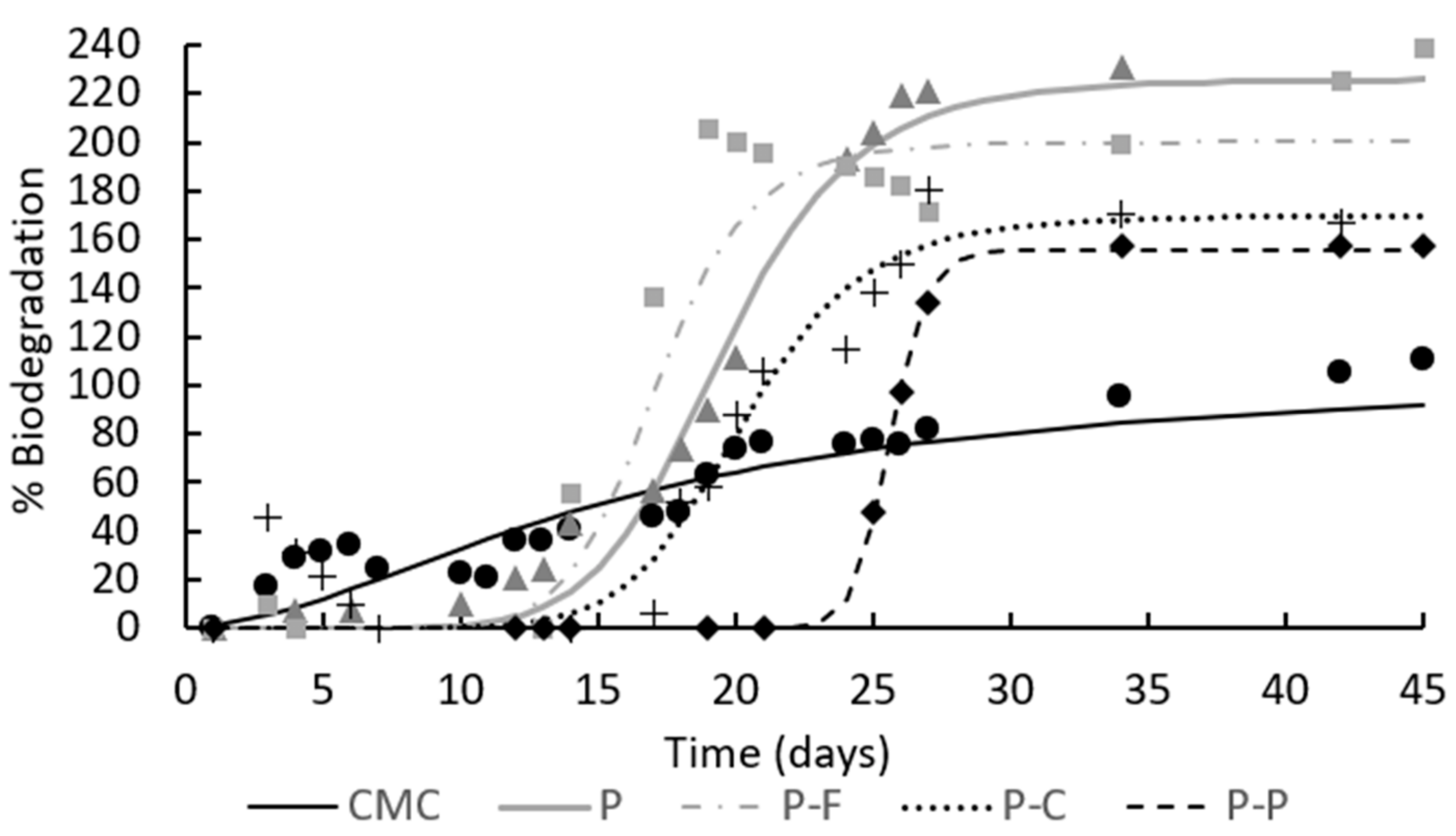
| Sample/Reactor | MC (%) | Thickness (µm) | C (%) |
|---|---|---|---|
| P | 0.1600 ± 0.0006 a | 146 ± 0.015 b | 52.0 ± 0.2 a |
| P-F | 0.1500 ± 0.0010 b | 153 ± 0.018 a | 52.0 ± 0.6 a |
| P-C | 0.0300 ± 0.0002 d | 144 ± 0.016 c | 52.1 ± 0.3 a |
| P-P | 0.1000 ± 0.0008 c | 137 ± 0.018 d | 52.0 ± 0.4 a |
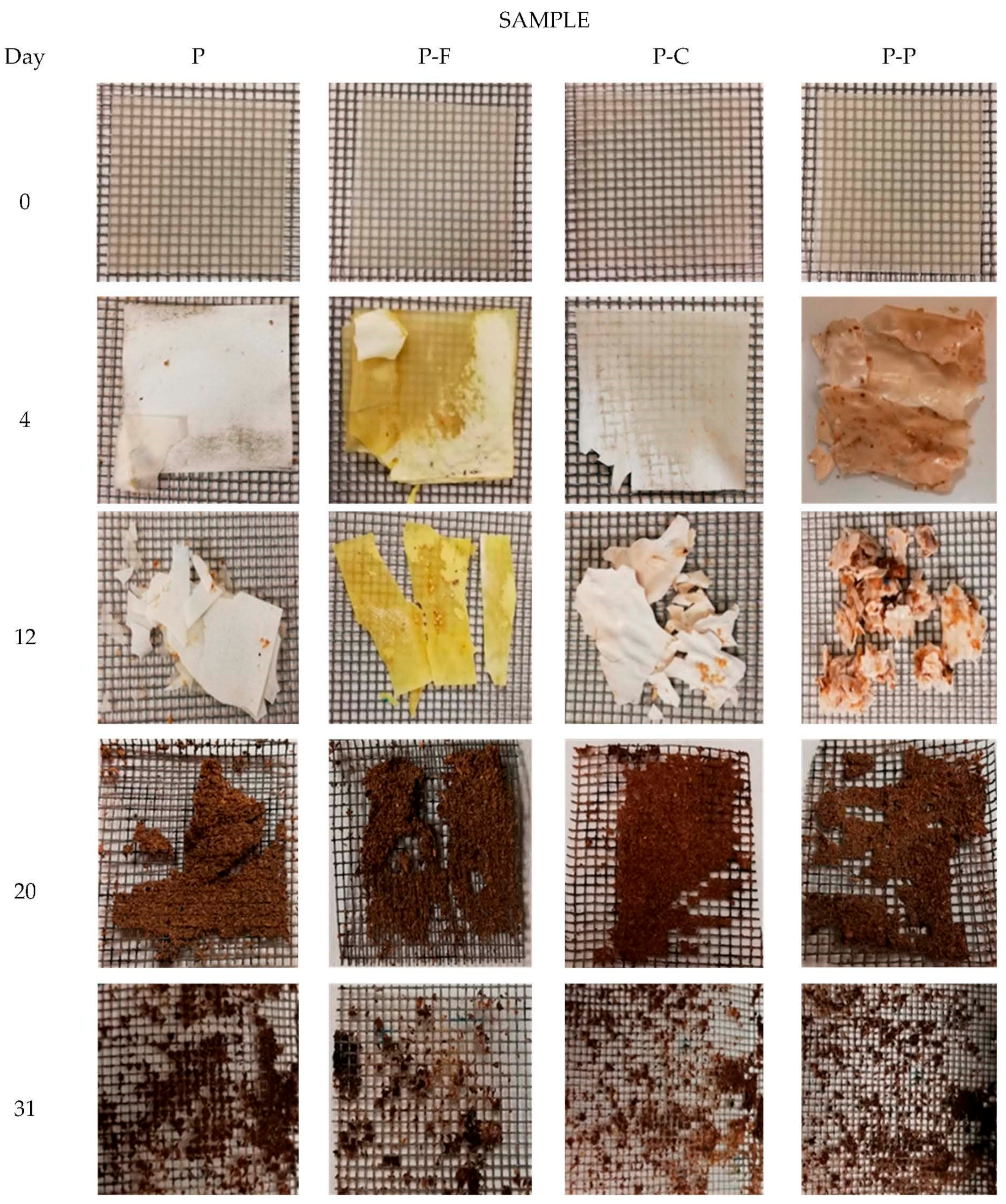
| Reactor | VS (g Volatiles/100 g DS) Post-Composting | R (%) | Disintegration D35 (%) |
|---|---|---|---|
| SSR (blank) | 90.5 ± 0.9 a | 40 ± 1.8 d | - |
| P | 86 ± 1.3 b | 58 ± 1.5 b | 89 ± 2 ab |
| P-F | 82 ± 1.0 c | 64 ± 1.6 a | 92 ± 5 a |
| P-C | 85 ± 1.2 b | 53 ± 0.6 c | 85 ± 3 b |
| P-P | 89 ± 1.6 a | 54 ± 2.0 c | 88 ± 4 b |
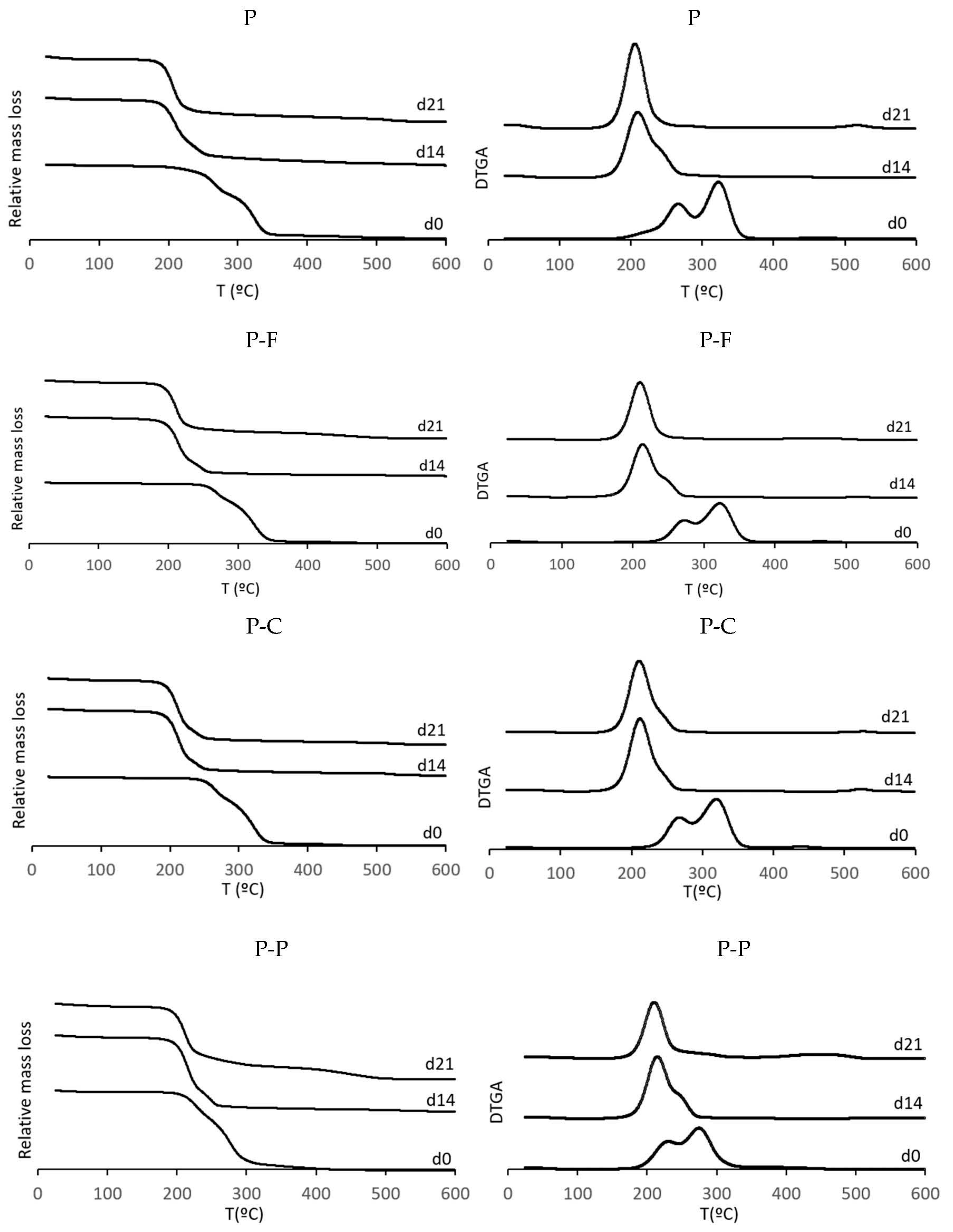
| Sample | Day | To | Tp1 | Tp2 | Residual Mass (%) |
|---|---|---|---|---|---|
| P | 0 | 197.0 ± 3.0 c,1 | 268.0 ± 3.0 b,1 | 320.0 ± 3.0 a,1 | 0.4 ± 0.1 b,2 |
| 14 | 181.0 ± 4.0 a,2 | 210.0 ± 4.0 bc,2 | 240.0 ± 4.0 c,2 | 2.5 ± 0.5 b,1 | |
| 21 | 180.8 ± 0.3 b,2 | 207.4 ± 1.1 c,2 | - | 4.0 ± 1.5 abc,1 | |
| P-F | 0 | 242.8 ± 2.0 a,1 | 273.0 ± 1.0 a,1 | 324.0 ± 3.0 a,1 | 0.5 ± 0.1 ab,3 |
| 14 | 186.5 ± 0.1 b,2 | 214.7 ± 0.2 b,2 | 248.0 ± 0.7 ab,2 | 1.1 ± 0.1 c,2 | |
| 21 | 184.5 ± 1.0 a,3 | 210.8 ± 2.0 b,3 | - | 3.6 ± 0.2 b,1 | |
| P-C | 0 | 207.0 ± 3.0 b,1 | 269.0 ± 1.0 b,1 | 321.0 ± 1.0 a,1 | 0.7 ± 0.1 a,2 |
| 14 | 184.9 ± 0.1 b,2 | 212.9 ± 0.4 c,2 | 247.3 ± 1.0 b,2 | 1.9 ± 0.1 b,1 | |
| 21 | 184.2 ± 0.5 a,2 | 211.8 ± 0.5 a,3 | - | 2.3 ± 0.5 c,1 | |
| P-P | 0 | 176.0 ± 2.0 d,3 | 232.0 ± 3.0 c,1 | 275.0 ± 1.0 b,1 | 0.6 ± 0.1 ab,3 |
| 14 | 186.9 ± 0.2 b,1 | 215.2 ± 0.1 a,2 | 249.1 ± 0.5 a,2 | 3.8 ± 0.6 a,2 | |
| 21 | 183.6 ± 1.0 a,2 | 210.6 ± 0.1 b,3 | - | 5.0 ± 0.5 a,1 |
| Sample | n | k (days) | Bmax (%) | R2 |
|---|---|---|---|---|
| CMC | 1.8 | 15.5 | 105 | 0.92 |
| P | 8.0 | 19.5 | 226 | 0.89 |
| P-F | 10.1 | 17.1 | 200 | 0.80 |
| P-C | 9.0 | 20.3 | 170 | 0.83 |
| P-P | 39.1 | 25.6 | 156 | 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-García, E.; Vargas, M.; Chiralt, A.; González-Martínez, C. Biodegradation of PLA-PHBV Blend Films as Affected by the Incorporation of Different Phenolic Acids. Foods 2022, 11, 243. https://doi.org/10.3390/foods11020243
Hernández-García E, Vargas M, Chiralt A, González-Martínez C. Biodegradation of PLA-PHBV Blend Films as Affected by the Incorporation of Different Phenolic Acids. Foods. 2022; 11(2):243. https://doi.org/10.3390/foods11020243
Chicago/Turabian StyleHernández-García, Eva, Maria Vargas, Amparo Chiralt, and Chelo González-Martínez. 2022. "Biodegradation of PLA-PHBV Blend Films as Affected by the Incorporation of Different Phenolic Acids" Foods 11, no. 2: 243. https://doi.org/10.3390/foods11020243
APA StyleHernández-García, E., Vargas, M., Chiralt, A., & González-Martínez, C. (2022). Biodegradation of PLA-PHBV Blend Films as Affected by the Incorporation of Different Phenolic Acids. Foods, 11(2), 243. https://doi.org/10.3390/foods11020243






